Abstract
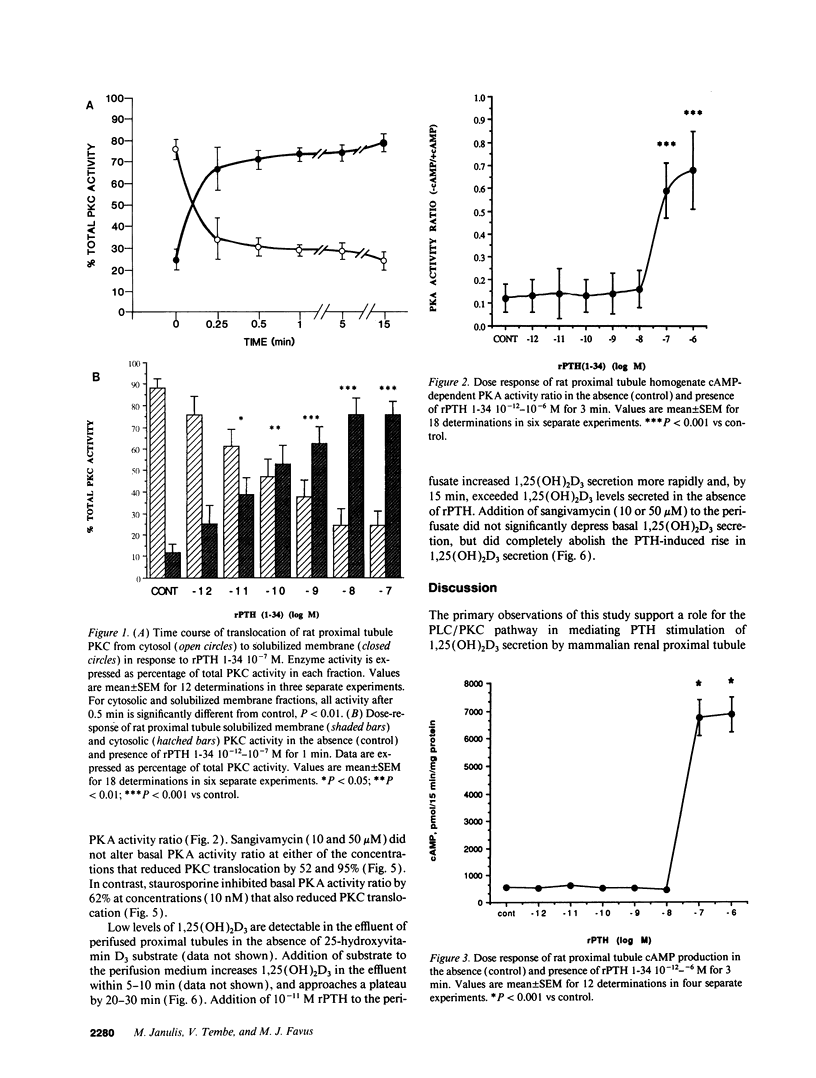
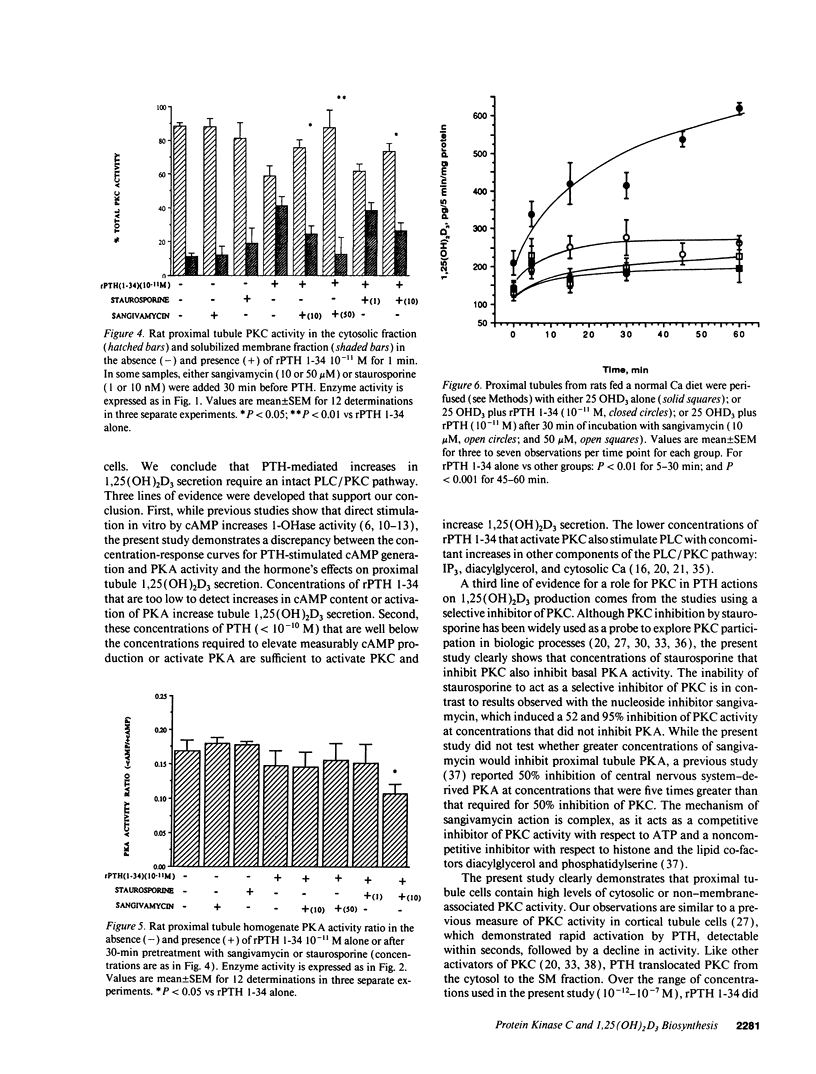
PTH is a major regulator of renal proximal tubule 1,25(OH)2D3 biosynthesis. However, the intracellular pathways involved in PTH activation of the mitochondrial 25-hydroxyvitamin D3-1 alpha-hydroxylase (1-OHase) remain unknown. PTH can activate both the adenylate cyclase/protein kinase A (PKA) and the plasma membrane phospholipase C/protein kinase C (PKC) pathways. The present study was undertaken to determine whether PKC may mediate PTH activation of renal 25-hydroxyvitamin D3-1 alpha-hydroxylase activity. Rat PTH 1-34 fragment in vitro translocated PKC activity from cytosolic to soluble membrane fraction from freshly prepared rat proximal tubules. Physiologic concentrations (10(-11)-10(-10) M) of rat PTH 1-34 fragment increased PKC translocation three- to fourfold while PKA activity ratio increased at PTH 10(-7) M. PTH stimulation of PKC and PKA was reduced in the presence of staurosporine (10 nM) by 41 and 29%, respectively. Sangivamycin (10 and 50 microM) also reduced PTH-stimulated PKC translocation, but did not alter PKA activity ratio. In vitro perifusion of renal proximal tubules with PTH (10(-11) M) increased 1,25(OH)2D3 steady-state secretion two- to fourfold. Sangivamycin at the same concentration that inhibited PKC translocation by 52% completely inhibited PTH-stimulated 1,25(OH)2D3 secretion. The present studies indicate that the phospholipase C/PKC pathway may mediate PTH stimulation of mammalian renal proximal tubule 1,25(OH)2D3 secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbrecht H. J., Forte L. R., Wongsurawat N., Zenser T. V., Davis B. B. Forskolin increases 1,25-dihydroxyvitamin D3 production by rat renal slices in vitro. Endocrinology. 1984 Feb;114(2):644–649. doi: 10.1210/endo-114-2-644. [DOI] [PubMed] [Google Scholar]
- Armbrecht H. J., Wongsurawat N., Zenser T. V., Davis B. B. Effect of PTH and 1,25(OH)2D3 on renal 25(OH)D3 metabolism, adenylate cyclase, and protein kinase. Am J Physiol. 1984 Jan;246(1 Pt 1):E102–E107. doi: 10.1152/ajpendo.1984.246.1.E102. [DOI] [PubMed] [Google Scholar]
- Baksi S. N., Kenny A. D. Acute effects of parathyroid extract on renal vitamin D hydroxylases in Japanese quail. Pharmacology. 1979;18(4):169–174. doi: 10.1159/000137248. [DOI] [PubMed] [Google Scholar]
- Bar A., Hurwitz S., Maoz A. The 25-hydroxycholecalciferol-1-hydroxylase activity of chick kidney cells: direct effect of parathyroid. FEBS Lett. 1980 May 5;113(2):328–330. doi: 10.1016/0014-5793(80)80620-x. [DOI] [PubMed] [Google Scholar]
