Abstract
Duchenne Muscular Dystrophy (DMD) is a fatal X-linked genetic disease, caused by mutations in the dystrophin gene, which cause functional loss of this protein. DMD pathology is associated with an increased production of reactive oxygen and nitrogen species (ROS and RNS). The aim of this work was to study the alterations in NF-κB activation and Interleukin-6 (IL-6) expression induced by membrane depolarization in dystrophic mdx myotubes. Membrane depolarization elicited by electrical stimulation (ES) increased p65 phosphorylation, NF-κB transcriptional activity and NF-κB-dependent IL-6 expression in wt myotubes, whereas in mdx myotubes it had the opposite effect. We have previously shown that depolarization-induced intracellular Ca2+ increases and ROS production are necessary for NF-κB activation and stimulation of gene expression in wt myotubes. Dystrophic myotubes showed a reduced amplitude and area under the curve of the Ca2+ transient elicited by ES. On the other hand, ES induced higher ROS production in mdx than wt myotubes, which were blocked by NOX2 inhibitors. Moreover, mRNA expression and protein levels of the NADPH oxidase subunits; p47phox and gp91phox were increased in mdx myotubes. Looking at ROS-dependence of NF-κB activation we found that in wt myotubes external administration of 50µM H2O2 increased NF-κB activity; after administration of 100 and 200 µM H2O2 there was no effect. In mdx myotubes there was a dose-dependent reduction in NF-κB activity in response to external administration of H2O2, with a significant effect of 100 µM and 200 µM, suggesting that ROS levels are critical for NF-κB activity. Prior blockage with NOX2 inhibitors blunted the effects of ES in both NF-κB activation and IL-6 expression.
Finally, to ascertain whether stimulation of NF-κB and IL-6 gene expression by the inflammatory pathway is also impaired in mdx myotubes, we studied the effect of lipopolysaccharide (LPS) on both NF-κB activation and IL-6 expression. Exposure to LPS induced a dramatic increase in both NF-κB and IL-6 expression in both wt and mdx myotubes, suggesting that the altered IL-6 gene expression after ES in mdx muscle cells is due to dysregulation of Ca2+ release and ROS production, both of which impinge on NF-κB signaling. IL-6 is a key metabolic modulator that is released by skeletal muscle to coordinate a multi-systemic response (liver, muscle, and adipocytes) during physical exercise; the alteration of this response in dystrophic muscles may contribute to an abnormal response to contraction and exercise.
Keywords: Duchenne Muscular Dystrophy, NF-κB, Reactive oxygen species, Interleukin-6, membrane depolarization, calcium
1. INTRODUCTION
Duchenne muscular dystrophy (DMD) is a lethal X-linked human genetic muscular disorder caused by mutations in the dystrophin gene that lead to absence of dystrophin protein [1]. DMD is a severe and progressive muscle wasting disease leading to wheelchair dependence and premature death [2]. DMD patients have an abnormal response to exercise compared to control subjects [3, 4] and it has been shown that running exercise induces rapid muscle damage [5], cell death and worsening of the muscle pathology [4]. However, beneficial effects of exercise through utrophin upregulation in dystrophic patients have been reported as well [6].
A hallmark feature of DMD pathology is the excessive inflammation observed in skeletal muscles due to immune cell infiltrates and an increase in inflammatory mediators [7, 8]. Interleukin-6 (IL-6) is a ubiquitously expressed cytokine with either pro- or anti-inflammatory effects depending on the tissue microenvironment and the concentration [9]. In DMD patients, as well as in the mdx mouse (a DMD model) elevated IL-6 levels have been found both in plasma and muscle [10, 11]. Recently, we reported that there were no differences in IL-6 gene expression between wt and mdx myotubes [12], suggesting that it is the immune cell infiltrates rather than the skeletal muscles themselves that produce the elevated levels of this cytokine.
In normal patients and animals, IL-6 plasma levels are increased by exercise and it appears that contracting muscles are the source of this increase [13]. IL-6 plays a central role in muscle communication with several tissues as liver, adipose cells, and other muscles to increase energy supply during exercise, making it an essential metabolic regulator [13, 14]. Several intracellular signaling pathways have been related to IL-6 expression in skeletal muscle including NF-κB activation, and ROS production [14, 15]. In skeletal muscle cells, membrane depolarization activates NF-κB by a mechanism involving Ca2+ release from the sarcoplasmic reticulum (SR) and ROS production [16]. ROS and RNS have long been implicated in cellular damage and we have previously reported an increase in iNOS expression and nitric oxide (NO) production in mdx myotubes [12]. ROS and RNS have also emerged as important physiological signaling molecules that modify receptor stimulation, enzymatic activity and gene expression in skeletal muscle cells [17]. In normal skeletal muscle NADPH oxidase (NOX) is a major source of ROS both at rest and during contraction [18–20]. NOX is found mainly in transverse tubules [21] and consists in regulatory cytosolic units: p47phox, p67phox, p40phox, a GTPase protein (rac 1 and 2) and the catalytic membrane-bound subunits: gp91phox (also called NOX2) and p22phox. Several studies have shown that NOX2 activity is altered in mdx muscle and could play an important role in dystrophic pathophysiology [22, 23].
Until now, there have been no studies addressing the molecular mechanism involved in NF-κB activation and IL-6 production elicited by membrane depolarization in dystrophic skeletal muscle cells. The aim of this work was to study the alterations in both NF-κB activation and IL-6 gene expression induced by ES between wt and mdx myotubes.
2. MATERIAL AND METHODS
2.1. Cell culture
Primary myotubes from wild type (C57BL/6) and mdx (C57BL/10ScSn-Dmd<mdx>/J) mice were isolated according to the method of Rando and Blau [24]. The myoblasts were grown and differentiated as described previously [25]. For experiments myoblasts were seeded in matrigel-coated dishes or coverslips. When cells reached approximately 70% confluence, growth medium was replaced with differentiation medium (DMEM low glucose, 4% heat inactivated horse serum and 1× Pen-Strep-Glutamine solution) to induce myoblast differentiation. The medium was changed every two days and cells were used for experimental determinations at day 3–4 of differentiation.
2.2. Swimming exercise protocol
A 1-liter beaker filled with water at room temperature was used as swimming pool. Briefly, wt and mdx mice were gently placed in the water to perform 1h of swimming (without any weight on the tail). Later, mice were removed immediately from the swimming pool, dried gently with paper towels and returned to their cages. After 2h the animals were euthanized, their diaphragm muscles were dissected and RNA was extracted using the Trizol reagent according to the manufacturer’s instructions.
2.3. Electric stimulation protocol
Myotubes were stimulated with a stimulation device, that consists of six rows of platinum wires, intercalated 0.5 cm apart, with alternate polarity across a circular Teflon holder that fits in the dish. This was connected to a Grass S48 pulse generator, as described previously [26]. The protocol for electrical stimulation (ES) used was a single train of 250 square wave pulses of 0.5 ms duration at a frequency of 20 Hz (12.5 s total). This protocol was previously shown to be effective in inducing both Ca2+ signaling and gene expression in skeletal muscle cells [26]. For some experiments, high potassium solution (85 NaCl, 60 KCl, 2.5 CaCl2, 1 MgCl2, 5.6 glucose, 10 HEPES-Tris, pH 7.4) was used to study the magnitude of NF-κB activation. Depolarization proceeded during 1 min in the high potassium solution, returning to normal physiological solution (140 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 5.6 glucose, 10 HEPES-Tris, pH 7.4). Protein samples were obtained 30 or 60 min after high potassium depolarization protocol.
