Abstract
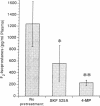
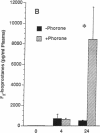
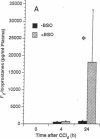
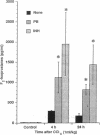
These studies examine the in vivo formation of a unique series of PGF2-like compounds (F2-isoprostanes) derived from free radical-catalyzed nonenzymatic peroxidation of arachidonic acid. We have previously shown that levels of these compounds increase up to 50-fold in rats administered CCl4. To understand further the formation of these compounds in vivo, we carried out a series of experiments assessing factors influencing their generation. After CCl4 (2 ml/kg) was administered to rats, plasma F2-isoprostanes increased 55-fold by 4 h. Levels declined thereafter, but at 24 h, they were still elevated 21-fold, indicating continued lipid peroxidation. Pretreatment of rats with isonicotinic acid hydrazide and phenobarbital to induce cytochrome P-450 enhanced the production of F2-isoprostanes after CCl4 administration eightfold and fivefold, respectively, whereas inhibition of the cytochrome P-450 system with SKF-525A and 4-methylpyrazole decreased formation of F2-isoprostanes after CCl4 by 55 and 82%, respectively. Further, the glutathione-depleting agents buthionine sulfoximine and phorone augmented the F2-isoprostane response to CCl4 by 22- and 11-fold, respectively. F2-isoprostanes are formed in situ esterified to lipids and, in addition to increases in levels of free F2-isoprostanes in the circulation, levels of F2-isoprostanes esterified to lipids in various organs and plasma also increase sharply during CCl4 poisoning. The measurement of F2-isoprostanes may facilitate investigation of the role of lipid peroxidation in human diseases.
Full text
PDF
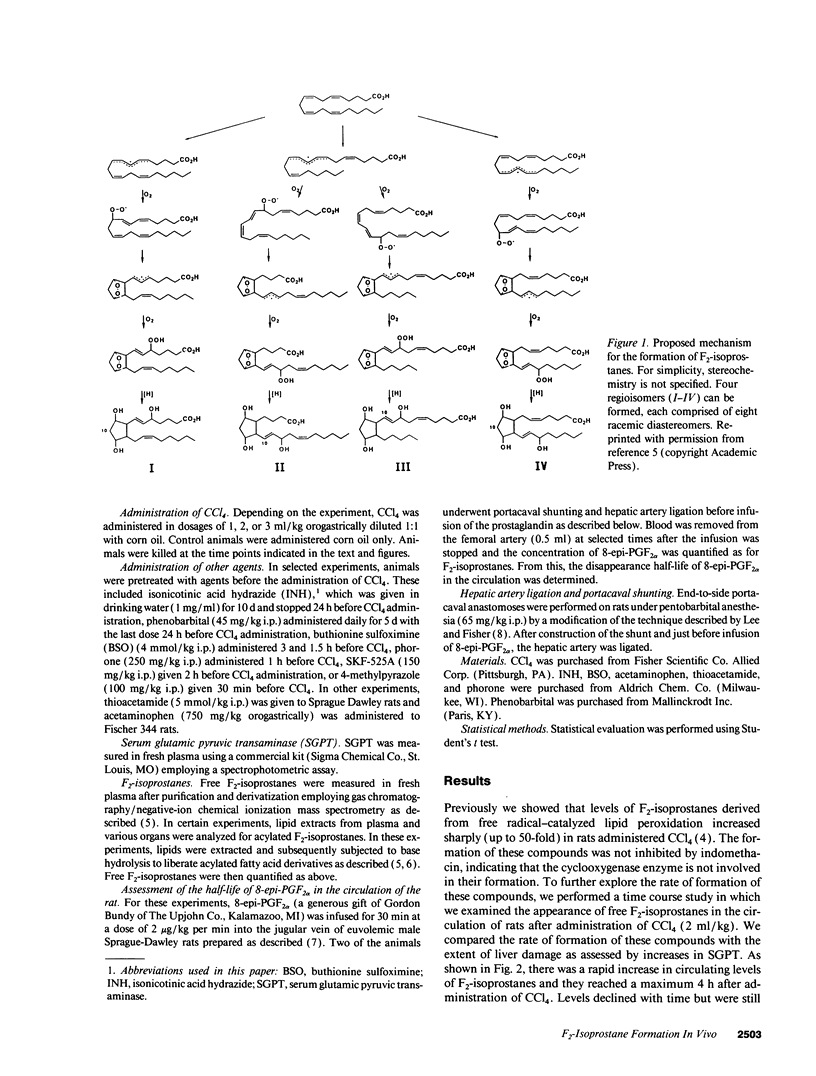
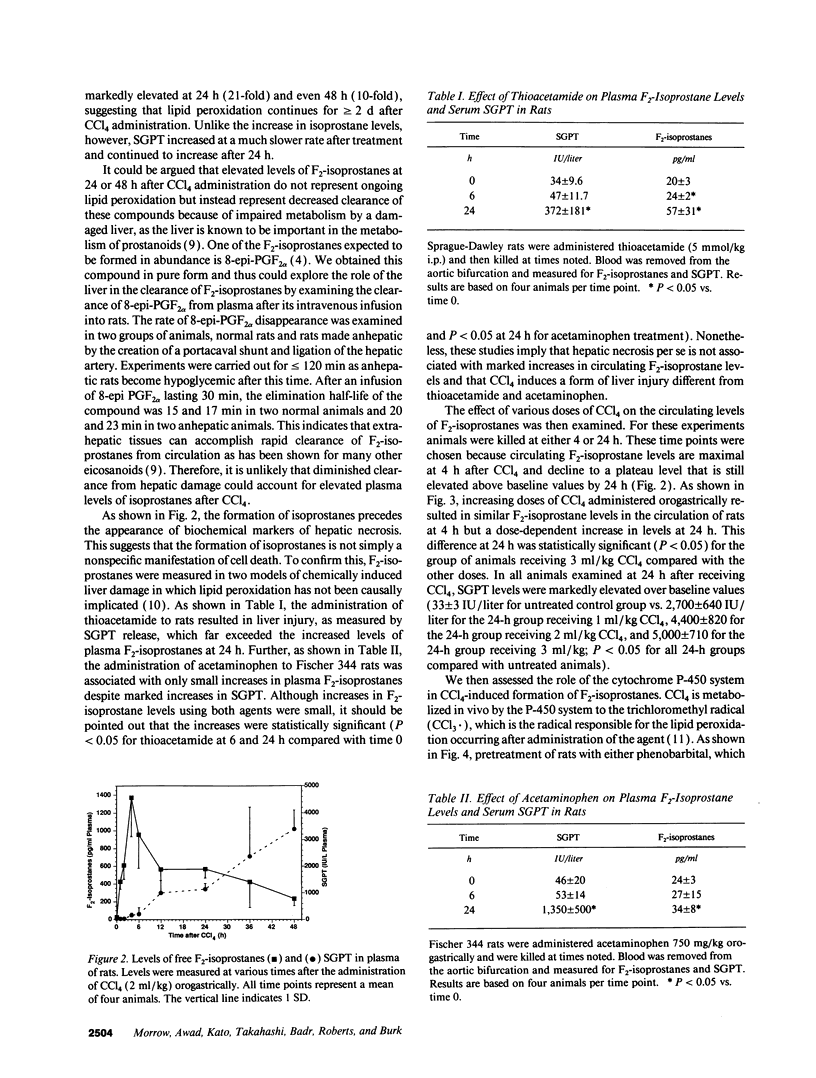
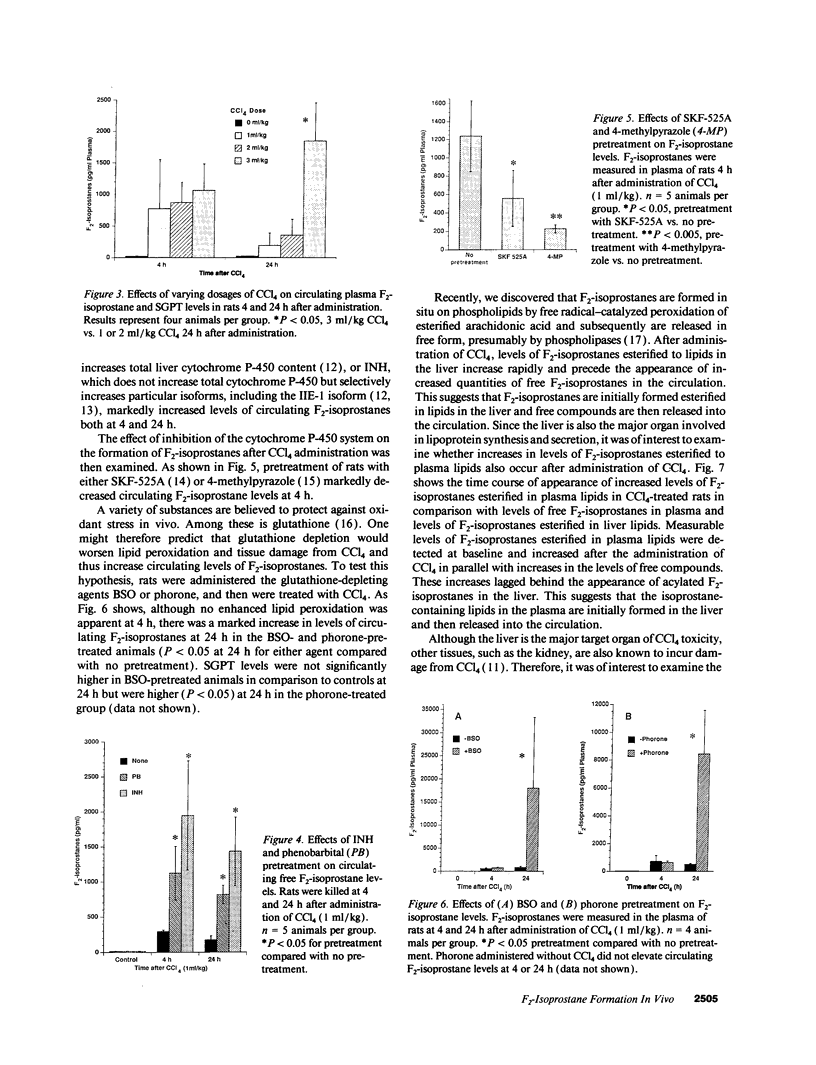
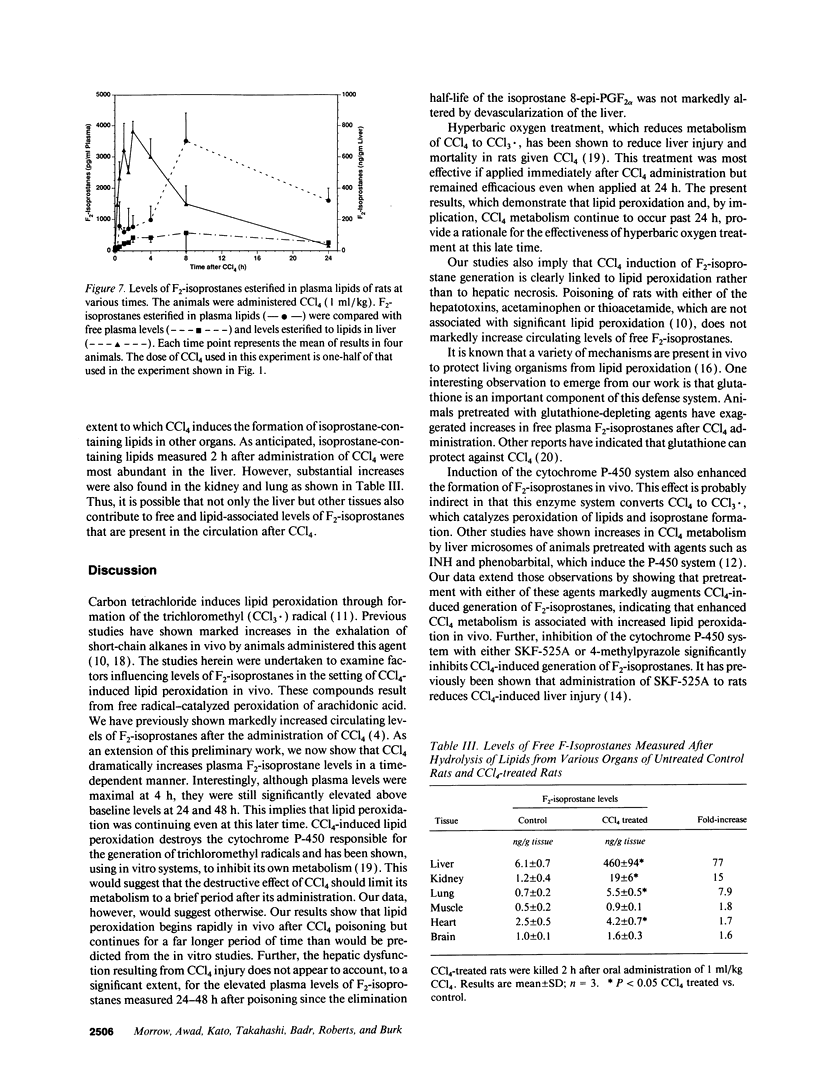

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burk R. F., Hill K. E., Lane J. M. Inhibition of CCl4 metabolism by oxygen varies between isoenzymes of cytochrome P-450. Biochem Biophys Res Commun. 