Abstract
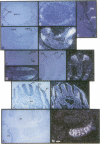
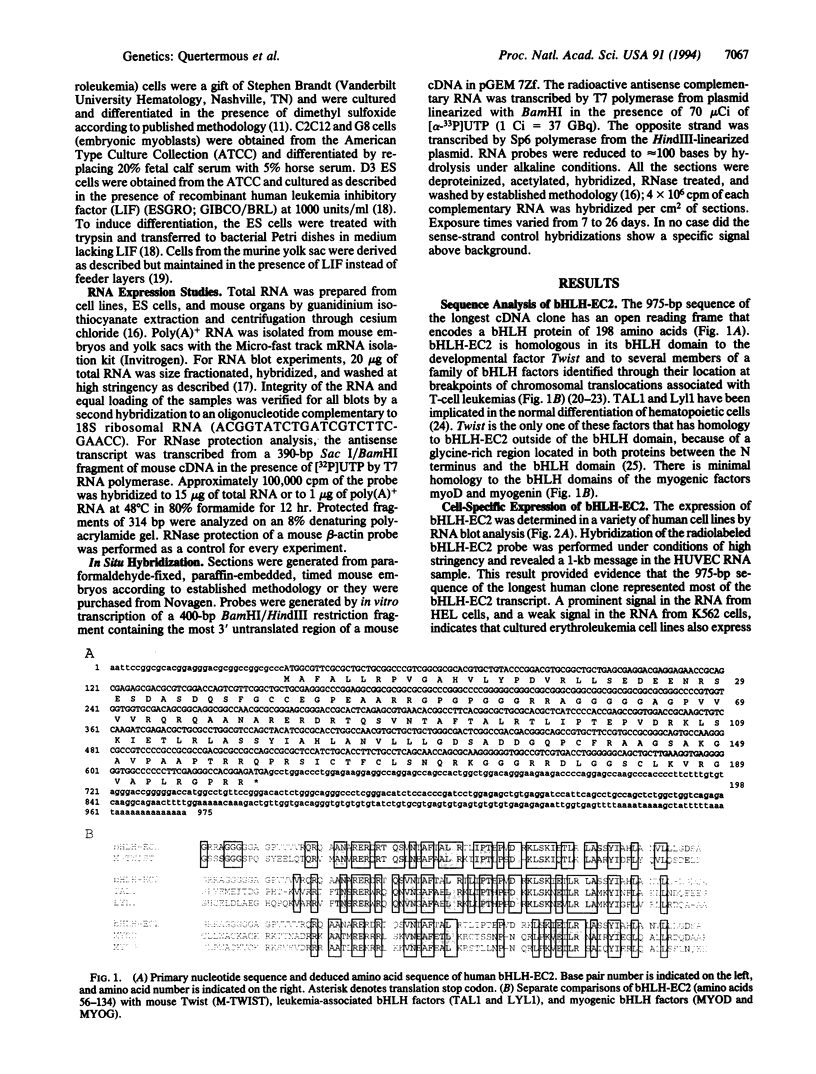
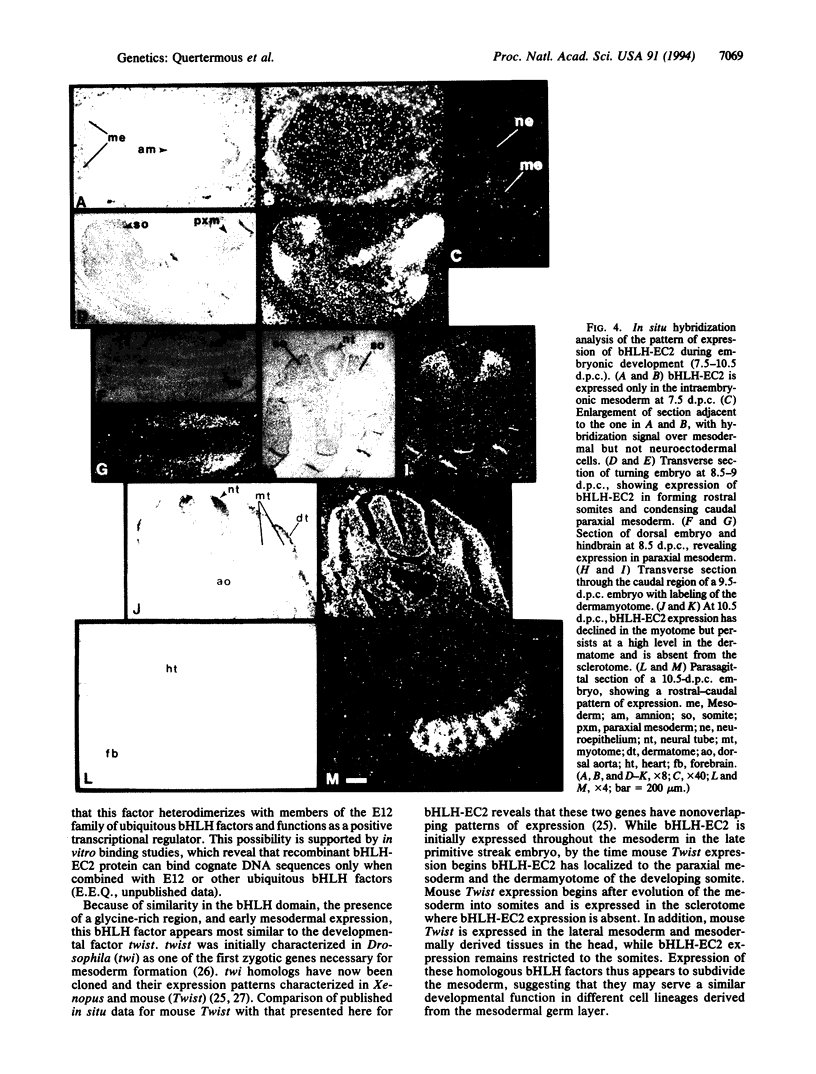
Basic helix-loop-helix (bHLH) heterodimer protein complexes regulate transcription of genes during the processes of differentiation and development. To study the molecular basis of early mesodermal differentiation, we sought to identify bHLH proteins from cells of mesodermal origin. By using an interaction cloning strategy with a radiolabled recombinant bHLH protein, E12, a clone encoding a potential heterodimer partner was isolated from an endothelial cell library. This gene (bHLH-EC2) is most homologous to Twist but shares similarity within the bHLH domain with TAL1 and other members of this family. bHLH-EC2 is expressed in cultured endothelial cells and in embryonic stem cell, erythroleukemia, and muscle cell lines in a differentiation-dependent manner. In situ hybridization studies of mouse embryos reveal that bHLH-EC2 is expressed throughout the primitive mesoderm as early as 7.5 days postcoitum. Expression then becomes restricted to the paraxial mesoderm and to the dermamyotome of the developing somite. Expression of bHLH-EC2 in cells destined to become myoblasts thus predates expression of myogenic bHLH factors. bHLH-EC2 is expressed in early endothelial and hematopoietic cells in vivo, as shown by RNA studies of embryonic yolk sac and cultured cells derived from yolk sac explants. These findings suggest that bHLH-EC2 plays a role in the development of multiple cell types derived from the primitive mesoderm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplan P. D., Nakahara K., Orkin S. H., Kirsch I. R. The SCL gene product: a positive regulator of erythroid differentiation. EMBO J. 1992 Nov;11(11):4073–4081. doi: 10.1002/j.1460-2075.1992.tb05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate M., Rushton E., Currie D. A. Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development. 1991 Sep;113(1):79–89. doi: 10.1242/dev.113.1.79. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- German M. S., Blanar M. A., Nelson C., Moss L. G., Rutter W. J. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991 Feb;5(2):292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Gurdon J. B. Gene activation in the amphibian mesoderm. Dev Suppl. 1991;1:95–104. [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell. 1989 Dec 1;59(5):893–903. doi: 10.1016/0092-8674(89)90612-0. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993 Dec 3;75(5):827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991 Aug 25;266(24):16188–16192. [PubMed] [Google Scholar]
