Abstract
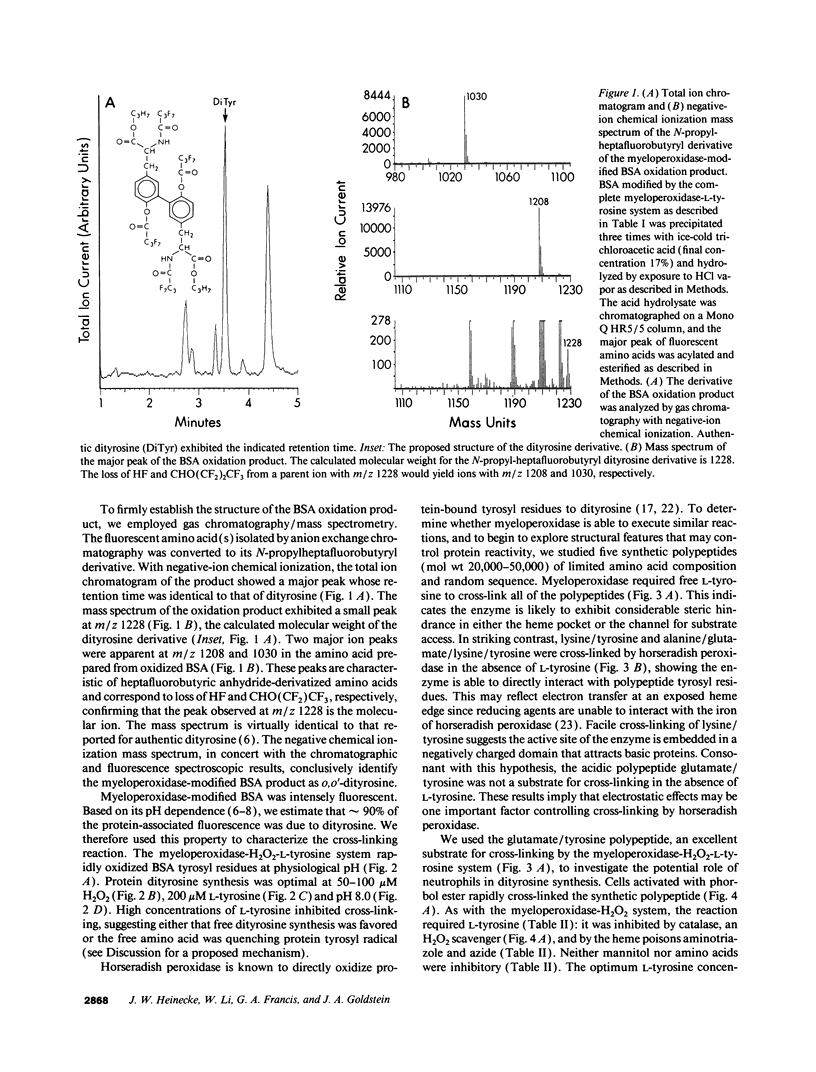
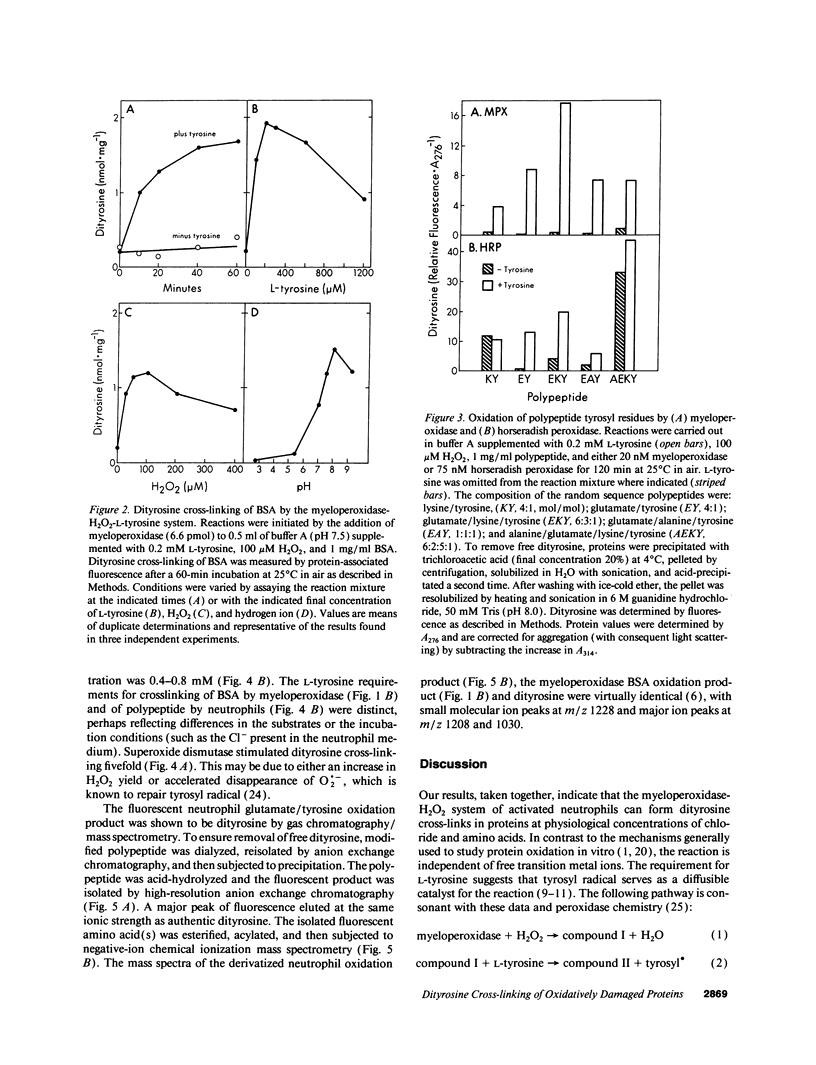
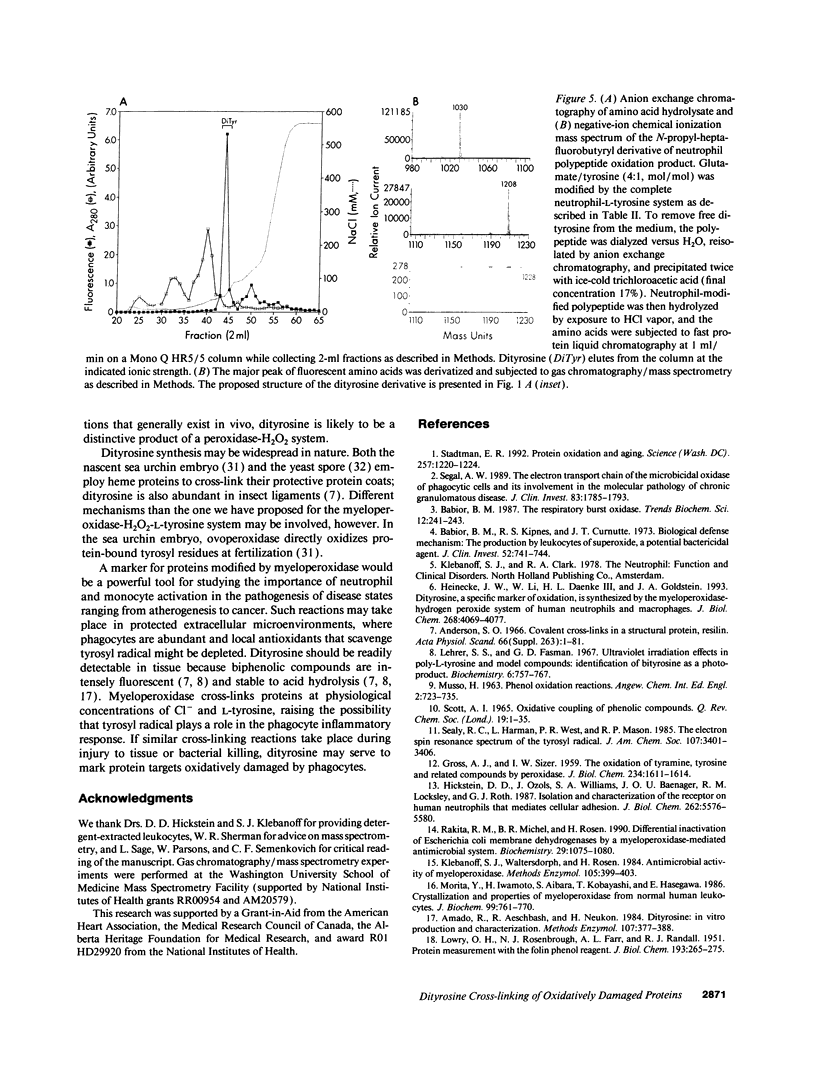
Phagocytes generate H2O2 for use by a secreted heme enzyme, myeloperoxidase, to kill invading bacteria, viruses, and fungi. We have explored the possibility that myeloperoxidase might also convert L-tyrosine to a radical catalyst that cross-links proteins. Protein-bound tyrosyl residues exposed to myeloperoxidase, H2O2, and L-tyrosine were oxidized to o,o'-dityrosine, a stable product of the tyrosyl radical. The cross-linking reaction required L-tyrosine but was independent of halide and free transition metal ions; the heme poisons azide and aminotriazole were inhibitory. Activated neutrophils likewise converted polypeptide tyrosines to dityrosine. The pathway for oxidation of peptide tyrosyl residues was dependent upon L-tyrosine and was inhibited by heme poisons and catalase. Dityrosine synthesis was little affected by plasma concentrations of Cl- and amino acids, suggesting that the reaction pathway might be physiologically relevant. The requirement for free L-tyrosine and H2O2 for dityrosine formation and the inhibition by heme poisons support the hypothesis that myeloperoxidase catalyzes the cross-linking of proteins by a peroxidative mechanism involving tyrosyl radical. In striking contrast to the pathways generally used to study protein oxidation in vitro, the reaction does not require free metal ions. We speculate that protein dityrosine cross-linking by myeloperoxidase may play a role in bacterial killing or injuring normal tissue. The intense fluorescence and stability of biphenolic compounds may allow dityrosine to act as a marker for proteins oxidatively damaged by myeloperoxidase in phagocyte-rich inflammatory lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeschbach R., Amadò R., Neukom H. Formation of dityrosine cross-links in proteins by oxidation of tyrosine residues. Biochim Biophys Acta. 1976 Aug 9;439(2):292–301. doi: 10.1016/0005-2795(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Amadò R., Aeschbach R., Neukom H. Dityrosine: in vitro production and characterization. Methods Enzymol. 1984;107:377–388. doi: 10.1016/0076-6879(84)07026-9. [DOI] [PubMed] [Google Scholar]
