Abstract
Study Objectives:
To analyze statistically the association between periodic leg movements during sleep (PLMS) and arousals, in order to eventually support or challenge the current scoring rules and to further understand their reciprocal influence.
Setting:
Sleep research center.
Patients:
Twenty untreated consecutive patients with restless legs syndrome (RLS) (13 women and 7 males, mean age 60.9 y).
Methods:
In each recording, we selected all PLMS/arousal pairs that met the following inclusion criteria: (a) PLMS events that were separated from another PLMS event (preceding or following) by at least 10 s of EMG inactivity; (b) arousal events separated from another arousal event (preceding or following) by at least 10 s of stable EEG baseline activity; (c) PLMS/arousal pairs were then selected among events identified according to the previous two criteria, when PLMS and arousals were separated (offset-to-onset) by no more than 10 s, regardless of which was first.
Measurements and Results:
We selected a mean of 46.1 (SD 25.55) PLMS/arousal pairs per subject; in these pairs, average PLMS duration was 3.2 s (0.65) and average arousal duration was 6.5 s (0.92). Within these event pairs, the great majority (on average 98.4%, SD 3.88) was separated by less than 0.5 s (i.e., between the end of one event and the onset of the other, regardless of which was first). Arousal onsets preceded PLMS onset in 41.2% of pairs, while the opposite was true for the remaining 58.8% of pairs. A significant correlation between PLMS duration and arousal duration was also found (r = 0.447, P < 0.000001).
Conclusion:
The results of this study support the current rule for the definition of the association between periodic leg movements during sleep (PLMS) and arousals. The tight time relationship between PLMS and arousals and their correlated durations seem to indicate that both events might be regulated by a complex mechanism, rather than being connected by a simple reciprocal cause/effect relationship.
Citation:
Ferri R, Rundo F, Zucconi M, Manconi M, Bruni O, Ferini-Strambi L, Fulda S. An evidence-based analysis of the association between periodic leg movements during sleep and arousals in restless legs syndrome. SLEEP 2015;38(6):919–924.
Keywords: periodic leg movements during sleep, PLMS, arousals, restless legs syndrome, sleep scoring
INTRODUCTION
The first polysomnographic (PSG) EMG recordings of periodic leg movements during sleep (PLMS) were carried out in Bologna (Italy) by Lugaresi et al. in 1965,1,2 but it was only almost 20 years later that duration, amplitude, periodicity, and symmetry of PLMS were defined and manually measured on paper recordings by Coleman in 1982,3 and constituted reference data for the first scoring criteria introduced by the American Sleep Disorders Association (ASDA) in 1993.4 These criteria were then used for more than 20 years.
Subsequently, two sets of similar rules for scoring PLMS and periodic leg movements during wakefulness (PLMW) have been established that were based, at least in part, on algorithms proposed for the automatic detection of leg movements (LMs) in PSG, including mathematically defined parameters such as thresholds, intervals, and amplitude.5,6 The first of these two sets of rules was introduced in 2006 by a task force of the International Restless Legs Syndrome (RLS) Study Group, endorsed by the World Association of Sleep Medicine (WASM/IRLSSG).7 Subsequently, the majority of these rules (but not all) were adopted by the American Academy of Sleep Medicine (AASM) in 2007 and confirmed in 2012.8,9
Notwithstanding the fact that the current rules are largely based on data-driven measures, they still contain some unchallenged criteria that had been introduced during the “paper era” of sleep medicine and maintained throughout the years, or that had been introduced without a formal assessment of their validity. Among these, the rule to define the association between a PLMS event and an arousal has been established by expert consensus but the statistical properties of this association have not been explored before. The current WASM/IRLSSG7 and AASM9 sets of rules both agree that PLMS are considered to be associated with an arousal event when the two events are separated by less than 0.5 s; i.e., between the end of one event and the onset of the other, regardless of which is first. The PLMS arousal index indicates the number of PLMS associated with arousals divided by the number of hours of sleep with PLMS and arousal recording.
The aim of this study was to analyze statistically the association between PLMS and arousals to eventually support or challenge the current scoring rules and to further understand their reciprocal influence.
