Abstract
We studied the effects of fibrinogen degradation product (FDP) fragment D on endothelial monolayer integrity and the mechanisms of fragment D-induced endothelial cell detachment from the substratum. Incubation of bovine pulmonary artery endothelial cells (BPAEC) with fragment D caused concentration- and time-dependent cell detachment from the substratum. The optimal response occurred at fragment D concentrations of 2 microM and required an incubation time of 24 h. BPAEC challenged with fragment D increased the concentration and activity of urokinase-type plasminogen activator (uPA) in the conditioned medium within 2 to 4 h of incubation. Fragment D also induced the release of tissue-type plasminogen activator, but to a lesser extent than uPA. Fragment D concurrently increased plasminogen activator (PA) activity in a concentration-dependent manner. Increased PA activity was followed by augmentation of cell-associated plasmin activity and subsequent increase in the degradation of 125I-fibrinogen and 125I-vitronectin precoated in the subendothelial matrix. Pretreatment of BPAEC with anti-uPA antibody, and inhibitors of uPA (dansyl-GGACK) and plasmin (aprotinin) prevented approximately 60% of the fragment D-induced endothelial cell detachment. We conclude that FDP fragment D increases secretion of endothelial PAs and enhances the generation of plasmin, thereby contributing to proteolysis of extracellular matrix and endothelial cell detachment. Fragment D may be a critical mediator linking activation of fibrinolysis to vascular endothelial injury in inflammatory disorders.
Full text
PDF




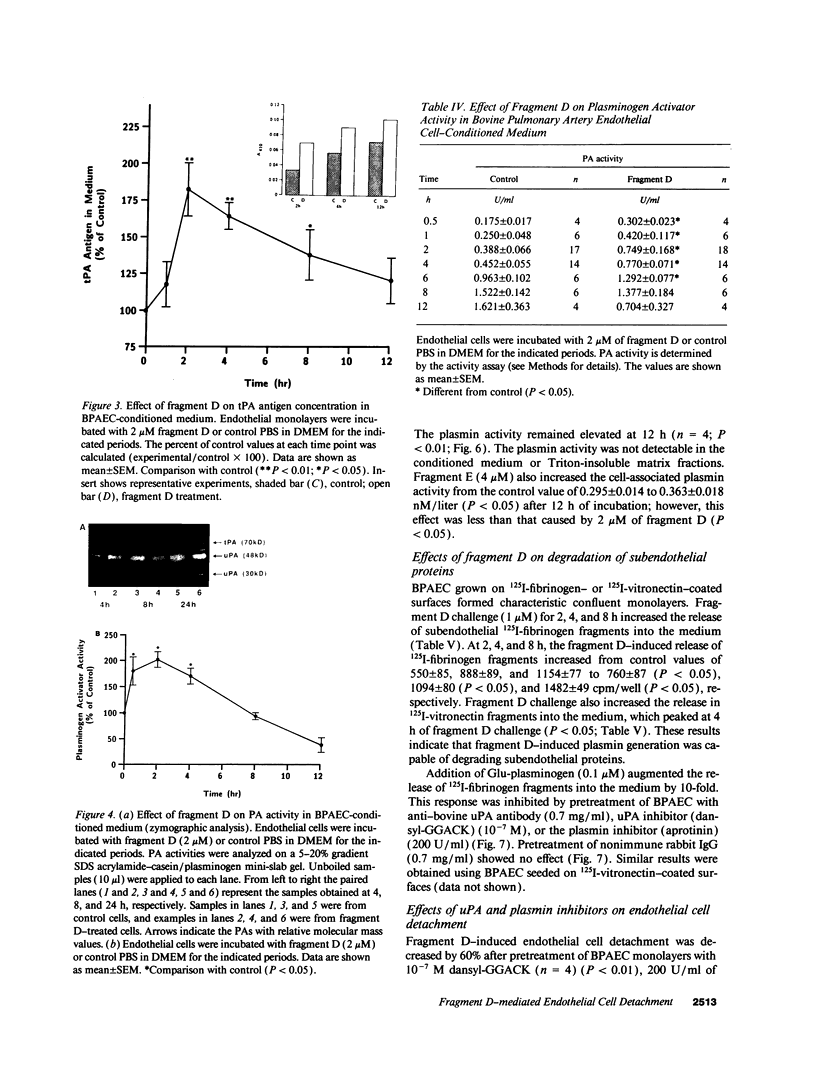

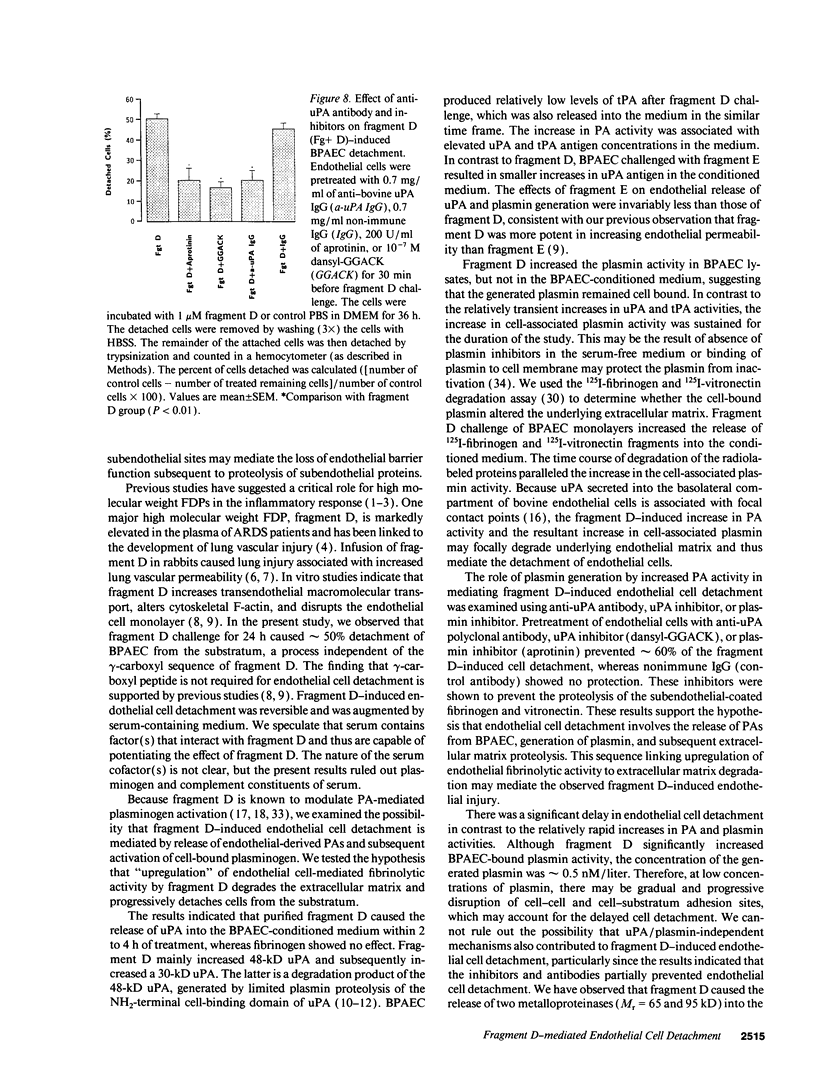

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer P. I., Machovich R., Büki K. G., Csonka E., Koch S. A., Horváth I. Interaction of plasmin with endothelial cells. Biochem J. 1984 Feb 15;218(1):119–124. doi: 10.1042/bj2180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykowska K., Levin E. G., Rijken D. C., Loskutoff D. J., Collen D. Characterization of a plasminogen activator secreted by cultured bovine aortic endothelial cells. Biochim Biophys Acta. 1982 Apr 21;703(1):113–115. doi: 10.1016/0167-4838(82)90018-8. [DOI] [PubMed] [Google Scholar]
