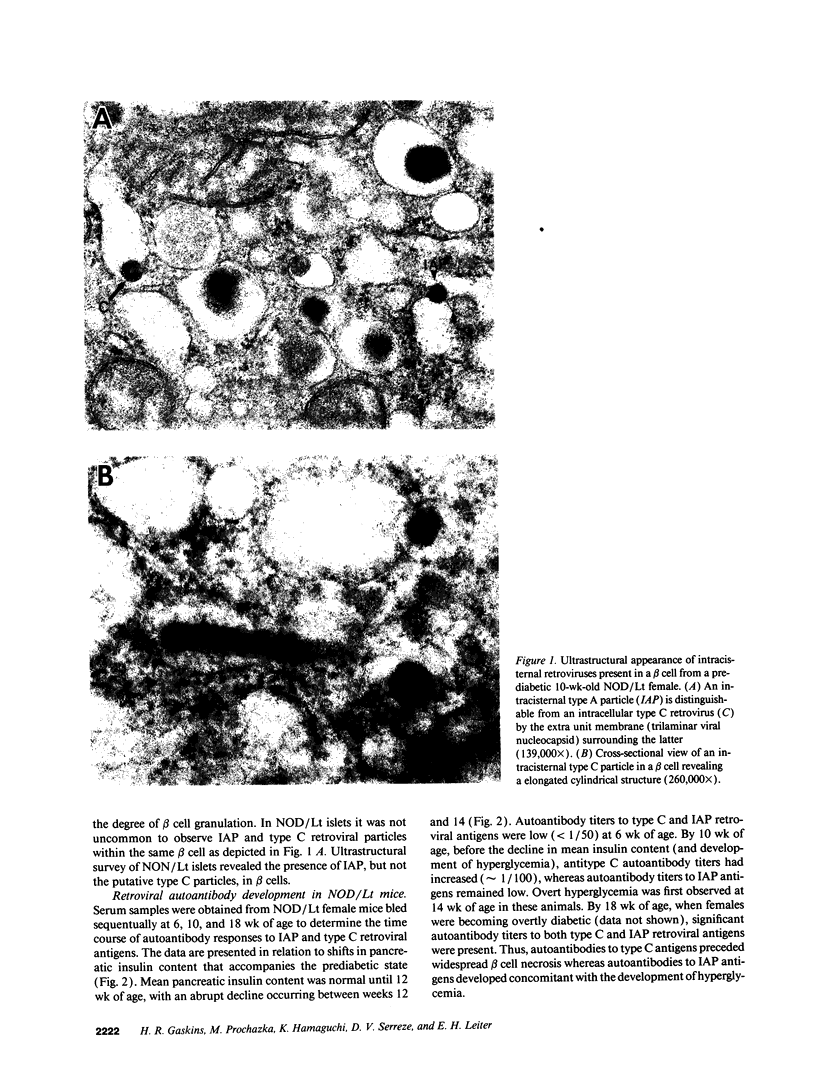
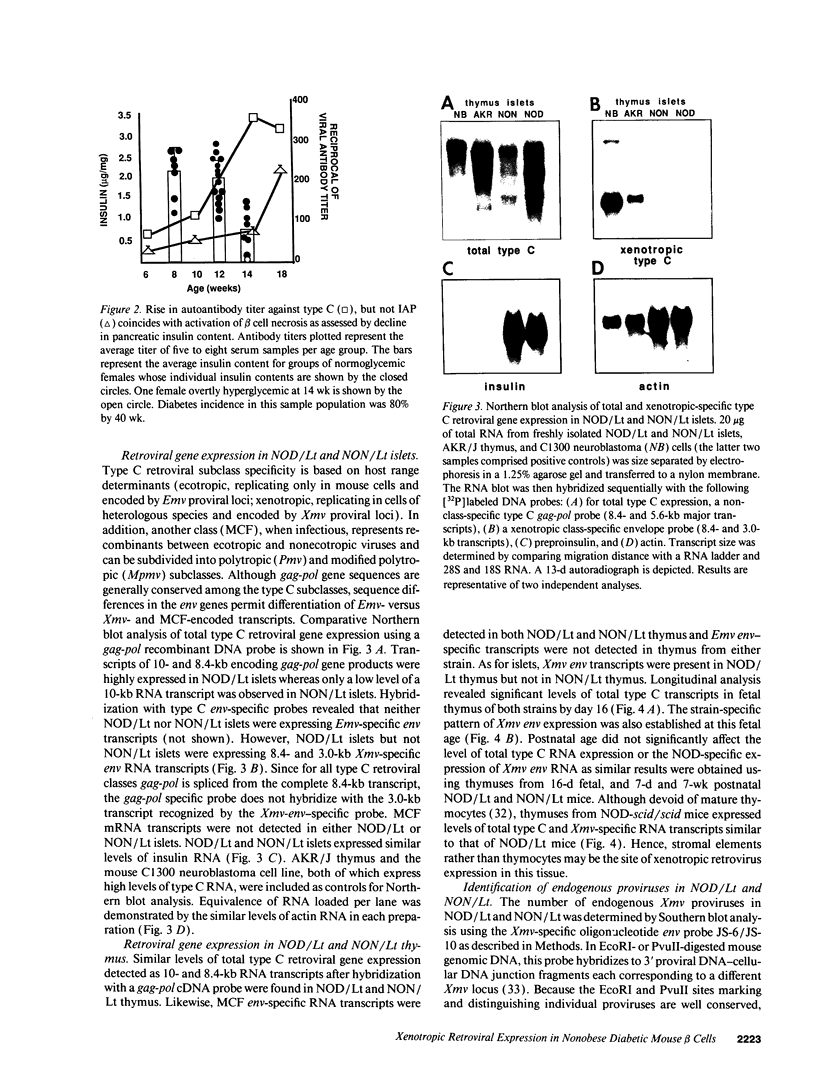
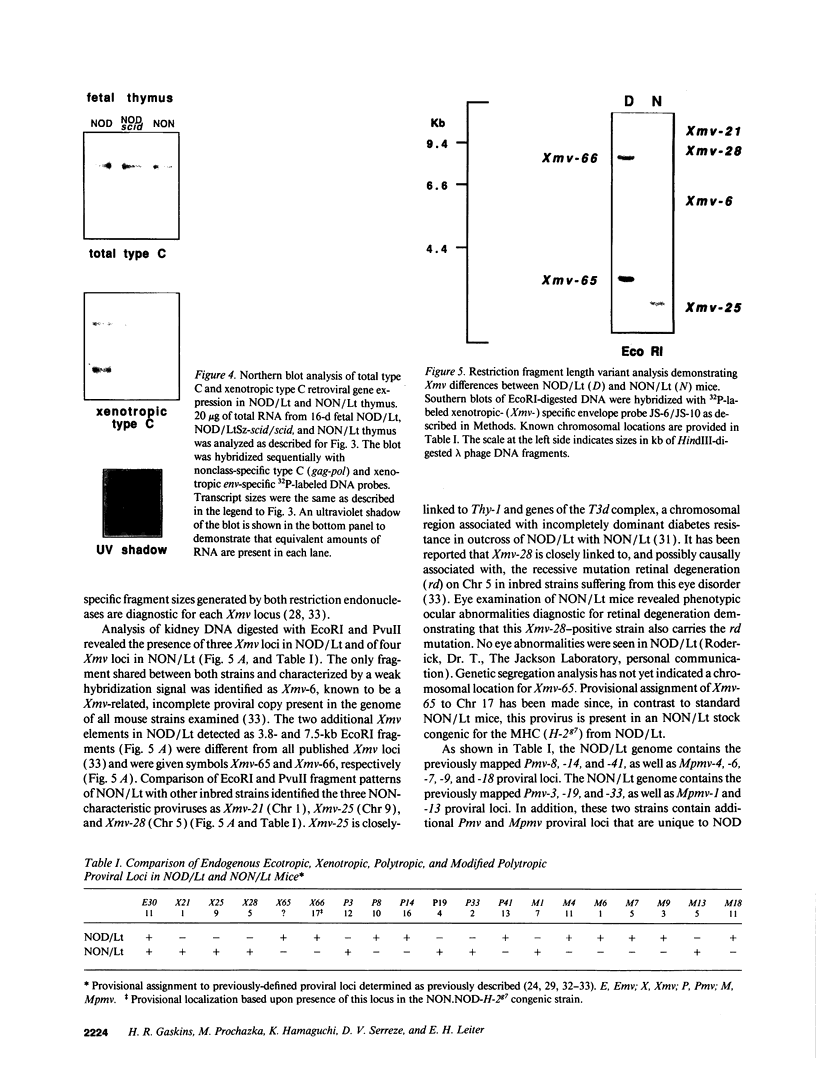
Abstract
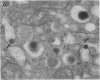
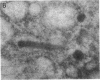
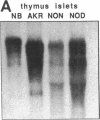
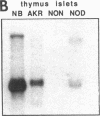
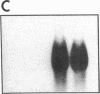
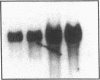
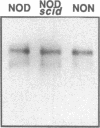
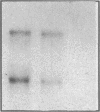
Endogeneous retroviral expression in beta cells is a feature of prediabetes in nonobese diabetic (NOD) mice. The purpose of this study was to characterize the class-specific pattern of retroviral gene expression in NOD/Lt beta cells versus a related, but diabetes-resistant strain, NON/Lt. Electron microscopic comparison of beta cells from both strains indicated low constitutive expression of the intracisternal type A (IAP) retroviral class. However, NOD beta cells, in contrast to NON beta cells, expressed an additional intracisternal retroviral form resembling a type C particle. Antibodies against both IAP and type C were detected in NOD, with the humoral response to type C, but not IAP, preceding decline in beta cell function. RNA was extracted from freshly isolated islets from NOD and NON males. Comparative Northern blot analysis of total type C retroviral gene expression using a gag-pol DNA probe corroborated expression of endogenous type C proviruses in both NOD and NON islet cells and thymus. Use of class-specific retroviral probes identified the class of expressed endogenous retrovirus distinguishing the two inbred strains. The single ecotropic provirus present in both the NOD and NON genome (Emv-30) was not expressed in islets or thymus of either strain. Comparison of endogenous xenotropic provirus content by Southern blot analysis revealed two unique xenotropic loci (Xmv-65, -66) in NOD; 8.4 and 3.0 kb xenotropic envelope (env) RNA transcripts were detected in NOD, but not NON islets and thymus. NON contained three xenotropic loci common to other inbred strains (Xmv-21, -25, and -28). Both strains were partially characterized for content of recombinant (polytropic and modified polytropic) proviruses. IAP RNA expression was common to both NOD and NON islets and hence could not be specifically associated