2.4. Western blot
Total protein lysates were prepared from differentiated myotubes by homogenizing them in a lysis buffer containing 20mM Tris-HCl (pH 7,5), 1% Triton X-100, 1mM EDTA, 1mM EGTA, 20mM NaF, 1mM Na2P2O7, 10% glycerol, 150mM NaCl, 10mM Na3VO4, 1mM PMSF and protease inhibitors (Complete™, Roche Applied Science). Proteins were separated using SDS-PAGE and transferred to PVDF membranes. The following primary antibodies and their dilutions were used: gp91phox (1:1000; Santa Cruz Biotechnology); p-p65 (Ser536; 1:2000; Cell Signaling), p65 (total; 1:2000; Cell Signaling), IκBα (1:1000; Cell Signaling), and GAPDH (1:2000; Cell Signaling) The protein bands in the blots were visualized using either HRP secondary antibodies and autoradiography films or quantified with Odyssey Imaging System (Li-COR Biosciences).
2.5. NF-κB luciferase reporter activity determinations
Both wt and mdx myoblasts were transduced with a lentivirus containing 5 tandem NF-κB binding site repeats cloned upstream of a luciferase reporter gene and populations that stably expressed the transgene were selected using G418, as described previously [12]. Myotubes were stimulated in differentiation media. Luciferase activity was determined using the luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Light detection was carried out in a Berthold F12 luminometer. Results were normalized with total protein and the ratio “luciferase mg−1” was shown.
2.6. ELISA
Culture media (DMEM with 5% heat inactivated horse serum and antibiotics) was changed one day before the experiment to allow basal IL-6 release stabilization. The ES protocol was applied as described above and after 2 hrs media samples (1mL) were collected from both an ES-stimulated dish and a control dish (no media change before the experiment). Supernatants were spun at 10,000×g for 1 min to remove any cellular debris and kept on ice. ELISA was carried out immediately using a Mouse IL-6 High Sensitivity ELISA Kit (Affymetrix eBioscience #BMS603HS) according to the manufacturer’s instructions. Total proteins were extracted with lysis buffer and the protein concentration was assessed with the BCA method. IL-6 levels (pg/mL) were normalized to the total protein (mg).
2.7. Ca2+ transients
Differentiated myotubes were loaded with 5 µM with Fluo3-AM at 37°C, 30 min in normal physiological solution. Image series during stimulation experiments were obtained with an inverted microscope (Olympus T041) with epifluorescence illumination (XCite® Series 120) equipped with a CCD cooled camera (QImaging, Retiga 2000R) every 0.7 s at room temperature (RT). A filter Wheel (Lambda 10-2, Sutter Instruments) was used to filter 484-nm wavelength. The experiments were performed in Krebs-Ringer's solution (in mM: 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 5.5 glucose, 10 Hepes, pH 7.4). Fluorescence images were analyzed with ImageJ software (NIH, Bethesda) and the average cell fluorescence, F, was calculated from selected regions of interest (ROI). Fluorescence data (F) normalized with respect to basal fluorescence (F0) were expressed as [F−F0]/F0 (ΔF/F0).
2.8. ROS production
Myotubes were loaded with 5 µM CM-H2DCFA for 15 min at 37°C in imaging solution. The cells on coverslips were washed with Ringer solution (in mM: 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 5.5 glucose, 10 Hepes, pH 7.4) and placed on the stage of confocal microscope (Carl Zeiss Pascal 5, LSM) at RT. CM-H2DCFA fluorescence was detected using excitation at 488 nm and emission at 510–540 nm. In all measurements, a control with laser excitation only was performed. The laser illumination was kept at a minimum to prevent light activation of the dye. Fluorescence images were analyzed with ImageJ software (NIH, Bethesda) and the average cell fluorescence, F, was calculated from selected regions of interest (ROI). Fluorescence data (F) normalized with respect to basal fluorescence (F0) of wt myotubes were expressed as [F−F0]/F0 (ΔF/F0).
2.9. Real time PCR
Total RNA from myotubes cultures was isolated with Trizol reagent (Invitrogen) and cDNA was prepared using SuperScript II (Invitrogen) according to manufacturer’s protocol. Real-time PCR was performed using a Stratagene Mx3000P as follows: Primers were used at 400 nM final concentrations and 2 µl of cDNA reaction together with the appropriate primers was added to 10 µl Brilliant III UltraFast SYBR green QPCR Master mix (Agilent Technologies) to a total volume of 20 µl. No-template control (NTC) reactions were also prepared for each gene. The cycling parameters for all genes were the following: 95°C for 3 min, then 50 cycles of 95°C for 20 s, and 60°C for 20 s. Expression values were normalized to GAPDH and are reported in units of 2−ΔΔCt [27]. PCR products was verified by melting-curve analysis and resolved by electrophoresis on 2% agarose gel and stained with ethidium bromide. The p47phox, gp91phox, IL-6 and GAPDH mRNA transcripts were quantified using oligonucleotide primers designed based on sequences published in NCBI GenBank with the opensource PerlPrimer software [28], using the following primers: gp91phox 5’-TCACATCCTCTACCAAAACC-3’ and 5’-CCTTTATTTTTCCCCATTCT-3’; p47phox 5’-AGAACAGAGTCATCCCACAC-3’ and 5’-GCTACGTTATTCTTGCCATC-3’; gapdh 5’-CTCATGACCACAGTCCATGC-3’ and 5’-TTCAGCTCTGGGATGACCTT-3’; IL-6 5’- CCAATTTCCAATGCTCTCCT 3’ and 5’- ACCACAGTGAGGAATGTCCA-3’
2.10. Statistics
All values are expressed as mean ± SEM from at least 3 different independent determinations. Data were normalized to wt values at control conditions. Statistical analysis was performed using either unpaired two-tailed t test or ANOVA-with Dunnett’s correction to determine significance (P < 0.05).
3. RESULTS
3.1. Depolarization-induced NF-κB activation is impaired in mdx myotubes
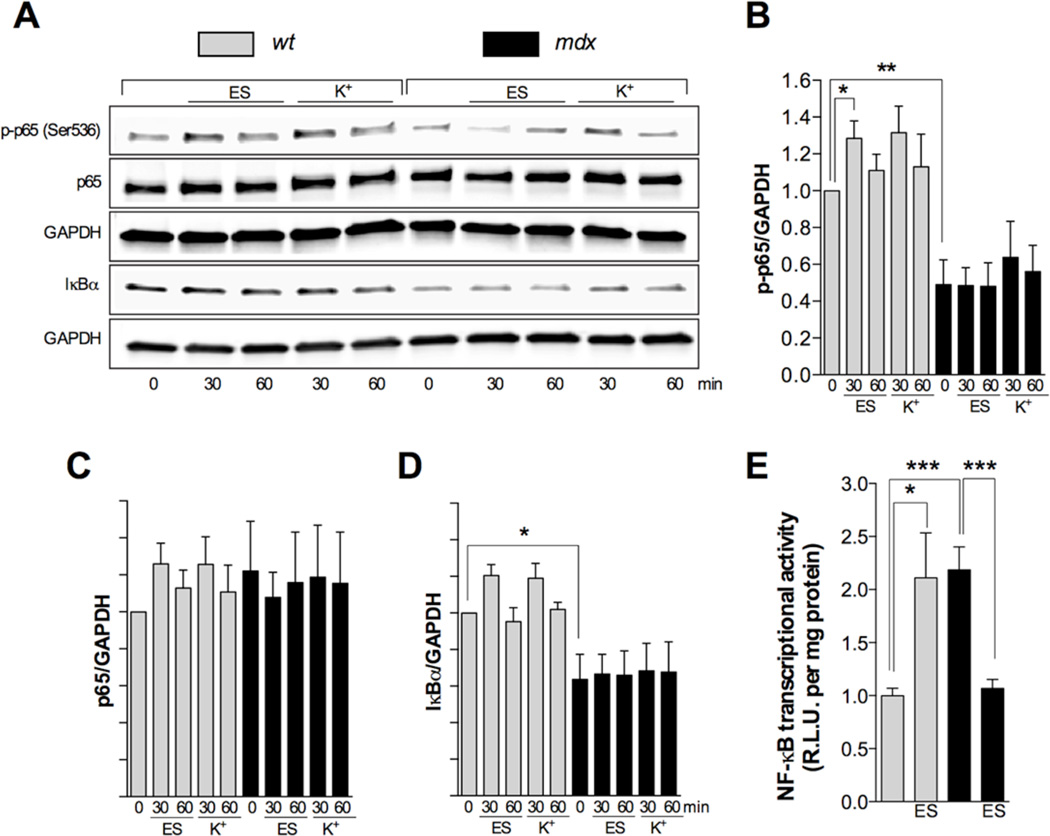
Membrane depolarization by either ES or high extracellular potassium has been shown to activate NF-κB in wt skeletal muscle cells [16]. In order to study any alterations in NF-κB activation we studied the levels of p-p65 (Ser536), p65 and IκBα by western blot as well as NF-κB transcriptional activity. In wt myotubes electrical stimulation (250 pulses of 0.5 ms of duration at 20 Hz frequency) increased p-p65 levels at 30 min (P<0.05), which returned to non significant levels above baseline at 60 min (Figure 1A and 1B) but no significant changes in total p65 and IκBα levels were observed at any time point (Figure 1C and 1D). Similar results were observed in wt myotubes after potassium depolarization protocol at 30 min, however the increase in p-p65 levels did not reach statistical significance, probably due to the differences in the duration of the depolarization protocol and its effects in the time course of NF-κB activation (Figure 1). In mdx myotubes, electrical stimuli did not modify p-p65, p65 or IκBα levels (Figure 1A–1D). NF-κB transcriptional activity, assessed by a luciferase reporter, increased by 2-fold at 12 h post ES in wt myotubes (P<0.05) (Figure 1E), and had the opposite effects on NF-κB transcriptional activity, reducing it by 2-fold after stimulation at 12 h (P<0.05) in dystrophic myotubes (Figure 1E). NF-κB transcriptional activity was elevated in mdx myotubes at resting conditions, and this was correlated with reduced IκBα levels, reduced p-p65 (Figure 1B, 1D and 1E).