METHODS
Subjects
Twenty untreated, drug-naïve, consecutive patients (13 women, 7 males, mean age 60.9 y) affected by idiopathic RLS were included in this study. The patients were recruited at the Sleep Research Centre, Oasi Research Institute, Troina (Italy). In agreement with the International RLS Study Group,10,11 the minimal criteria accepted for the diagnosis of RLS were: (a) an urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs; (b) the urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting; (c) the urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching; (d) the urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night; (e) the occurrence of the above features is not solely accounted for as symptoms primary to another medical or a behavioral condition. Routine blood tests and neurophysiological investigation (EMG and electroneurography of the lower limbs) were also normal. The sleep respiratory pattern of each patient was assessed by means of oral and nasal airflow (thermistor and/ or nasal pressure cannula), thoracic and abdominal respiratory effort (strain gauge), and oxygen saturation (pulse oximetry), in a previous recording (within 1 w) or during this study recording; subjects with an apnea-hypopnea index > 5 were not included. Neurological examination resulted unremarkable in all patients. For each patient included, the International RLS Severity Scale (IRLS) score12 was also obtained.
This study was approved by the local ethics committee and all subjects provided informed consent before entering the study.
Polysomnographic Sleep Recording
Each subject underwent a polysomnographic full-night recording, after an adaptation night, carried out in a standard sound-attenuated (noise level to a maximum of 30 dB nHL) sleep laboratory. Subjects were not allowed to have beverages containing caffeine during the afternoon preceding the recording and were allowed to sleep until their spontaneous awakening in the morning.
The following parameters were included in the polysomnographic study: EEG (≥ 3 channels, one frontal, one central, and one occipital, referred to the contralateral earlobe); electrooculogram (electrodes placed 1 cm above the right outer canthus and 1 cm below the left outer canthus and referred to A1), electromyogram (EMG) of the submentalis muscle, EMG of the right and left tibialis anterior muscles (bipolar derivations with 2 electrodes placed 3 cm apart on the belly of the anterior tibialis muscle of each leg, with impedance less than 10 KΩ), and ECG (one derivation). EMG signals, in particular, were digitally band-pass filtered at 10–100 Hz, with a notch filter at 50 Hz.
At the beginning of each session and before the start of recording, the sleep technician checked that the amplitude of the EMG signal from the 2 tibialis anterior muscles was < 2 μV at rest.
Sleep and Arousal Scoring and Detection of Leg Movements
Sleep stages were visually scored following standard criteria on 30-s epochs.13 Arousals were also visually detected during sleep following the standard criteria: an abrupt shift of the electroencephalogram frequency (alpha, theta, and/or frequencies > 16 Hz, but not spindles) lasting ≥ 3 s and preceded by ≥ 10 s of stable sleep; in REM sleep, a concurrent increase of the submental EMG lasting ≥ 1 s was also required.9 LMs during sleep were first detected by the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy). With this software, detection is performed by means of a human-supervised automatic approach controlled by the scorer. For this study, one scorer (R.F.) visually edited the detections proposed by the automatic analysis before the computation of the various parameters which were automatically generated by the same software adopting the criteria set by the WASM/IRLSSG.7 In particular, LMs recorded in the 2 limbs were considered to be a single bilateral LM when they were overlapping or separated (offset-to-onset) by < 0.5 s; in a few cases this resulted in the identification of PLMS > 10 s (but always < 15 s), in agreement with the above cited criteria.7 The PLMS index was calculated as the number of LMs included in a series ≥ 4, separated by ≥ 5 and ≤ 90 s, per hour of sleep. Subsequently, the number of intervals included in sequences of ≥ 3 (all 10–90 s long) was divided by the total number of intervals. We will refer to this ratio as the periodicity index (PI); this index can vary between 0 (absence of periodicity, with none of the intervals having a length between 10 and 90 s) to 1 (complete periodicity, with all intervals having a length between 10 and 90 s).14,15
Analysis of the PLMS/Arousal Association
In each recording, we selected all PLMS/arousal pairs that met the following inclusion criteria: (a) PLMS events separated from another PLMS event (preceding or following) by ≥ 10 s of EMG inactivity; (b) arousal events identified according to the American Academy of Sleep Medicine criteria9 and separated from another arousal event (preceding or following) by ≥ 10 s of stable EEG baseline activity; (c) among the events detected according to the above criteria, PLMS/arousal pairs were included when PLMS and arousal were separated (offset-to-onset) by ≤ 10 s, regardless of which was first.