with the unique intracisternal type C particle found in NOD, but not NON beta cells. In conclusion, this study shows that expression of xenotropic type C but not IAP distinguishes retroviral activity in NOD/Lt versus NON/Lt beta cells. The potential pathogenic role of retroviral gene expression in NOD beta cells is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel M. C., Rossini A. A., Williams R. M., Like A. A. Viral studies in streptozotocin-induced pancreatic insulitis. Diabetologia. 1978 Oct;15(4):327–336. doi: 10.1007/BF02573827. [DOI] [PubMed] [Google Scholar]
- Brilliant M. H., Gondo Y., Eicher E. M. Direct molecular identification of the mouse pink-eyed unstable mutation by genome scanning. Science. 1991 Apr 26;252(5005):566–569. doi: 10.1126/science.1673574. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic analysis of endogenous xenotropic murine leukemia viruses: association with two common mouse mutations and the viral restriction locus Fv-1. J Virol. 1989 Apr;63(4):1763–1774. doi: 10.1128/jvi.63.4.1763-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino-Kurihara H., Fujita H., Hakura A., Nonaka K., Tarui S. Morphological aspects on pancreatic islets of non-obese diabetic (NOD) mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(2):107–120. doi: 10.1007/BF02912089. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K., Gaskins H. R., Leiter E. H. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes. 1991 Jul;40(7):842–849. doi: 10.2337/diab.40.7.842. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K., Leiter E. H. Comparison of cytokine effects on mouse pancreatic alpha-cell and beta-cell lines. Viability, secretory function, and MHC antigen expression. Diabetes. 1990 Apr;39(4):415–425. doi: 10.2337/diab.39.4.415. [DOI] [PubMed] [Google Scholar]
- Harrison A. K., Murphy F. A. Murine oncornavirus activation in the pancreas during infection with Venezuelan equine encephalitis virus. J Natl Cancer Inst. 1975 Oct;55(4):917–923. doi: 10.1093/jnci/55.4.917. [DOI] [PubMed] [Google Scholar]