- Camiolo S. M., Markus G., Englander L. S., Siuta M. R., Hobika G. H., Kohga S. Plasminogen activator content and secretion in explants of neoplastic and benign human prostate tissues. Cancer Res. 1984 Jan;44(1):311–318. [PubMed] [Google Scholar]
- Chen J. P., Shurley H. M., Vickroy M. A facile separation of fragments D and E from the fibrinogen-fibrin degradation products of three mammalian species. Biochem Biophys Res Commun. 1974 Nov 6;61(1):66–71. doi: 10.1016/0006-291x(74)90534-8. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Green G. D. A sensitive, coupled assay for plasminogen activator using a thiol ester substrate for plasmin. Ann N Y Acad Sci. 1981;370:617–626. doi: 10.1111/j.1749-6632.1981.tb29768.x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Bell W. R., Kaiser D., Wong A. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 1985 Mar 22;227(4693):1487–1490. doi: 10.1126/science.4038818. [DOI] [PubMed] [Google Scholar]
- Ge M., Ryan T. J., Lum H., Malik A. B. Fibrinogen degradation product fragment D increases endothelial monolayer permeability. Am J Physiol. 1991 Oct;261(4 Pt 1):L283–L289. doi: 10.1152/ajplung.1991.261.4.L283. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Radnik R. A., Garoni J. A., Kreisberg J. I. Urokinase-dependent adhesion loss and shape change after cyclic adenosine monophosphate elevation in cultured rat mesangial cells. J Clin Invest. 1988 Dec;82(6):1992–2000. doi: 10.1172/JCI113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Kirkeby L. T., Ralfkiaer E., Kristensen P., Lund L. R., Danø K. Urokinase-type plasminogen activator in endothelial cells during acute inflammation of the appendix. Am J Pathol. 1989 Oct;135(4):631–636. [PMC free article] [PubMed] [Google Scholar]
- Haynes J. B., Hyers T. M., Giclas P. C., Franks J. J., Petty T. L. Elevated fibrin(ogen) degradation products in the adult respiratory distress syndrome. Am Rev Respir Dis. 1980 Dec;122(6):841–847. doi: 10.1164/arrd.1980.122.6.841. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol. 1982 Sep;94(3):631–636. doi: 10.1083/jcb.94.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. N., Gurewich V. A comparative study of the promotion of tissue plasminogen activator and pro-urokinase-induced plasminogen activation by fragments D and E-2 of fibrin. J Clin Invest. 1991 Dec;88(6):2012–2017. doi: 10.1172/JCI115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M. A., Straight D. L., Fretto L. J., McKee P. A. The effects of fibrinogen and its cleavage products on the kinetics of plasminogen activation by urokinase and subsequent plasmin activity. J Biol Chem. 1983 Oct 25;258(20):12171–12177. [PubMed] [Google Scholar]
