Abstract
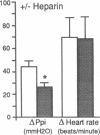
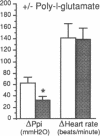
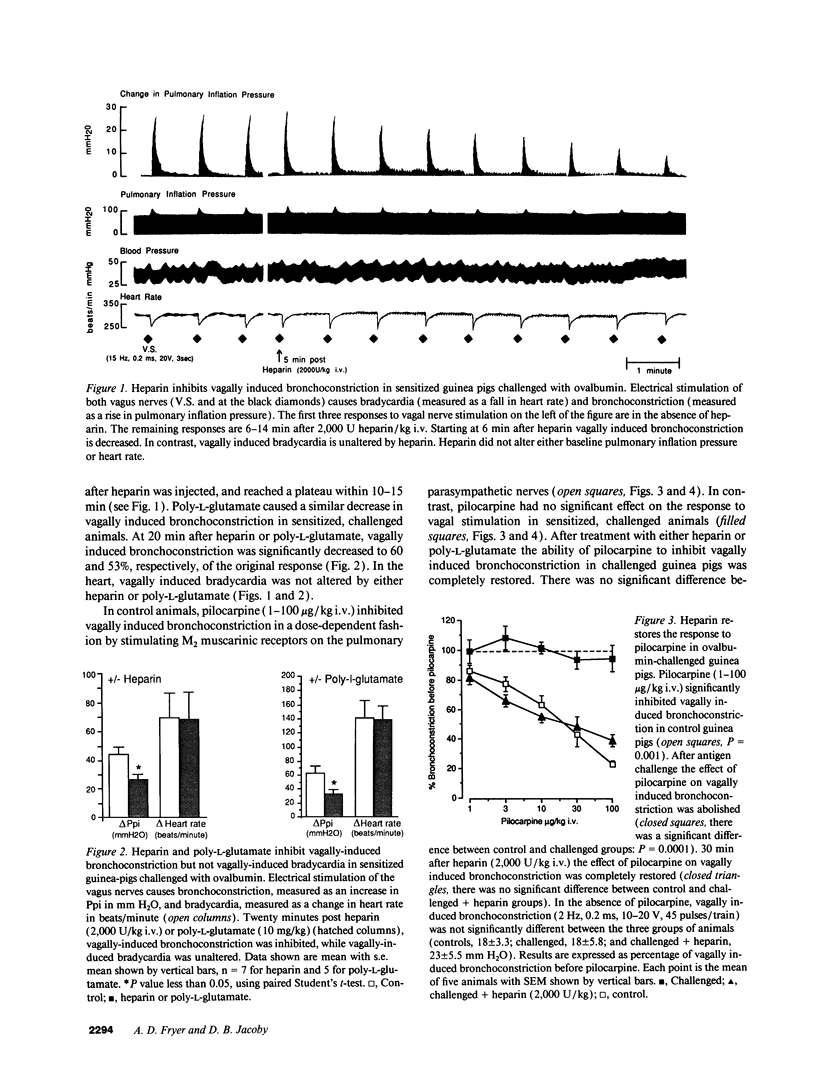
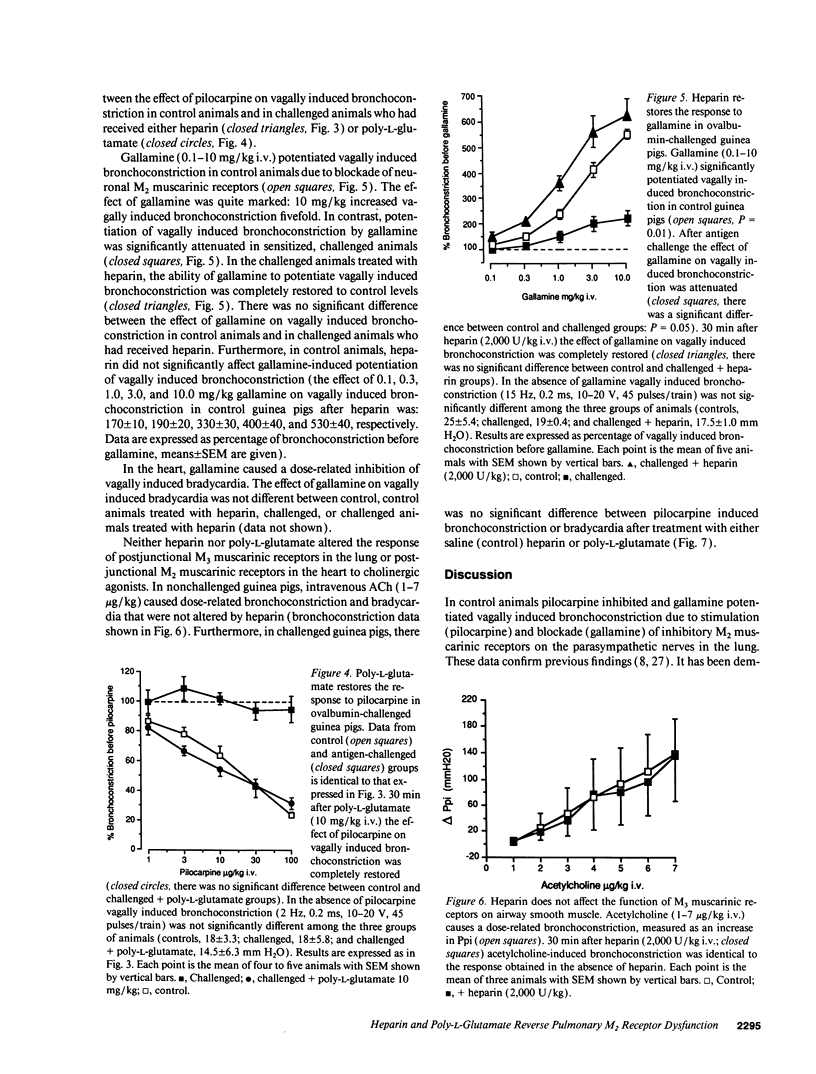
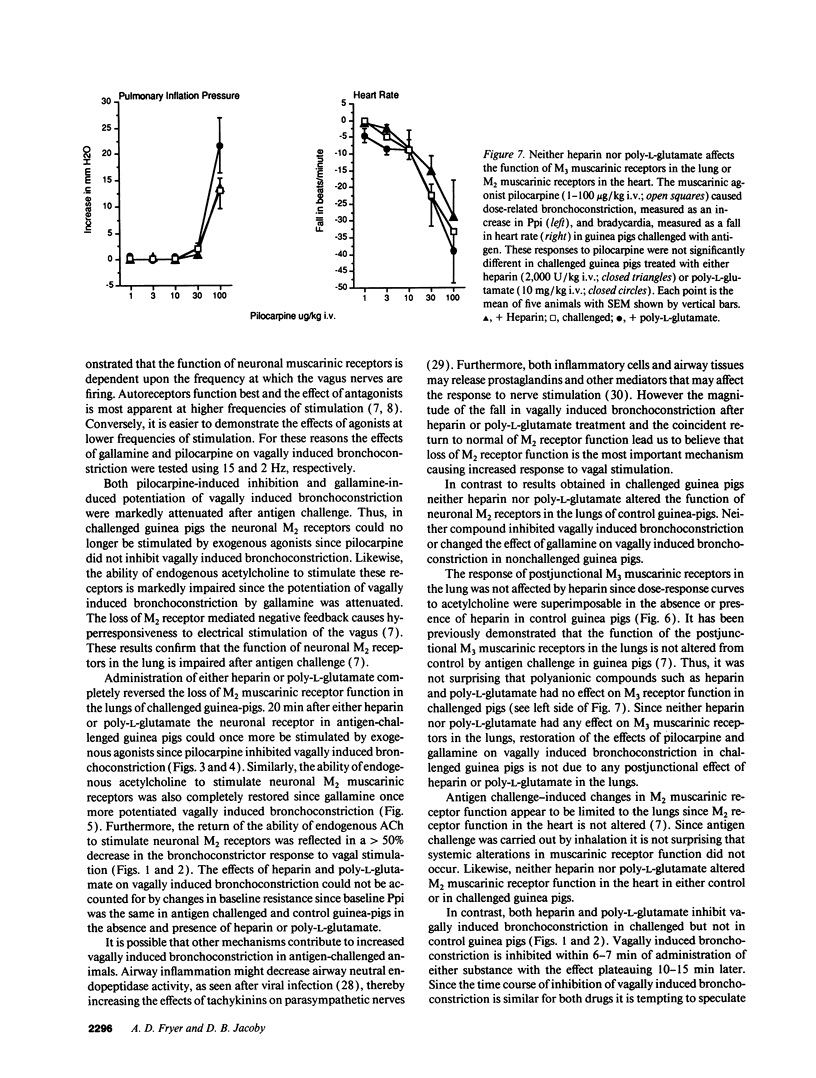
The effect of heparin and poly-L-glutamate on the function of inhibitory M2 muscarinic autoreceptors on parasympathetic nerves in the lung was tested in antigen-challenged guinea pigs. After antigen challenge, M2 receptor function is decreased, thus increasing release of acetylcholine from the vagus and potentiating vagally induced bronchoconstriction. Guinea pigs were anesthetized, tracheostomized, vagotomized, paralyzed, and ventilated. Electrical stimulation of the vagi caused bronchoconstriction and bradycardia. In controls, pilocarpine attenuated vagally induced bronchoconstriction by stimulating neuronal M2 muscarinic receptors. Conversely, blocking these autoreceptors with gallamine potentiated vagally induced bronchoconstriction. In challenged animals the effects of both drugs were markedly reduced, confirming M2 receptor dysfunction. 20 min after heparin or poly-L-glutamate, the effects of both pilocarpine and gallamine on vagally induced bronchoconstriction were restored, demonstrating recovery of M2 receptor function. Neither heparin nor poly-L-glutamate affected vagally induced responses in control animals. Thus antigen-induced dysfunction of M2 receptors can be reversed by polyanionic polysaccharides (heparin) or polyanionic peptides (poly-L-glutamate). This suggests that a polycationic substance such as eosinophil major basic protein, cationic protein, or peroxidase may be responsible for antigen-induced pulmonary M2 receptor dysfunction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas P., Maclagan J. Evidence for prejunctional M2 muscarinic receptors in pulmonary cholinergic nerves in the rat. Br J Pharmacol. 1990 Sep;101(1):73–76. doi: 10.1111/j.1476-5381.1990.tb12091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala L. E., Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma? Chest. 1989 Dec;96(6):1285–1291. doi: 10.1378/chest.96.6.1285. [DOI] [PubMed] [Google Scholar]
- Barker R. L., Gundel R. H., Gleich G. J., Checkel J. L., Loegering D. A., Pease L. R., Hamann K. J. Acidic polyamino acids inhibit human eosinophil granule major basic protein toxicity. Evidence of a functional role for ProMBP. J Clin Invest. 1991 Sep;88(3):798–805. doi: 10.1172/JCI115379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. L., Loegering D. A., Ten R. M., Hamann K. J., Pease L. R., Gleich G. J. Eosinophil cationic protein cDNA. Comparison with other toxic cationic proteins and ribonucleases. J Immunol. 1989 Aug 1;143(3):952–955. [PubMed] [Google Scholar]