- Haskins K., Portas M., Bergman B., Lafferty K., Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R. Improved oligonucleotide labeling and hybridization assay for endogenous nonecotropic murine leukemia proviruses. Mamm Genome. 1991;1(4):260–262. doi: 10.1007/BF00352334. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gause W. C., Gourley M. F., Steinberg A. D. A role for endogenous retroviral sequences in the regulation of lymphocyte activation. J Immunol. 1989 Oct 15;143(8):2448–2451. [PubMed] [Google Scholar]
- Krieg A. M., Gourley M. F., Steinberg A. D. Association of murine lupus and thymic full-length endogenous retroviral expression maps to a bone marrow stem cell. J Immunol. 1991 May 1;146(9):3002–3005. [PubMed] [Google Scholar]
- Krieg A. M., Steinberg A. D. Analysis of thymic endogenous retroviral expression in murine lupus. Genetic and immune studies. J Clin Invest. 1990 Sep;86(3):809–816. doi: 10.1172/JCI114778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Christianson G. J., Serreze D. V., Ting A. T., Worthen S. M. MHC antigen induction by interferon gamma on cultured mouse pancreatic beta cells and macrophages. Genetic analysis of strain differences and discovery of an "occult" class I-like antigen in NOD/Lt mice. J Exp Med. 1989 Oct 1;170(4):1243–1262. doi: 10.1084/jem.170.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter E. H., Hamaguchi K. Viruses and diabetes: diabetogenic role for endogenous retroviruses in NOD mice? J Autoimmun. 1990 Apr;3 (Suppl 1):31–40. doi: 10.1016/s0896-8411(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. The genetics of diabetes susceptibility in mice. FASEB J. 1989 Sep;3(11):2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. The influence of genetic background on the expression of mutations at the diabetes locus in the mouse IV. Male lethal syndrome in CBA/Lt mice. Diabetes. 1981 Dec;30(12):1035–1044. doi: 10.2337/diab.30.12.1035. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Type C retrovirus production by pancreatic beta cells. Association with accelerated pathogenesis in C3H-db/db ("Diabetes") mice. Am J Pathol. 1985 Apr;119(1):22–32. [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Merregaert J., Janowski M., Reddy E. P. Nucleotide sequence of a radiation leukemia virus genome. Virology. 1987 May;158(1):88–102. doi: 10.1016/0042-6822(87)90241-8. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Rossomando A., Offer M., Buxbaum J., Pellicer A. Association of endogenous viral loci with genes encoding murine histocompatibility and lymphocyte differentiation antigens. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5032–5036. doi: 10.1073/pnas.80.16.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Bedigian H. G., Shull M. M., Copeland N. G., Jenkins N. A. Comparative molecular genetic analysis of lymphomas from six inbred mouse strains. J Virol. 1988 Mar;62(3):839–846. doi: 10.1128/jvi.62.3.839-846.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. B., Schmidt J., Rieke L. Retrovirus-induced osteopetrosis in mice. Ultrastructural evidence of early virus production in osteoblasts and osteocytes. Am J Pathol. 1986 Aug;124(2):319–323. [PMC free article] [PubMed] [Google Scholar]
- Nakagawa C., Hanafusa T., Miyagawa J., Yutsudo M., Nakajima H., Yamamoto K., Tomita K., Kono N., Hakura A., Tarui S. Retrovirus gag protein p30 in the islets of non-obese diabetic mice: relevance for pathogenesis of diabetes mellitus. Diabetologia. 1992 Jul;35(7):614–618. doi: 10.1007/BF00400251. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Officer J. E., Tecson N., Estes J. D., Fontanilla E., Rongey R. W., Gardner M. B. Isolation of a neurotropic type C virus. Science. 1973 Sep 7;181(4103):945–947. doi: 10.1126/science.181.4103.945. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Gaskins H. R., Shultz L. D., Leiter E. H. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Frankel W. N., Leiter E. H. NOR/Lt mice: MHC-matched diabetes-resistant control strain for NOD mice. Diabetes. 1992 Jan;41(1):98–106. doi: 10.2337/diab.41.1.98. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Worthen S. M., Leiter E. H. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes. 1989 Nov;38(11):1446–1455. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Bottazzo G. F. Puzzling diabetic transgenic mice: a lesson for human type 1 diabetes? Immunol Today. 1988 Oct;9(10):303–306. doi: 10.1016/0167-5699(88)91322-9. [DOI] [PubMed] [Google Scholar]
- Reuss F. U., Schaller H. C. cDNA sequence and genomic characterization of intracisternal A-particle-related retroviral elements containing an envelope gene. J Virol. 1991 Nov;65(11):5702–5709. doi: 10.1128/jvi.65.11.5702-5709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A., Meruelo D. Viral sequences are associated with many histocompatibility genes. Immunogenetics. 