- Bringhurst F. R., Zajac J. D., Daggett A. S., Skurat R. N., Kronenberg H. M. Inhibition of parathyroid hormone responsiveness in clonal osteoblastic cells expressing a mutant form of 3',5'-cyclic adenosine monophosphate-dependent protein kinase. Mol Endocrinol. 1989 Jan;3(1):60–67. doi: 10.1210/mend-3-1-60. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Favus M. J., Coe F. L. Mechanism of chronic hypocalciuria with chlorthalidone: reduced calcium absorption. Am J Physiol. 1984 Nov;247(5 Pt 2):F746–F752. doi: 10.1152/ajprenal.1984.247.5.F746. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Forte L. R., Eber S., Thorne P. K., Poelling R. E. Regulation of sodium-dependent phosphate transport by parathyroid hormone in opossum kidney cells: adenosine 3',5'-monophosphate-dependent and -independent mechanisms. Endocrinology. 1988 Jun;122(6):2981–2989. doi: 10.1210/endo-122-6-2981. [DOI] [PubMed] [Google Scholar]
- Di Bella F. P., Dousa T. P., Miller S. S., Arnaud C. D. Parathyroid hormone receptors of renal cortex: specific binding of biologically active, 125I-labeled hormone and relationship to adenylate cyclase activation. Proc Natl Acad Sci U S A. 1974 Mar;71(3):723–726. doi: 10.1073/pnas.71.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlay R., Hruska K. PTH receptor coupling to phospholipase C is an alternate pathway of signal transduction in bone and kidney. Am J Physiol. 1990 Feb;258(2 Pt 2):F223–F231. doi: 10.1152/ajprenal.1990.258.2.F223. [DOI] [PubMed] [Google Scholar]
- Favus M. J., Langman C. B. Evidence for calcium-dependent control of 1,25-dihydroxyvitamin D3 production by rat kidney proximal tubules. J Biol Chem. 1986 Aug 25;261(24):11224–11229. [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fukase M., Birge S. J., Jr, Rifas L., Avioli L. V., Chase L. R. Regulation of 25 hydroxyvitamin D3 1-hydroxylase in serum-free monolayer culture of mouse kidney. Endocrinology. 1982 Mar;110(3):1073–1075. doi: 10.1210/endo-110-3-1073. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligorsky M. S., Loftus D. J., Hruska K. A. Cytoplasmic calcium in individual proximal tubular cells in culture. Am J Physiol. 1986 Nov;251(5 Pt 2):F938–F944. doi: 10.1152/ajprenal.1986.251.5.F938. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Influence of a tumor promoting phorbol ester on the metabolism of 25-hydroxyvitamin D3. Biochem Biophys Res Commun. 1986 Sep 14;139(2):495–500. doi: 10.1016/s0006-291x(86)80018-3. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Luntao E. M. Interactions between intracellular signals involved in the regulation of 25-hydroxyvitamin D3 metabolism. Endocrinology. 1989 May;124(5):2228–2234. doi: 10.1210/endo-124-5-2228. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Parathyroid hormone modulation of 25-hydroxyvitamin D3 metabolism by cultured chick kidney cells is mimicked and enhanced by forskolin. Endocrinology. 1985 Feb;116(2):503–510. doi: 10.1210/endo-116-2-503. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem. 1979 Apr 25;254(8):2722–2729. [PubMed] [Google Scholar]