- Barnes P. J., Minette P., Maclagan J. Muscarinic receptor subtypes in airways. Trends Pharmacol Sci. 1988 Nov;9(11):412–416. doi: 10.1016/0165-6147(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Blaber L. C., Fryer A. D., Maclagan J. Neuronal muscarinic receptors attenuate vagally-induced contraction of feline bronchial smooth muscle. Br J Pharmacol. 1985 Nov;86(3):723–728. doi: 10.1111/j.1476-5381.1985.tb08951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet L. P., Cartier A., Thomson N. C., Roberts R. S., Dolovich J., Hargreave F. E. Asthma and increases in nonallergic bronchial responsiveness from seasonal pollen exposure. J Allergy Clin Immunol. 1983 Apr;71(4):399–406. doi: 10.1016/0091-6749(83)90069-6. [DOI] [PubMed] [Google Scholar]
- Cartier A., Thomson N. C., Frith P. A., Roberts R., Hargreave F. E. Allergen-induced increase in bronchial responsiveness to histamine: relationship to the late asthmatic response and change in airway caliber. J Allergy Clin Immunol. 1982 Sep;70(3):170–177. doi: 10.1016/0091-6749(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Becker A. B., Lazarus S. C., Frick O. L., Nadel J. A., Gold W. M. Antigen-induced airway hyperresponsiveness and pulmonary inflammation in allergic dogs. J Appl Physiol (1985) 1985 Apr;58(4):1347–1353. doi: 10.1152/jappl.1985.58.4.1347. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Osborne M. L., Corrales R. J., Evans T. W., McCabe L., Frick O. L., Nadel J. A., Gold W. M. Histamine release and circulating cells after antigen inhalation in allergic dogs. J Appl Physiol (1985) 1987 Jan;62(1):253–258. doi: 10.1152/jappl.1987.62.1.253. [DOI] [PubMed] [Google Scholar]
- Dixon W. E. Contributions to the physiology of the lungs: Part I. The bronchial muscles, their innervation, and the action of drugs upon them. J Physiol. 1903 Mar 16;29(2):97–173. doi: 10.1113/jphysiol.1903.sp000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusser D. J., Jacoby D. B., Djokic T. D., Rubinstein I., Borson D. B., Nadel J. A. Virus induces airway hyperresponsiveness to tachykinins: role of neutral endopeptidase. J Appl Physiol (1985) 1989 Oct;67(4):1504–1511. doi: 10.1152/jappl.1989.67.4.1504. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Solley G. O., Farrow G. M., Gleich G. J. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc. 1981 Jun;56(6):345–353. [PubMed] [Google Scholar]