1986;23(4):233–245. doi: 10.1007/BF00373018. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Development of diabetogenic T cells from NOD/Lt marrow is blocked when an allo-H-2 haplotype is expressed on cells of hemopoietic origin, but not on thymic epithelium. J Immunol. 1991 Aug 15;147(4):1222–1229. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Kuff E. L., Jardieu P., Ishizaka K. Molecular mimicry between insulin and retroviral antigen p73. Development of cross-reactive autoantibodies in sera of NOD and C57BL/KsJ db/db mice. Diabetes. 1988 Mar;37(3):351–358. doi: 10.2337/diab.37.3.351. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Shultz L. D. Transplantation analysis of B cell destruction in (NOD x CBA)F1 mouse bone marrow chimeras. Diabetologia. 1990 Feb;33(2):84–92. doi: 10.1007/BF00401045. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Worthen S. M., Shultz L. D. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD x NON)F1 mice. Diabetes. 1988 Feb;37(2):252–255. doi: 10.2337/diab.37.2.252. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988 Jan;62(1):168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga K., Yoon J. W. Association of beta-cell-specific expression of endogenous retrovirus with development of insulitis and diabetes in NOD mouse. Diabetes. 1988 Dec;37(12):1722–1726. doi: 10.2337/diab.37.12.1722. [DOI] [PubMed] [Google Scholar]
- Thurston S. J., Saffer J. D. Ultraviolet shadowing nucleic acids on nylon membranes. Anal Biochem. 1989 Apr;178(1):41–42. doi: 10.1016/0003-2697(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Waters S. H., O'Neil J. J., Melican D. T., Appel M. C. Multiple TCR V beta usage by infiltrates of young NOD mouse islets of Langerhans. A polymerase chain reaction analysis. Diabetes. 1992 Mar;41(3):308–312. doi: 10.2337/diab.41.3.308. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Chai A., Terada M., Mullen Y. Expression of genetically determined diabetes and insulitis in the nonobese diabetic (NOD) mouse at the level of bone marrow-derived cells. Transfer of diabetes and insulitis to nondiabetic (NOD X B10) F1 mice with bone marrow cells from NOD mice. J Exp Med. 1988 Jun 1;167(6):1801–1810. doi: 10.1084/jem.167.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]