- Ho A. K., Thomas T. P., Chik C. L., Anderson W. B., Klein D. C. Protein kinase C: subcellular redistribution by increased Ca2+ influx. Evidence that Ca2+-dependent subcellular redistribution of protein kinase C is involved in potentiation of beta-adrenergic stimulation of pineal cAMP and cGMP by K+ and A23187. J Biol Chem. 1988 Jul 5;263(19):9292–9297. [PubMed] [Google Scholar]
- Horiuchi N., Suda T., Takahashi H., Shimazawa E., Ogata E. In vivo evidence for the intermediary role of 3',5'-cyclic AMP in parathyroid hormone-induced stimulation of 1alpha,25-dihydroxyvitamin D3 synthesis in rats. Endocrinology. 1977 Sep;101(3):969–974. doi: 10.1210/endo-101-3-969. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Goligorsky M., Scoble J., Tsutsumi M., Westbrook S., Moskowitz D. Effects of parathyroid hormone on cytosolic calcium in renal proximal tubular primary cultures. Am J Physiol. 1986 Aug;251(2 Pt 2):F188–F198. doi: 10.1152/ajprenal.1986.251.2.F188. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Moskowitz D., Esbrit P., Civitelli R., Westbrook S., Huskey M. Stimulation of inositol trisphosphate and diacylglycerol production in renal tubular cells by parathyroid hormone. J Clin Invest. 1987 Jan;79(1):230–239. doi: 10.1172/JCI112788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkor A. B., Gray R. W., Henry H. L., Kleinman J. G., Blumenthal S. S., Garancis J. C. Evidence that stimulation of 1,25(OH)2D3 production in primary cultures of mouse kidney cells by cyclic AMP requires new protein synthesis. J Bone Miner Res. 1987 Dec;2(6):517–524. doi: 10.1002/jbmr.5650020608. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., Rapoport A., Martin T. J., Tulloch B. R., Byfield P. G., Matthews E. W., MacIntyre I. Effects of nucleotides, hormones, ions, and 1,25-dihydroxycholecalciferon on 1,25-dihydroxycholecalciferol production in isolated chick renal tubules. Clin Sci Mol Med. 1974 May;46(5):569–582. doi: 10.1042/cs0460569. [DOI] [PubMed] [Google Scholar]
- Loomis C. R., Bell R. M. Sangivamycin, a nucleoside analogue, is a potent inhibitor of protein kinase C. J Biol Chem. 1988 Feb 5;263(4):1682–1692. [PubMed] [Google Scholar]
- Martin K. J., McConkey C. L., Garcia J. C., Montani D., Betts C. R. Protein kinase-A and the effects of parathyroid hormone on phosphate uptake in opossum kidney cells. Endocrinology. 1989 Jul;125(1):295–301. doi: 10.1210/endo-125-1-295. [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Dobre V., Rickmeyer M., Cole J., Forte L., Hruska K. A. Stimulation of transient elevations in cytosolic Ca2+ is related to inhibition of Pi transport in OK cells. Am J Physiol. 1990 Sep;259(3 Pt 2):F485–F493. doi: 10.1152/ajprenal.1990.259.3.F485. [DOI] [PubMed] [Google Scholar]