- Malik A. B. Pulmonary microembolism. Physiol Rev. 1983 Jul;63(3):1114–1207. doi: 10.1152/physrev.1983.63.3.1114. [DOI] [PubMed] [Google Scholar]
- Manwaring D., Curreri P. W. The role of platelet aggregation and release in fragment D-induced pulmonary dysfunction. Ann Surg. 1980 Jul;192(1):103–107. doi: 10.1097/00000658-198007000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwaring D., Thorning D., Curreri P. W. Mechanisms of acute pulmonary dysfunction induced by fibrinogen degradation product D. Surgery. 1978 Jul;84(1):45–54. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985 Apr 10;260(7):4303–4311. [PubMed] [Google Scholar]
- Murata T., Nakashima Y., Yasunaga C., Maeda K., Sueishi K. Extracellular and cell-associated localizations of plasminogen activators and plasminogen activator inhibitor-1 in cultured endothelium. Exp Mol Pathol. 1991 Oct;55(2):105–118. doi: 10.1016/0014-4800(91)90046-z. [DOI] [PubMed] [Google Scholar]
- Ryan T. J., Keegan M. C. Photoaffinity labeling of functionally different lysine-binding sites in human plasminogen and plasmin. Biochim Biophys Acta. 1985 Aug 8;830(2):187–194. doi: 10.1016/0167-4838(85)90027-5. [DOI] [PubMed] [Google Scholar]
- Ryan T. J., Seeger J. I., Kumar S. A., Dickerman H. W. Estradiol preferentially enhances extracellular tissue plasminogen activators of MCF-7 breast cancer cells. J Biol Chem. 1984 Dec 10;259(23):14324–14327. [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Saldeen T. Vasoactive peptides derived from degradation of fibrinogen and fibrin. Ann N Y Acad Sci. 1983 Jun 27;408:424–437. doi: 10.1111/j.1749-6632.1983.tb23262.x. [DOI] [PubMed] [Google Scholar]
- Stief T. W., Marx R., Heimburger N. Oxidized fibrin(ogen) derivatives enhance the activity of tissue type plasminogen activator. Thromb Res. 1989 Oct 15;56(2):221–228. doi: 10.1016/0049-3848(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., van den Berg E. A., Fiers W., Dooijewaard G. Tumor necrosis factor induces the production of urokinase-type plasminogen activator by human endothelial cells. Blood. 1990 May 15;75(10):1991–1998. [PubMed] [Google Scholar]