- Fryer A. D., Jacoby D. B. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1991 Jan;102(1):267–271. doi: 10.1111/j.1476-5381.1991.tb12164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984 Dec;83(4):973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A. D., Maclagan J. Pancuronium and gallamine are antagonists for pre- and post-junctional muscarinic receptors in the guinea-pig lung. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):367–371. doi: 10.1007/BF00165549. [DOI] [PubMed] [Google Scholar]
- Fryer A. D., Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol (1985) 1991 Dec;71(6):2255–2261. doi: 10.1152/jappl.1991.71.6.2255. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Maldonado J. E. Identification of a major basic protein in guinea pig eosinophil granules. J Exp Med. 1973 Jun 1;137(6):1459–1471. doi: 10.1084/jem.137.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbenkian A. R., Fernandez X., Kreutner W., Minnicozzi M., Watnick A. S., Kung T., Egan R. W. Anaphylactic challenge causes eosinophil accumulation in bronchoalveolar lavage fluid of guinea pigs. Modulation by betamethasone, phenidone, indomethacin, WEB 2086, and a novel antiallergy agent, SCH 37224. Am Rev Respir Dis. 1990 Sep;142(3):680–685. doi: 10.1164/ajrccm/142.3.680. [DOI] [PubMed] [Google Scholar]
- Gundel R. H., Letts L. G., Gleich G. J. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991 Apr;87(4):1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Wang S. Z., Forray C., el-Fakahany E. E. Complex allosteric modulation of cardiac muscarinic receptors by protamine: potential model for putative endogenous ligands. Mol Pharmacol. 1992 Aug;42(2):311–321. [PubMed] [Google Scholar]
- Ishida K., Kelly L. J., Thomson R. J., Beattie L. L., Schellenberg R. R. Repeated antigen challenge induces airway hyperresponsiveness with tissue eosinophilia in guinea pigs. J Appl Physiol (1985) 1989 Sep;67(3):1133–1139. doi: 10.1152/jappl.1989.67.3.1133. [DOI] [PubMed] [Google Scholar]