FIGURE 1. NF-κB activation after membrane depolarization is impaired in mdx myotubes.
Cells were stimulated at 250 pulses of 0.5 ms of duration at 20 Hz or with 60 mM K+ solution for 1 min. Then, myotubes were returned to imaging solution and lysed at 30 and 60 min. A. Representative western blots Quantification of p-p65/GAPDH (B), p65/GAPDH (C) and IκBα/GAPDH (D) ratios (n=4). E. NF-κB transcriptional activity (luciferase assay) at 12 hrs (n=7–9). *=P<0.05, **=P<0.01 (indicated or versus basal wt value).
3.2. Depolarization-induced IL-6 expression is altered in mdx myotubes
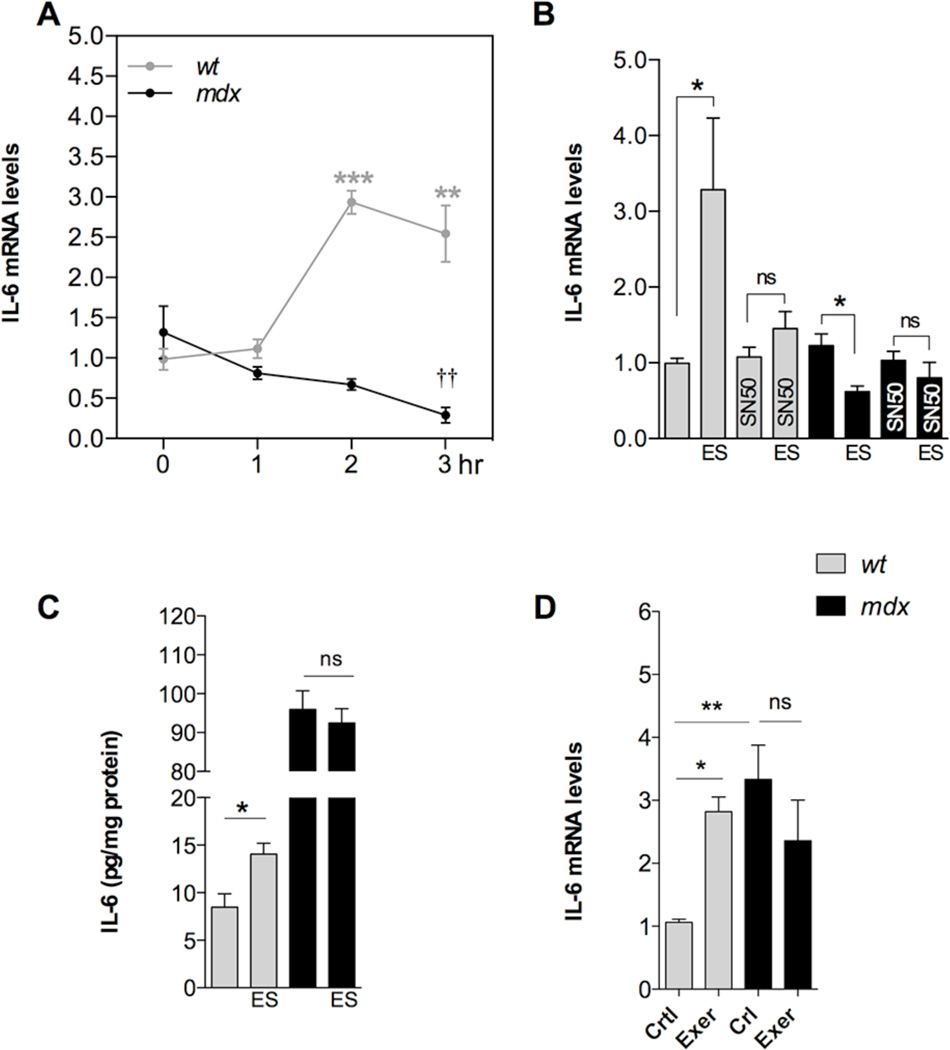
Membrane depolarization increases IL-6 gene expression in human and rat myotubes [14, 29] and this increased expression requires activation of NF-κB [14]. Here we studied the changes in IL-6 mRNA levels induced by ES by real time PCR. In wt myotubes ES significantly increased IL-6 mRNA levels by 2.9 and 2.5-fold at 2 and 3h post-stimuli, respectively (P<0.001 and P<0.01 compared to basal value) (Figure 2A). On the contrary, ES reduced IL-6 mRNA levels in mdx myotubes, reaching a 78% reduction at 3 hours post-stimuli (P<0.01) (Figure 2A).
FIGURE 2. IL-6 expression and secretion is defective in mdx myotubes.
A. IL-6 expression determined by real time PCR at 1, 2 and 3 h post-stimuli (n=3). B. Effect of SN50 (NF-κB inhibitor, 50 µM) on depolarization-induced IL-6 expression (n=3–7). C. IL-6 secreted levels measured after ES in wt and mdx myotubes (n=6). D. IL-6 expression in diaphragms from either swim-exercised or control mice (n=6). *=P<0.05, **=P<0.01, ***=P<0.001 (indicated or versus basal wt value). ††=P<0.01 (versus basal mdx value) and ns, no significant difference.
