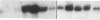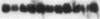Abstract
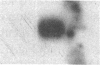
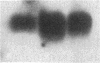
Proliferation and functional activation of endothelial cells within a tissue site of inflammation are regulated by humoral factors released by cells, such as T lymphocytes and monocytes, infiltrating the perivascular space. In the present study we investigated the effects of interleukin 3 (IL-3), an activated T lymphocyte-derived cytokine, on cultured human umbilical vein endothelial cells (HUVEC). Proliferative activity, evaluated both by estimation of the fraction of cells in the S phase and by direct cell count demonstrated that IL-3, at the dose of 25 ng/ml, enhances more than threefold both DNA synthesis and cell proliferation above baseline control conditions. Binding studies with radioiodinated ligand demonstrated that HUVEC constitutively express a smaller number of IL-3 binding sites (approximately 99 binding sites per cell, with an apparent Kd of 149 pM). Accordingly, molecular analysis showed the presence of transcripts for both alpha and beta subunits of the IL-3 receptor. Functional activation of endothelial cells was evaluated by the expression of the endothelial-leukocyte adhesion molecule 1 (ELAM-1) transcript and by leukocyte adhesion. The ELAM-1 gene transcript was clearly detectable 4 h after IL-3 addition and started to decrease after 12 h. Moreover, IL-3-induced ELAM-1 transcription was followed by enhanced adhesion of neutrophils and CD4+ T cells to HUVEC. The findings that IL-3 can stimulate both proliferation and functional activation of endothelial cells suggest that this cytokine can be involved in sustaining the process of chronic inflammation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Brizzi M. F., Avanzi G. C., Veglia F., Clark S. C., Pegoraro L. Expression and modulation of IL-3 and GM-CSF receptors in human growth factor dependent leukaemic cells. Br J Haematol. 1990 Oct;76(2):203–209. doi: 10.1111/j.1365-2141.1990.tb07872.x. [DOI] [PubMed] [Google Scholar]
- Budel L. M., Elbaz O., Hoogerbrugge H., Delwel R., Mahmoud L. A., Löwenberg B., Touw I. P. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood. 1990 Apr 1;75(7):1439–1445. [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Defilippi P., Turrini F., Sanavio F., Edgell C. J., Aglietta M., Arese P., Mantovani A. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989 Feb 2;337(6206):471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Ziche M., Wang J. M., Alessi D., Morbidelli L., Cremona O., Bosia A., Marchisio P. C., Mantovani A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991 Mar;87(3):986–995. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Dobrina A., Schwartz B. R., Carlos T. M., Ochs H. D., Beatty P. G., Harlan J. M. CD11/CD18-independent neutrophil adherence to inducible endothelial-leucocyte adhesion molecules (E-LAM) in vitro. Immunology. 1989 Aug;67(4):502–508. [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone D. G., Wahl S. M., Tsokos M., Cattell H., Decker J. L., Wilder R. L. Immune function in severe, active rheumatoid arthritis. A relationship between peripheral blood mononuclear cell proliferation to soluble antigens and synovial tissue immunohistologic characteristics. J Clin Invest. 1984 Oct;74(4):1173–1185. doi: 10.1172/JCI111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazi A., Cattoretti G., Schiró R., Siena S., Bregni M., Di Nicola M., Gianni A. M. Recombinant human interleukin-3 and recombinant human granulocyte-macrophage colony-stimulating factor administered in vivo after high-dose cyclophosphamide cancer chemotherapy: effect on hematopoiesis and microenvironment in human bone marrow. Blood. 1992 May 15;79(10):2610–2619. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Razin E., Leslie K. B., Schrader J. W. Connective tissue mast cells in contact with fibroblasts express IL-3 mRNA. Analysis of single cells by polymerase chain reaction. J Immunol. 1991 Feb 1;146(3):981–987. [PubMed] [Google Scholar]
- Saegusa Y., Ziff M., Welkovich L., Cavender D. Effect of inflammatory cytokines on human endothelial cell proliferation. J Cell Physiol. 1990 Mar;142(3):488–495. doi: 10.1002/jcp.1041420307. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Watt S. L., Auerbach R. A mitogenic factor for endothelial cells obtained from mouse secondary mixed leukocyte cultures. J Immunol. 1986 Jan;136(1):197–202. [PubMed] [Google Scholar]
- Wimperis J. Z., Niemeyer C. M., Sieff C. A., Mathey-Prevot B., Nathan D. G., Arceci R. J. Granulocyte-macrophage colony-stimulating factor and interleukin-3 mRNAs are produced by a small fraction of blood mononuclear cells. Blood. 1989 Oct;74(5):1525–1530. [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]