- Ito Y., Yoshitomi T. Autoregulation of acetylcholine release from vagus nerve terminals through activation of muscarinic receptors in the dog trachea. Br J Pharmacol. 1988 Mar;93(3):636–646. doi: 10.1111/j.1476-5381.1988.tb10321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B. "Helper" (CD4+) T cells and eosinophils in allergy and asthma. Am Rev Respir Dis. 1992 Feb;145(2 Pt 2):S22–S26. doi: 10.1164/ajrccm/145.2_Pt_2.S22. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Marsh W. R., Irvin C. G., Murphy K. R., Behrens B. L., Larsen G. L. Increases in airway reactivity to histamine and inflammatory cells in bronchoalveolar lavage after the late asthmatic response in an animal model. Am Rev Respir Dis. 1985 Jun;131(6):875–879. doi: 10.1164/arrd.1985.131.6.875. [DOI] [PubMed] [Google Scholar]
- McCaig D. J. Comparison of autonomic responses in the trachea isolated from normal and albumin-sensitive guinea-pigs. Br J Pharmacol. 1987 Dec;92(4):809–816. doi: 10.1111/j.1476-5381.1987.tb11385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minette P. A., Barnes P. J. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J Appl Physiol (1985) 1988 Jun;64(6):2532–2537. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- Minette P. A., Lammers J. W., Dixon C. M., McCusker M. T., Barnes P. J. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol (1985) 1989 Dec;67(6):2461–2465. doi: 10.1152/jappl.1989.67.6.2461. [DOI] [PubMed] [Google Scholar]
- Murphy K. R., Wilson M. C., Irvin C. G., Glezen L. S., Marsh W. R., Haslett C., Henson P. M., Larsen G. L. The requirement for polymorphonuclear leukocytes in the late asthmatic response and heightened airways reactivity in an animal model. Am Rev Respir Dis. 1986 Jul;134(1):62–68. doi: 10.1164/arrd.1986.134.1.62. [DOI] [PubMed] [Google Scholar]
- Sekizawa K., Tamaoki J., Graf P. D., Basbaum C. B., Borson D. B., Nadel J. A. Enkephalinase inhibitor potentiates mammalian tachykinin-induced contraction in ferret trachea. J Pharmacol Exp Ther. 1987 Dec;243(3):1211–1217. [PubMed] [Google Scholar]
- Walters E. H., O'Byrne P. M., Fabbri L. M., Graf P. D., Holtzman M. J., Nadel J. A. Control of neurotransmission by prostaglandins in canine trachealis smooth muscle. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):129–134. doi: 10.1152/jappl.1984.57.1.129. [DOI] [PubMed] [Google Scholar]
- Wasmoen T. L., Bell M. P., Loegering D. A., Gleich G. J., Prendergast F. G., McKean D. J. Biochemical and amino acid sequence analysis of human eosinophil granule major basic protein. J Biol Chem. 1988 Sep 5;263(25):12559–12563. [PubMed] [Google Scholar]