3.3. NF-κB is involved in depolarization-induced IL-6 expression
Some studies have shown that IL-6 gene expression can be controlled by NFAT and AP-1 rather than NF-κB [13]. To determine the effects of NF-κB inhibition on IL-6 gene expression, we applied 50 µM SN50, a cell permeable NF-κB inhibitory peptide [30], 30 min before ES and kept it on the cells during the entire experiment. IL-6 was significantly increased 2 hours after ES in wt myotubes, compared to un-stimulated myotubes (P<0.05) (Figure 2B) and SN50 treatment prevented the increase in IL-6 mRNA. In addition, SN50 treatment prevented the reduction in IL-6 mRNA levels after membrane depolarization in mdx myotubes (Figure 2B). Secreted IL-6 levels were measured in culture supernatants from wt and mdx myotubes using a high sensitivity ELISA kit. ES significantly increased the IL-6 secretion from 8.5±1.4 to 14.1±1.1 pg/mg protein (P<0.05) in wt myotubes (Figure 2C). Surprisingly, we found that the basal IL-6 release was augmented in mdx myotubes to 95.9±4.8 pg/mL despite the fact that basal IL-6 mRNA was not different from wt myotubes. However, after ES there was no significant change in mdx myotubes (P>0.05), supporting our hypothesis that dystrophic skeletal muscle cells fail to increase the expression and release of IL-6 after membrane depolarization (Figure 2D). This suggests that there is an altered secretion route for IL-6 that is normally pre-pooled inside the cell and released during exercise [31].
3.4. Dystrophic diaphragms fail to express IL-6 expression after swimming exercise in mice
To examine whether the alteration of IL-6 expression was relevant to exercised skeletal muscles of dystrophic mice we used a swimming protocol to assess this phenomena in vivo. Mice were allowed to swim for 1 hr and then, 2h post-exercise, they were euthanized, mRNA was extracted and the IL-6 expression was assessed by real time PCR. As we expected, swimming exercise increased IL-6 expression by 2.9-fold (P<0.05) in diaphragm muscles from wt mice (Figure 2E). Basal IL-6 expression was elevated in dystrophic diaphragms by 3.7-fold compared to wt, and swimming exercise did not modify its expression level (P>0.05).
3.5. Ca2+ transients after membrane depolarization
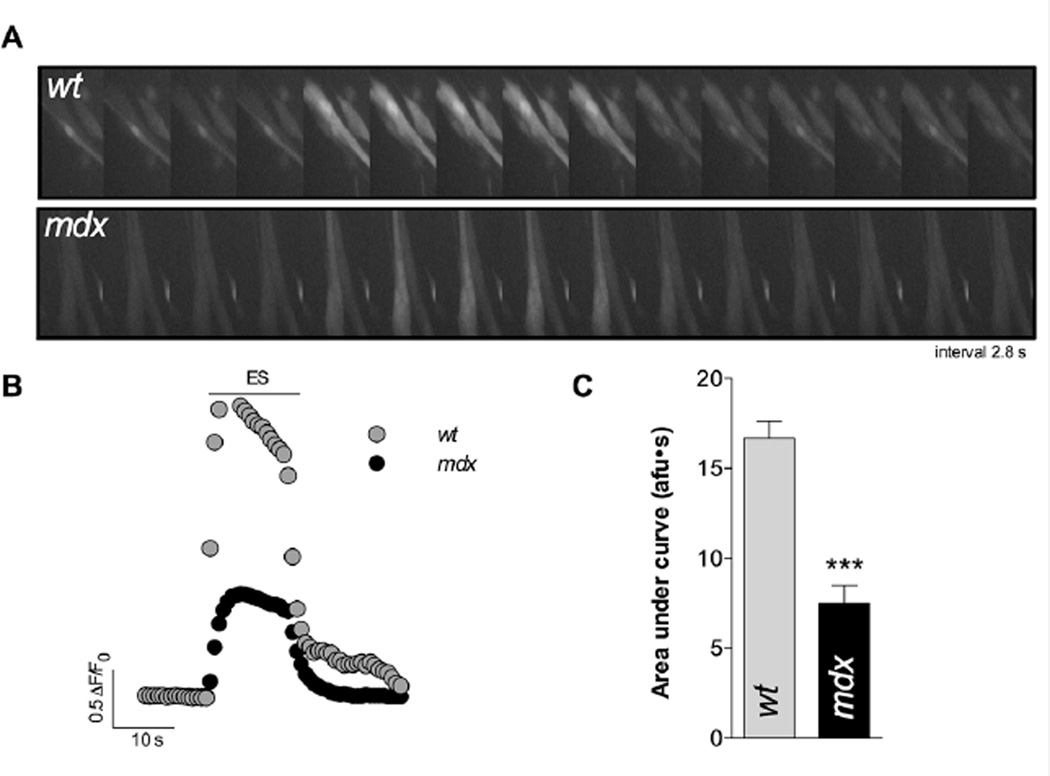
We have previously demonstrated that depolarization-induced NF-κB activation depends on changes in both intracellular Ca2+ concentration and ROS production [16, 32]. We studied the Ca2+ transient elicited by ES using Fluo3-AM and epifluorescence microscopy. Dystrophic mdx myotubes showed a reduced area under the curve of the Ca2+ transient elicited by ES (≈ 50%, P<0.001 see Figure 3).
FIGURE 3. Ca2+ transients elicited by ES in both wt and mdx myotubes.
A. Representative fluorescence images and B. representative traces obtained with Fluo3-AM and epifluorescence microscopy. C. Quantification of the area under the curve of the evoked transients. (n=40 cells for wt and n=40 for mdx myotubes, from at least 3 independent cultures, respectively). ***=P<0.001
3.6. ROS production induced by depolarization is higher in mdx myotubes
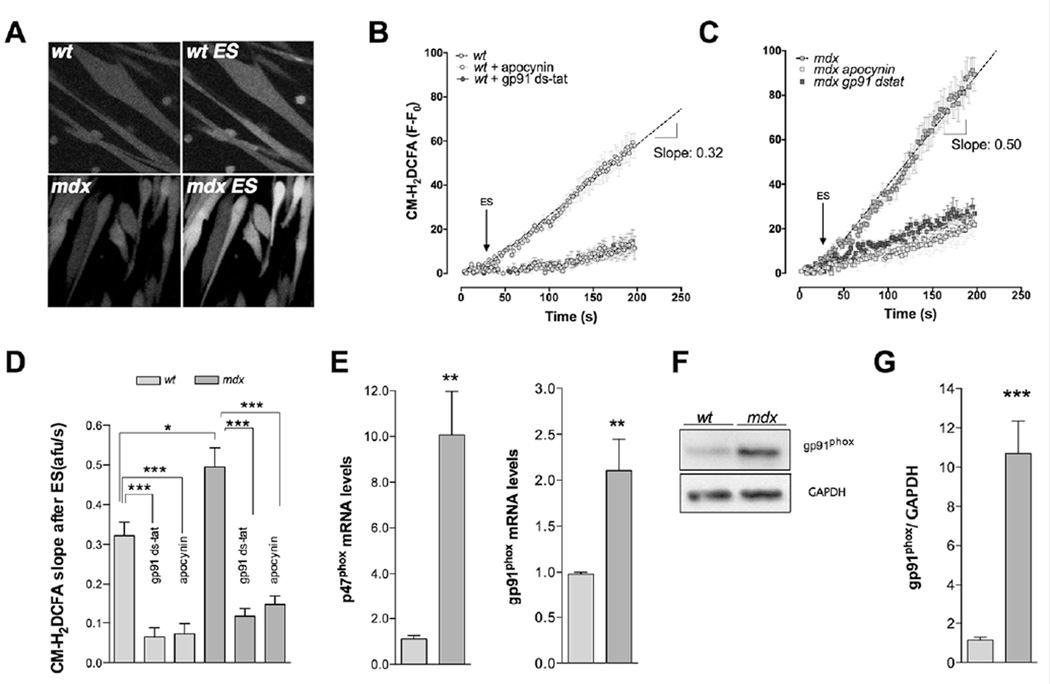
To study the ROS production induced by ES, myotubes were loaded with CM-H2DCFDA (5µM), a nonspecific redox probe that emits fluorescence when oxidized. The fluorescence changes over time were determined by confocal microscopy. Basal florescence was increased in mdx myotubes (Figure S1). Membrane depolarization by ES increased ROS production in both wt and mdx myotubes (Figure 4A and 4B) but the rate of ROS production was significantly higher in mdx myotubes compared with wt myotubes (0.49 vs 0.32 afu/s, P<0.05) (Figure 4C).