- Anderson S. O. Covalent cross-links in a structural protein, resilin. Acta Physiol Scand Suppl. 1966;263:1–81. [PubMed] [Google Scholar]
- Ator M. A., David S. K., Ortiz de Montellano P. R. Structure and catalytic mechanism of horseradish peroxidase. Regiospecific meso alkylation of the prosthetic heme group by alkylhydrazines. J Biol Chem. 1987 Nov 5;262(31):14954–14960. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayse G. S., Michaels A. W., Morrison M. The peroxidase-catalyzed oxidation of tyrosine. Biochim Biophys Acta. 1972 Sep 19;284(1):34–42. doi: 10.1016/0005-2744(72)90043-5. [DOI] [PubMed] [Google Scholar]
- Briza P., Winkler G., Kalchhauser H., Breitenbach M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem. 1986 Mar 25;261(9):4288–4294. [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- DeFelippis M. R., Murthy C. P., Faraggi M., Klapper M. H. Pulse radiolytic measurement of redox potentials: the tyrosine and tryptophan radicals. Biochemistry. 1989 May 30;28(11):4847–4853. doi: 10.1021/bi00437a049. [DOI] [PubMed] [Google Scholar]
- Foerder C. A., Shapiro B. M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS A. J., SIZER I. W. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959 Jun;234(6):1611–1614. [PubMed] [Google Scholar]
- Garcia-Castineiras S., Dillon J., Spector A. Detection of bityrosine in cataractous human lens protein. Science. 1978 Feb 24;199(4331):897–899. doi: 10.1126/science.622574. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Li W., Daehnke H. L., 3rd, Goldstein J. A. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993 Feb 25;268(6):4069–4077. [PubMed] [Google Scholar]
- Hickstein D. D., Ozols J., Williams S. A., Baenziger J. U., Locksley R. M., Roth G. J. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J Biol Chem. 1987 Apr 25;262(12):5576–5580. [PubMed] [Google Scholar]