- Murer H., Werner A., Reshkin S., Wuarin F., Biber J. Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol. 1991 May;260(5 Pt 1):C885–C899. doi: 10.1152/ajpcell.1991.260.5.C885. [DOI] [PubMed] [Google Scholar]
- Pernalete N., Garcia J. C., Betts C. R., Martin K. J. Inhibitors of protein kinase-C modulate desensitization of the parathyroid hormone receptor-adenylate cyclase system in opossum kidney cells. Endocrinology. 1990 Jan;126(1):407–413. doi: 10.1210/endo-126-1-407. [DOI] [PubMed] [Google Scholar]
- Quamme G., Pfeilschifter J., Murer H. Parathyroid hormone inhibition of Na+/phosphate cotransport in OK cells: generation of second messengers in the regulatory cascade. Biochem Biophys Res Commun. 1989 Feb 15;158(3):951–957. doi: 10.1016/0006-291x(89)92814-3. [DOI] [PubMed] [Google Scholar]
- Quamme G., Pfeilschifter J., Murer H. Parathyroid hormone inhibition of Na+/phosphate cotransport in OK cells: requirement of protein kinase C-dependent pathway. Biochim Biophys Acta. 1989 Sep 19;1013(2):159–165. doi: 10.1016/0167-4889(89)90044-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro H. K., Tembe V., Favus M. J. Evidence that activation of protein kinase-C can stimulate 1,25-dihydroxyvitamin D3 secretion by rat proximal tubules. Endocrinology. 1992 Sep;131(3):1424–1428. doi: 10.1210/endo.131.3.1324162. [DOI] [PubMed] [Google Scholar]
- Ro H. K., Tembe V., Krug T., Yang P. Y., Bushinsky D. A., Favus M. J. Acidosis inhibits 1,25-(OH)2D3 but not cAMP production in response to parathyroid hormone in the rat. J Bone Miner Res. 1990 Mar;5(3):273–278. doi: 10.1002/jbmr.5650050311. [DOI] [PubMed] [Google Scholar]
- Shlatz L. J., Schwartz I. L., Kinne-Saffran E., Kinne R. Distribution of parathyroid hormone-stimulated adenylate cyclase in plasma membranes of cells of the kidney cortex. J Membr Biol. 1975 Nov 7;24(2):131–144. doi: 10.1007/BF01868619. [DOI] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Sakamoto H., Filburn C. R. Parathyroid hormone 1-34, but not 3-34 or 7-34, transiently translocates protein kinase C in cultured renal (OK) cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1352–1358. doi: 10.1016/0006-291x(89)92259-6. [DOI] [PubMed] [Google Scholar]
- Wali R. K., Baum C. L., Sitrin M. D., Brasitus T. A. 1,25(OH)2 vitamin D3 stimulates membrane phosphoinositide turnover, activates protein kinase C, and increases cytosolic calcium in rat colonic epithelium. J Clin Invest. 1990 Apr;85(4):1296–1303. doi: 10.1172/JCI114567. [DOI] [PMC free article] [PubMed] [Google Scholar]