FIGURE 4. Depolarization-induced ROS production is increased in mdx myotubes due to NOX2 overexpression.
Cells were preloaded with CM-H2DCFDA and stimulated at 250 pulses of 0.5 ms of duration at 20 Hz frequency in physiological solution at RT. Average traces obtained in both wt myotubes (A) and mdx myotubes (B) with either apocynin or gp91dstat. Arrow approximately indicates the ES starting time. C. Slope quantification of CM-DCFDA fluorescent signal in both wt and mdx myotubes (n=12 and n=4 cells, for ROIs and cells respectively, 3 different preparations.). mRNA levels of p47phox (D) and gp91phox (E) subunits assessed by real time PCR (n=3). F. Representative western blot showing gp91phox overexpression in mdx myotubes and G. quantification of gp91phox western blot (n=5). *=P<0.05, **=P<0.01, ***=P<0.001 (indicated or versus basal wt value)
3.7. Catalytic and regulatory subunits of NADPH oxidase are over-expressed in mdx myotubes
NOX2 is the main ROS source in skeletal muscle cells [33], previous studies have shown an overexpression in mdx muscles [22, 23]. In order to establish the contribution of NOX2 to depolarization-induced ROS production, we applied one of two NOX2 inhibitors (gp91-dstat peptide (1 µM) or apocynin (5 µM)) prior to and during ES. Both gp91-dstat and apocynin blunted the ROS production after ES (P<0.001), but the inhibitory effect of NOX2 inhibitors was higher in mdx myotubes than in wt. We then measured the mRNA expression of two key subunits of the NADPH oxidase enzyme complex; regulatory subunit p47phox and catalytic subunit gp91phox, and found that both the p47phox and gp91phox mRNA levels were increased by 9-fold (P<0.01) and 2-fold (P<0.05), respectively in mdx compared to wt myotubes under resting conditions (Figure 4E). To confirm this we quantitated the gp91phox catalytic subunit protein levels by western blot. In mdx myotubes, gp91phox expression was 8.5-fold higher compared with wt myotubes (P<0.05) (Figure 4F and 4G).
3.8. ROS differentially modulate NF-κB activation in wt and mdx myotubes
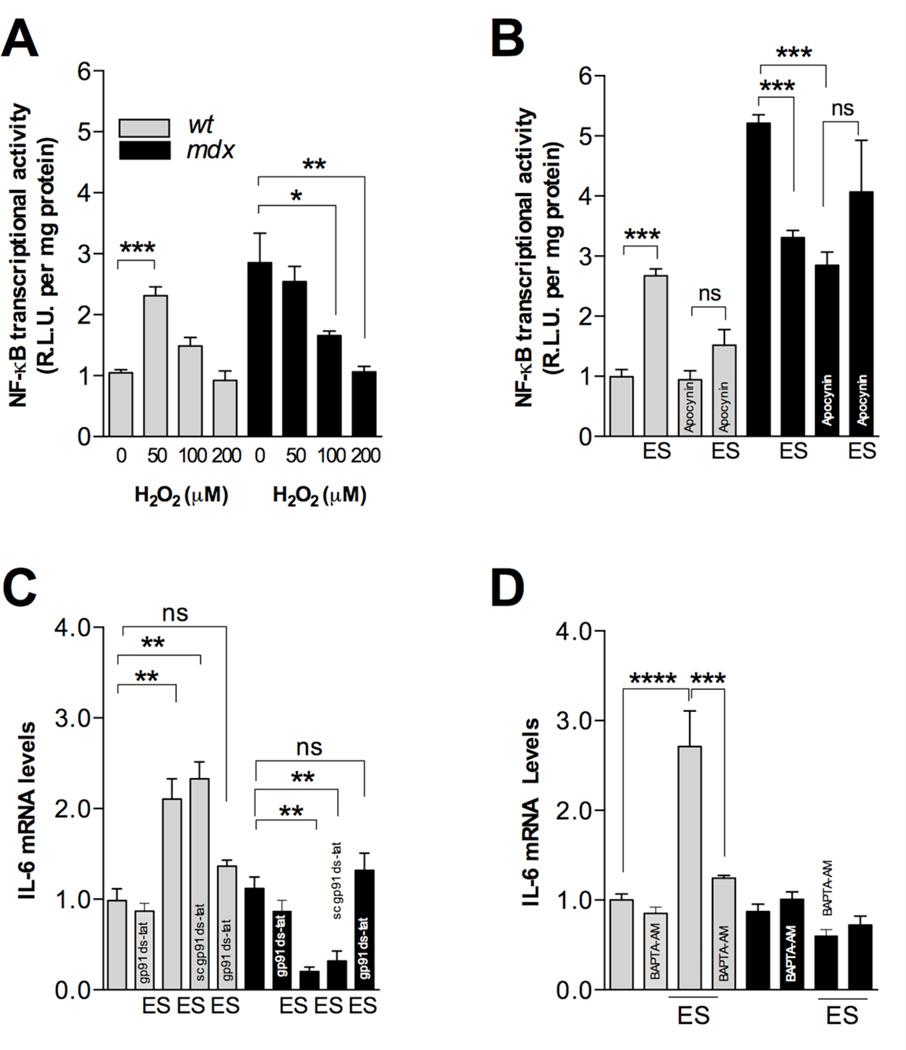
ROS have been reported to both activate and to repress NF-κB signaling depending on the intracellular pathway activated and the cell type [34]. To determine whether ROS play a role in NF-κB activity in wt and mdx myotubes, we evaluated their response to exogenous H2O2. NF-κB luciferase activity was then assessed 12 h after ROS stimulation at different doses of H2O2. In wt myotubes 50 µM H2O2 significantly increased NF-κB activity (P<0.05) but higher doses did not show any significant effect (Figure 5A). In mdx myotubes, 50 µM H2O2 did not induce any changes in NF-κB activity, whereas at 100 and 200 µM H2O2 there was a significant reduction in NF-κB activity (P<0.05, and P<0.01, respectively) (Figure 5A).
FIGURE 5. NF-κB dysregulation after membrane depolarization in mdx myotubes is mediated by excessive ROS production.
A. NF- κB activity induced by H2O2 in wt and mdx myotubes. Cells were stimulated with different concentrations of exogenous H2O2. Luciferase activity was determined after 12h (n=3). B. NOX2 inhibition (apocynin) blunted depolarization-induced changes in NF-κB activity in both wt and mdx myotubes (n=3). C. NOX2 inhibition blocked depolarization-induced changes in IL-6 expression in both wt and mdx myotubes (n=3). D. BAPTA-AM (30 µM) blocked depolarization-induced changes in IL-6 expression in both wt myotubes *=P<0.05, **=P<0.01, ***=P<0.001 (indicated or versus basal wt value), and ns, no significant difference.
3.9. NOX2 inhibition reduces the effects of ES on NF-κB activity
To establish a correlation between the NOX2-dependent ROS production and NF-κB activation, cells were treated with apocynin (5 µM) for 30 min before and during ES. Apocynin treatment reduced basal NF-κB activity by ~51% in mdx myotubes, without any significant effect in wt myotubes (Figure 5B). In addition, apocynin blunted the NF-κB activation after membrane depolarization in wt myotubes (P<0.001), while in mdx myotubes, apocynin treatment reduced the inhibitory effects of ES on NF-κB activity (P<0.05) (Figure 5B).