- Hunter E. P., Desrosiers M. F., Simic M. G. The effect of oxygen, antioxidants, and superoxide radical on tyrosine phenoxyl radical dimerization. Free Radic Biol Med. 1989;6(6):581–585. doi: 10.1016/0891-5849(89)90064-6. [DOI] [PubMed] [Google Scholar]
- Hurst J. K., Barrette W. C., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24(4):271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Waltersdorph A. M., Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer S. S., Fasman G. D. Ultraviolet irradiation effects in poly-L-tyrosine and model compounds. Identification of bityrosine as a photoproduct. Biochemistry. 1967 Mar;6(3):757–767. doi: 10.1021/bi00855a017. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Dityrosine formation in calmodulin. Biochemistry. 1987 Feb 10;26(3):695–704. doi: 10.1021/bi00377a006. [DOI] [PubMed] [Google Scholar]
- Meucci E., Mordente A., Martorana G. E. Metal-catalyzed oxidation of human serum albumin: conformational and functional changes. Implications in protein aging. J Biol Chem. 1991 Mar 15;266(8):4692–4699. [PubMed] [Google Scholar]
- Morita Y., Iwamoto H., Aibara S., Kobayashi T., Hasegawa E. Crystallization and properties of myeloperoxidase from normal human leukocytes. J Biochem. 1986 Mar;99(3):761–770. doi: 10.1093/oxfordjournals.jbchem.a135535. [DOI] [PubMed] [Google Scholar]
- Prince R. C. Tyrosine radicals. Trends Biochem Sci. 1988 Aug;13(8):286–288. doi: 10.1016/0968-0004(88)90119-3. [DOI] [PubMed] [Google Scholar]
- Rakita R. M., Michel B. R., Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990 Jan 30;29(4):1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- Segal A. W. The electron transport chain of the microbicidal oxidase of phagocytic cells and its involvement in the molecular pathology of chronic granulomatous disease. J Clin Invest. 1989 Jun;83(6):1785–1793. doi: 10.1172/JCI114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]