- Mellentin J. D., Smith S. D., Cleary M. L. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989 Jul 14;58(1):77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Klein W. H. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994 Jan;8(1):1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- Peault B. M., Thiery J. P., Le Douarin N. M. Surface marker for hemopoietic and endothelial cell lineages in quail that is defined by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(10):2976–2980. doi: 10.1073/pnas.80.10.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R. P., Arora K., Nüsslein-Volhard C., Gelbart W. M. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development. 1991 Sep;113(1):35–54. doi: 10.1242/dev.113.1.35. [DOI] [PubMed] [Google Scholar]
- Sawada S., Littman D. R. A heterodimer of HEB and an E12-related protein interacts with the CD4 enhancer and regulates its activity in T-cell lines. Mol Cell Biol. 1993 Sep;13(9):5620–5628. doi: 10.1128/mcb.13.9.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. M. Embryonic induction. Mech Dev. 1993 May;41(2-3):91–107. doi: 10.1016/0925-4773(93)90040-5. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Mesoderm-inducing factors in early vertebrate development. EMBO J. 1993 Dec;12(12):4463–4470. doi: 10.1002/j.1460-2075.1993.tb06135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B., Stoetzel C., Gorostiza-Thisse C., Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988 Jul;7(7):2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Thisse B. Dorsoventral development of the Drosophila embryo is controlled by a cascade of transcriptional regulators. Dev Suppl. 1992:173–181. [PubMed] [Google Scholar]
- Visvader J., Begley C. G., Adams J. M. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene. 1991 Feb;6(2):187–194. [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993 Dec 31;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Wilson R. B., Kiledjian M., Shen C. P., Benezra R., Zwollo P., Dymecki S. M., Desiderio S. V., Kadesch T. Repression of immunoglobulin enhancers by the helix-loop-helix protein Id: implications for B-lymphoid-cell development. Mol Cell Biol. 1991 Dec;11(12):6185–6191. doi: 10.1128/mcb.11.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C., Thisse C., Stoetzel C., Thisse B., Gerlinger P., Perrin-Schmitt F. The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev Biol. 1991 Feb;143(2):363–373. doi: 10.1016/0012-1606(91)90086-i. [DOI] [PubMed] [Google Scholar]
- Xia Y., Brown L., Yang C. Y., Tsan J. T., Siciliano M. J., Espinosa R., 3rd, Le Beau M. M., Baer R. J. TAL2, a helix-loop-helix gene activated by the (7;9)(q34;q32) translocation in human T-cell leukemia. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11416–11420. doi: 10.1073/pnas.88.24.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]