3.10. NOX2 inhibition reduces depolarization effects on IL-6 gene expression
To establish a correlation between NOX2-dependent ROS production and IL-6 gene expression, we treated myotubes either with gp91-dstat (1 µM) or the scramble version of gp91-dstat for 20 minutes before and during ES. In wt myotubes, gp91-dstat treatment blocked depolarization-induced IL-6 mRNA increases (Figure 5C) while the scramble version of gp91-dstat had no effect. In mdx myotubes gp91-dstat treatment prevented the previously observed decreases in IL-6 mRNA induced by ES (Figure 5C) and the scrambled version of gp91-dstat caused no significant change in IL-6 mRNA compared to control.
3.11 BAPTA-AM reduces IL-6 expression after ES in wt without any effect in mdx myotubes
To study the relationship between intracellular Ca2+ and IL-6 gene expression induced by ES, we pre-incubated the myotubes with BAPTA-AM (30 µM) 30 minutes after ES. In wt myotubes BAPTA-AM reduced depolarization-induced IL-6 mRNA increases (Figure 5D). In dystrophic muscle cells BAPTA-AM had no effect in IL-6 mRNA levels either at rest or in electrically stimulated conditions.
3.12. LPS increases NF-κB activity and IL-6 mRNA levels
Dystrophic mice have been shown to have high levels of serum IL-6 and increased skeletal muscle expression of IL-6 [10, 11]. To evaluate the role of the inflammatory pathway as a mechanism for this increase we studied the effect of lipopolysaccharide (LPS, 1ug/mL) on both NF-κB activity and IL-6 mRNA levels in dystrophic myotubes. LPS dramatically increased NF-κB transcriptional activity assessed by a luciferase reporter, at 12h in both wt and mdx myotubes (P<0.001), but the effect was larger in mdx myotubes than in wt (Figure 6). Furthermore, IL-6 expression was substantially increased 2h after stimulation in both wt and mdx muscle cells (P <0.001 and P <0.01 respectively) (Figure S1). These data suggest that despite the fact that NF-κB and IL-6 expression are impaired after ES in dystrophic myotubes, their ability to respond to the inflammatory pathway is intact.
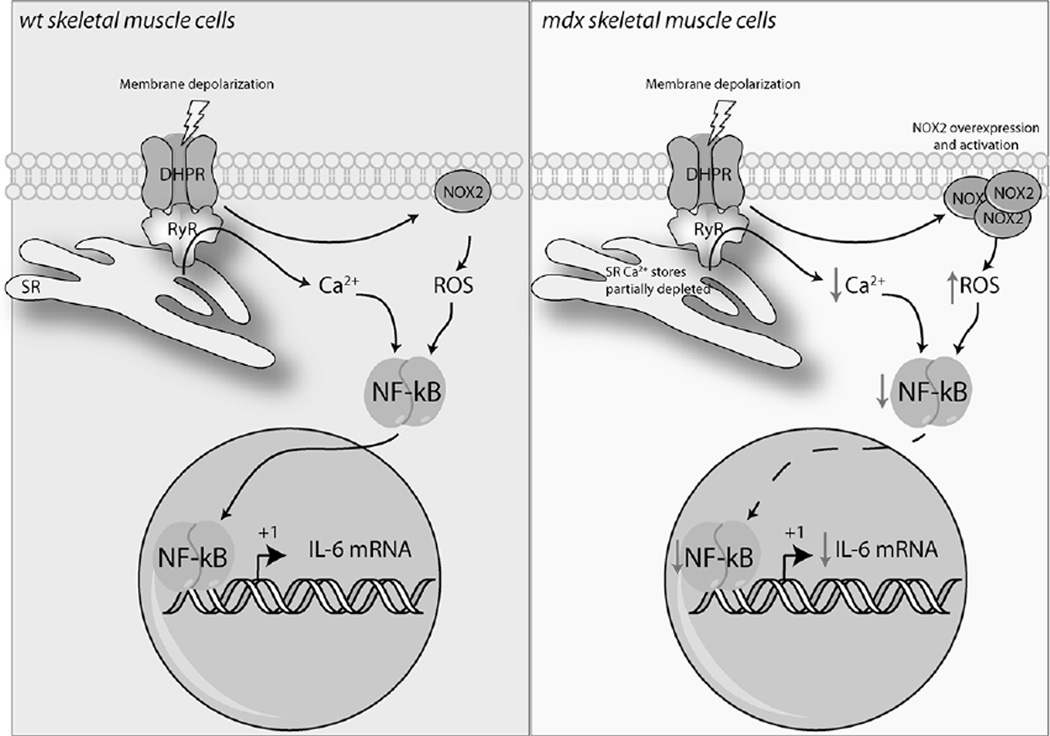
FIGURE 6. Proposed model for NF-κB and IL-6 dysregulation in dystrophic myotubes.
In wt skeletal muscle cells (Left panel) membrane depolarization increase NOX2-dependent ROS production and triggers Ca2+ release from intracellular reservoirs. Both Ca2+ and ROS levels are necessary to increase NF-κB transcriptional activity, and subsequently IL-6 gene expression. In mdx skeletal muscle cells (right panel), NOX2 overexpression increase ROS levels after membrane depolarization that negatively affects NF-κB transcriptional activity and IL-6 mRNA levels. In addition, Ca2+ signals elicited by membrane depolarization that are necessary for NF-κB activation are significantly reduced. IL-6 is a key regulator of metabolism and participates in the coordination of several organs during exercise. The impairment in IL-6 production might exacerbate muscle dysfunction in patients with DMD.
4. DISCUSION
IL-6 is an important myokine released during exercise by skeletal muscles, playing an important role in muscle metabolism and regeneration induced by exercise. DMD pathology is characterized by a persistent inflammatory response in skeletal muscles due to chronic damage. There is a basal increment of IL-6 levels in plasma and muscle lysates from DMD patients; however the contribution of skeletal muscle fibers to this pool after exercise is unknown. The aim of this research was to study the molecular mechanism involved in IL-6 expression evoked by ES in dystrophic muscle cells. Our main results are that 1) depolarization-induced IL-6 expression is controlled by NOX2/NF-κB pathway in normal skeletal muscle. 2) Dystrophic skeletal muscle cells fail to increase IL-6 after depolarization and exercise. 3) Both altered ROS production and NF-κB activity appear to be involved in the lack of IL-6 response to exercise in mdx myotubes.
NADPH oxidase complex is expressed in normal skeletal muscle and represents the main ROS source during resting and contracting conditions [18–20]. Here, we showed that ROS production after electrical stimuli was higher in mdx muscle cells and can be effectively blocked by NOX2 inhibitors (Figure 3C). The fact that the effect of NOX2 inhibitors appears to be higher in mdx cells suggest that the proportion of ROS produced by NOX2 is also higher in these cells. Our results are in agreement with previous findings that ROS production induced by muscle stretching is higher in mdx fibers compared to wt fibers [23, 35]. These data suggest that increments of ROS during muscle activity may contribute to oxidative stress and signaling dysregulation observed in DMD pathology [36]. Moreover, gene expression of key subunits for NADPH oxidase activity, p47phox and gp91phox were up regulated in mdx myotubes (Figure 4D and E). These data were supported by a 9-fold increase in protein expression for the catalytic subunit gp91phox in mdx myotubes at resting conditions (Figure 4F and G). Previous studies showed an increased expression of NADPH oxidase subunits in dystrophic mdx flexor digitorum brevis [23], tibialis anterior [37] and diaphragm [38], where a participation of immune system has been suggested [23]. In this study, we showed in an immune cell-free system that dystrophin deficiency leads to an increased NADPH oxidase expression.
NF-κB family plays an essential role in normal response to cellular stress in skeletal muscle including redox signaling [39]. We previously showed that basal NF-κB activity is increased in mdx muscle cells as a result of an elevated resting Ca2+ concentration [12]. Here, we confirmed these findings and we demonstrated that NF-κB signaling is up regulated under resting conditions in mdx myotubes through IκBα degradation (Figure 1D). The increased resting intracellular calcium concentration observed in dystrophic myotubes [12, 38] may involve calpain activation and the subsequent IκBα degradation [40]. On the other hand, a decreased basal p65 phosphorylation at Ser536 was observed in mdx myotubes (Figure 1B), these results suggest that basal NF-κB activity in mdx myotubes in the absence of immune mediators [41] does not require phosphorylation at Ser536.