1988 May 16;152(3):1463–1467. doi: 10.1016/s0006-291x(88)80450-9. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Lane J. M. Ethane production and liver necrosis in rats after administration of drugs and other chemicals. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):467–478. doi: 10.1016/0041-008x(79)90400-9. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Reiter R., Lane J. M. Hyperbaric oxygen protection against carbon tetrachloride hepatotoxicity in the rat. Association with altered metabolism. Gastroenterology. 1986 Apr;90(4):812–818. doi: 10.1016/0016-5085(86)90856-5. [DOI] [PubMed] [Google Scholar]
- Burk R. J., Ludden T. M., Lane J. M. Pentane clearance from inspired air by the rat: dependence on the liver. Gastroenterology. 1983 Jan;84(1):138–142. [PubMed] [Google Scholar]
- Ferreyra E. C., de Fenos O. M., Bernacchi A. S., de Castro C. R., Castro J. A. Treatment of carbon tetrachloride-induced liver necrosis with chemical compounds. Toxicol Appl Pharmacol. 1977 Dec;42(3):513–521. doi: 10.1016/s0041-008x(77)80036-7. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Koop D. R. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992 Jan 6;6(2):724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Harris T. M., Roberts L. J., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990 Jan;184(1):1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R., Burk R. F. Formation of glutathione adducts of carbon tetrachloride metabolites in a rat liver microsomal incubation system. Biochem Pharmacol. 1988 Jan 15;37(2):327–331. doi: 10.1016/0006-2952(88)90736-8. [DOI] [PubMed] [Google Scholar]
- Riely C. A., Cohen G., Lieberman M. Ethane evolution: a new index of lipid peroxidation. Science. 1974 Jan 18;183(4121):208–210. doi: 10.1126/science.183.4121.208. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nammour T. M., Fukunaga M., Ebert J., Morrow J. D., Roberts L. J., 2nd, Hoover R. L., Badr K. F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992 Jul;90(1):136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]