In human skeletal muscles, NF-κB has been shown to be activated after resistance exercise [42], cycling [43], eccentric muscle contraction [44], or ES [16, 45]. In this work, we showed that membrane depolarization increased NF-κB activity in normal cells (Figure 1B), as we previously reported in rat myotubes [16], but this physiological response is not observed in dystrophic muscle cells. In wt myotubes an increase in p-p65 was observed after membrane depolarization that was correlated with an increase in NF-κB transcriptional activity measured by NF-κB driven luciferase reporters (Figure 1E).
Our laboratory has reported that depolarization-induced NF-κB activation is partially blocked by the antioxidant N-acetyl cysteine (NAC) [16]. In the present study, apocynin treatment prevented depolarization-induced NF-κB activation in wt myotubes (Figure 5B), showing the importance of ROS production suggested in previous studies [23]. In contrast, in mdx cells the reduction of NF-κB activation after ES was blocked by apocynin treatment, showing that physiological NOX2-dependent ROS production is necessary to positively modulate NF-κB signaling (Figure 5B). We hypothesize that the elevated ROS levels at resting conditions and the increase produced by membrane depolarization impairs the NF-κB function in dystrophic cells. This hypothesis is supported by the fact that lower doses of H2O2 increased NF-κB activity whereas higher doses have no effect in in wt myotubes but that in dystrophic muscle cells, low doses had no effect but higher concentrations of H2O2 reduced NF-κB transcriptional activity (Figure 5A). ROS can both activate or repress NF-κB signaling depending on the cell type, levels and localization (reviewed in [34]). ROS stimulate NF-κB pathway in the cytosol, whereas inhibit its activity in the nucleus. Elevated ROS may inhibit NF-κB binding ability due to a cysteine oxidation in the p50 subunit. Moreover, oxidation of IKK on Cys-179 inactivates NF-κB signaling. There are many examples in the literature for NF-kB inhibition by ROS [34]. Considering the elevated levels of NO due to iNOS [12] in mdx muscles and the increase of ROS after membrane depolarization it is possible that these redox modifications may impair the NF-κB signaling pathway.
After depolarization with our stimulation protocol (one train, 250 pulses at 20 Hz), we found that IκBα levels remain similar to untreated cells (Figure 1A). There is some evidence showing that p65 phosphorylation at Ser536 targets to an IκBα independent pathway for NF-κB activation [46]. A reduced binding of NF-κB to IκB due to increased phosphorylation at ser536 of p65 subunit of NF-κB has been reported in response to H2O2 [52, 53]. Moreover, it has been reported that NF-κB activation without IκBα degradation, due to IκBα association to PI3K leading NF-κB to migrate to the nucleus [47]. Moreover, our lab previously demonstrated that Ikba degradation depends on the frequency and duration of the stimuli [16].
ROS have been linked with IL-6 expression [15] and there is evidence showing that antioxidant administration might reduce IL-6 release by skeletal muscle [54]. However, the source of ROS involved in this process is unclear. Our results showed that in wt cells, inhibition of NOX2 by the mimetic peptide gp91dstat reduces IL-6 expression. In contrast, in mdx cells inhibition of NOX2 prevents repression of IL-6 expresion after ES. Collectively, our data suggest that an excesive NOX2-mediated ROS production inhibits the NF-κB-dependent transcription of IL-6 gene, ablating its expression after membrane depolarization and impairing its secretion.
Our results demonstrate that IL-6 secretion is elevated in resting conditions in mdx myotubes, but mRNA levels are similar to those observed in wt myotubes. This can be achieved at different levels of regulation (e.g. post-transcriptional, translational, or secretion route). These basal differences might be due to an altered secretion of preformed IL-6 pool; although this was not the aim of this work, this is an interesting finding because it may reflect and altered IL-6 secretion similar to the one observed in plasma and muscle lysates in DMD [3, 4]. Our data suggest that dystrophic skeletal muscle cells fail to express and secrete IL-6 in response to membrane depolarization or exercise. IL- 6 is an endocrine, paracrine, and autocrine-signaling molecule. It is essential for a normal response to exercise, acting as an important metabolic regulator [13, 29]. Thus, its alteration in dystrophic muscle cells can affect the normal response to contractile activity and impair muscle function [55, 56]. Moreover, studies in rodents have shown that IL-6 contributes to muscle regeneration or hypertrophy after injury which may involve a similar stimulatory effect on satellite cell proliferation [57] and up-regulation of utrophin [58]. It seems that IL-6 has a dual effect in muscle physiology depending on its temporal release and concentration [31]. After membrane damage, muscle elevation of IL-6 appears to be due to leukocyte activation (mast cells, neutrophils, monocytes and macrophages) and it is necessary for muscle repair [59]. IL-6 null mice showed an abnormal muscle repair, pointing to the importance of this cytokine in the regeneration process [60]. Moreover, blockade of IL-6 in mdx mice is deleterious supporting this hypothesis [61]. On the other hand, during exercise IL-6 is a key energetic regulator that coordinates multiple organs to fulfill the energetic muscle requirements [13]. IL-6 release is transient, peaking at the end of exercise and then returning to basal levels [31]. The absence of an IL-6 increase after exercise due to altered NF-κB signaling in dystrophic muscles could induce metabolic dysfunctions and lack of adaptation in skeletal muscles that undergo physical activity. Contrary to ES response, stimulation with LPS induces activation of NF-κB and IL-6 expression in both skeletal myotubes (Figure S1). These data suggest that despite the fact that NF-κB and IL-6 expression are impaired after ES in dystrophic myotubes, they are able to respond to the inflammatory stimuli.
The NF-κB molecular mechanisms that explain the differences in the response to LPS should be explored in futures studies.
In summary, we have found that the IL-6 gene expression triggered by membrane depolarization is impaired in mdx myotubes, due to an increased NOX2-dependent ROS production and reduced NF-κB activation after depolarization (Figure 6). This altered pathway may well be on the basis of reduced muscle regeneration and increased cell death associated with the absence of dystrophin, and may be generalizable to other muscle diseases as well.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported with FONDECYT 1110467 (EJ) and ACT1111 (EJ) and FONDECYT 3140491 (DV). Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR43140 and AR052534 (JRL and PDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 2.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 3.Sockolov R, Irwin B, Dressendorfer RH, Bernauer EM. Exercise performance in 6-to-11-year-old boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1977;58:195–201. [PubMed] [Google Scholar]
- 4.Sandri M, Podhorska-Okolow M, Geromel V, Rizzi C, Arslan P, Franceschi C, Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. Journal of neuropathology and experimental neurology. 1997;56:45–57. doi: 10.1097/00005072-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle & nerve. 1998;21:567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Gordon BS, Lowe DA, Kostek MC. Exercise increases utrophin protein expression in the mdx mouse model of Duchenne muscular dystrophy. Muscle & nerve. 2014;49:915–918. doi: 10.1002/mus.24151. [DOI] [PubMed] [Google Scholar]
- 7.Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW. Immune-mediated mechanisms potentially regulate the disease time-course of duchenne muscular dystrophy and provide targets for therapeutic intervention. PM & R : the journal of injury, function, and rehabilitation. 2009;1:755–768. doi: 10.1016/j.pmrj.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tidball JG. Inflammatory processes in muscle injury and repair. American journal of physiology. Regulatory, integrative and comparative physiology. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 9.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Kurek JB, Nouri S, Kannourakis G, Murphy M, Austin L. Leukemia inhibitory factor and interleukin-6 are produced by diseased and regenerating skeletal muscle. Muscle & nerve. 1996;19:1291–1301. doi: 10.1002/(SICI)1097-4598(199610)19:10<1291::AID-MUS6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, Vita G. Activation of NF-kappaB pathway in Duchenne muscular dystrophy: relation to age. Acta myologica : myopathies and cardiomyopathies : official journal of the Mediterranean Society of Myology / edited by the Gaetano Conte Academy for the study of striated muscle diseases. 2011;30:16–23. [PMC free article] [PubMed] [Google Scholar]
- 12.Altamirano F, Lopez JR, Henriquez C, Molinski T, Allen PD, Jaimovich E. Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. The Journal of biological chemistry. 2012;287:20876–20887. doi: 10.1074/jbc.M112.344929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen BK. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc. 2012;44:392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 14.Juretic N, Garcia-Huidobro P, Iturrieta JA, Jaimovich E, Riveros N. Depolarization-induced slow Ca2+ transients stimulate transcription of IL-6 gene in skeletal muscle cells. Am J Physiol Cell Physiol. 2006;290:C1428–C1436. doi: 10.1152/ajpcell.00449.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C. Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. American journal of respiratory cell and molecular biology. 2002;26:587–593. doi: 10.1165/ajrcmb.26.5.4598. [DOI] [PubMed] [Google Scholar]
- 16.Valdes JA, Hidalgo J, Galaz JL, Puentes N, Silva M, Jaimovich E, Carrasco MA. NF-kappaB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am J Physiol Cell Physiol. 2007;292:C1960–C1970. doi: 10.1152/ajpcell.00320.2006. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free radical biology & medicine. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelson LP, Shi G, Ward CW, Rodney GG. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle & nerve. 2010;42:522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal R, Basu Thakur P, Li S, Minard C, Rodney GG. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PloS one. 2013;8:e63989. doi: 10.1371/journal.pone.0063989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. The Journal of biological chemistry. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 22.Pal R, Palmieri M, Loehr JA, Li S, Abo-Zahrah R, Monroe TO, Thakur PB, Sardiello M, Rodney GG. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nature communications. 2014;5:4425. doi: 10.1038/ncomms5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PloS one. 2010;5:e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casas M, Altamirano F, Jaimovich E. Myogenesis: Methods and Protocols. 1st ed. Humana Press; 2011. [Google Scholar]
- 26.Jorquera G, Altamirano F, Contreras-Ferrat A, Almarza G, Buvinic S, Jacquemond V, Jaimovich E, Casas M. Cav1.1 controls frequency-dependent events regulating adult skeletal muscle plasticity. Journal of cell science. 2013;126:1189–1198. doi: 10.1242/jcs.116855. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Marshall OJ. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics. 2004;20:2471–2472. doi: 10.1093/bioinformatics/bth254. [DOI] [PubMed] [Google Scholar]
- 29.Welc SS, Clanton TL. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol. 2013;98:359–371. doi: 10.1113/expphysiol.2012.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. The Journal of biological chemistry. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen BK. Muscle as a secretory organ. Comprehensive Physiology. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 32.Riquelme D, Alvarez A, Leal N, Adasme T, Espinoza I, Valdes JA, Troncoso N, Hartel S, Hidalgo J, Hidalgo C, Carrasco MA. High-frequency field stimulation of primary neurons enhances ryanodine receptor-mediated Ca2+ release and generates hydrogen peroxide, which jointly stimulate NF-kappaB activity. Antioxid Redox Signal. 2011;14:1245–1259. doi: 10.1089/ars.2010.3238. [DOI] [PubMed] [Google Scholar]
- 33.Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free radical research. 2014;48:12–29. doi: 10.3109/10715762.2013.830718. [DOI] [PubMed] [Google Scholar]
- 34.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Science signaling. 2012;5:ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragusa RJ, Chow CK, Porter JD. Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul Disord. 1997;7:379–386. doi: 10.1016/s0960-8966(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 37.Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N. Reciprocal amplification of ROS and Ca(2+) signals in stressed mdx dystrophic skeletal muscle fibers. Pflugers Arch. 2009;458:915–928. doi: 10.1007/s00424-009-0670-2. [DOI] [PubMed] [Google Scholar]
- 38.Altamirano F, Valladares D, Henriquez-Olguin C, Casas M, Lopez JR, Allen PD, Jaimovich E. Nifedipine Treatment Reduces Resting Calcium Concentration, Oxidative and Apoptotic Gene Expression, and Improves Muscle Function in Dystrophic mdx Mice. PloS one. 2013;8:e81222. doi: 10.1371/journal.pone.0081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourkioti F, Rosenthal N. NF-kappaB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med (Berl) 2008;86:747–759. doi: 10.1007/s00109-008-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochemical research. 2004;29:1443–1451. doi: 10.1023/b:nere.0000026410.56000.dd. [DOI] [PubMed] [Google Scholar]
- 41.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. The Journal of clinical investigation. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D. Resistance exercise increases NF-kappaB activity in human skeletal muscle. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R667–R673. doi: 10.1152/ajpregu.00336.2011. [DOI] [PubMed] [Google Scholar]
- 43.Tantiwong P, Shanmugasundaram K, Monroy A, Ghosh S, Li M, DeFronzo RA, Cersosimo E, Sriwijitkamol A, Mohan S, Musi N. NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab. 2010;299:E794–E801. doi: 10.1152/ajpendo.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyldahl RD, Xin L, Hubal MJ, Moeckel-Cole S, Chipkin S, Clarkson PM. Activation of nuclear factor-kappaB following muscle eccentric contractions in humans is localized primarily to skeletal muscle-residing pericytes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2956–2966. doi: 10.1096/fj.10-177105. [DOI] [PubMed] [Google Scholar]
- 45.Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, Hrabe de Angelis M, Haring HU, Weigert C. The cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol. 2013 doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki CY, Barberi TJ, Ghosh P, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. The Journal of biological chemistry. 2005;280:34538–34547. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- 47.Beraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. The Journal of biological chemistry. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 49.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic acids research. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. The Journal of biological chemistry. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 51.Byun MS, Jeon KI, Choi JW, Shim JY, Jue DM. Dual effect of oxidative stress on NF-kappakB activation in HeLa cells. Experimental & molecular medicine. 2002;34:332–339. doi: 10.1038/emm.2002.47. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima S, Kitamura M. Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free radical biology & medicine. 2013;65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Kefaloyianni E, Gaitanaki C, Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cellular signalling. 2006;18:2238–2251. doi: 10.1016/j.cellsig.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Fischer CP, Hiscock NJ, Penkowa M, Basu S, Vessby B, Kallner A, Sjoberg LB, Pedersen BK. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol. 2004;558:633–645. doi: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Call JA, McKeehen JN, Novotny SA, Lowe DA. Progressive resistance voluntary wheel running in the mdx mouse. Muscle & nerve. 2010;42:871–880. doi: 10.1002/mus.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle & nerve. 2008;38:1290–1303. doi: 10.1002/mus.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell metabolism. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Fujimori K, Itoh Y, Yamamoto K, Miyagoe-Suzuki Y, Yuasa K, Yoshizaki K, Yamamoto H, Takeda S. Interleukin 6 induces overexpression of the sarcolemmal utrophin in neonatal mdx skeletal muscle. Human gene therapy. 2002;13:509–518. doi: 10.1089/10430340252809801. [DOI] [PubMed] [Google Scholar]
- 59.Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304:E453–E465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. The Journal of biological chemistry. 2013;288:1489–1499. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kostek MC, Nagaraju K, Pistilli E, Sali A, Lai SH, Gordon B, Chen YW. IL-6 signaling blockade increases inflammation but does not affect muscle function in the mdx mouse. BMC musculoskeletal disorders. 2012;13:106. doi: 10.1186/1471-2474-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.