For all PLMS/arousal pairs detected according to the above criteria, 2 main parameters were assessed: (a) minimal distance between the PLMS and the arousal: the distance was set to 0 s when the 2 events overlapped; a positive value was assigned in the case that the arousal occurred after the PLMS and denotes the distance between the end of the PLMS and the onset of the arousal; likewise, a negative value characterizes the cases where the arousal occurred before the PLMS and describes the distance between the end of the arousal and the onset of the following PLMS; (b) the time lag between the onset of the PLMS and the onset of the arousal event (negative values indicated that the arousal onset occurred before the PLMS event onset, positive values indicate the opposite). All the above parameters were obtained automatically by means of a computer program arranged ad hoc for this purpose.
RESULTS
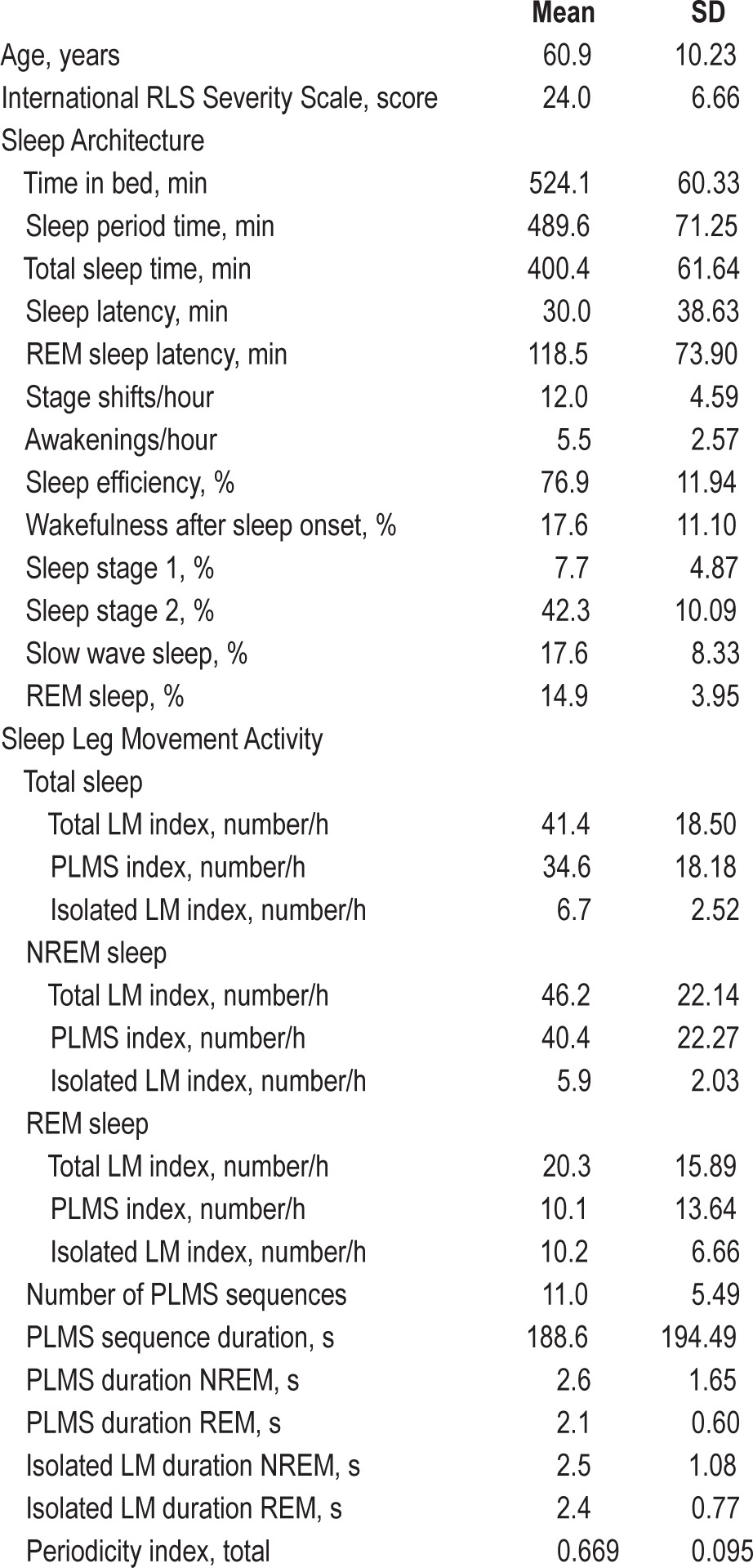
Table 1 shows the clinical features, sleep architecture, and sleep leg movement activity parameters obtained in the group of patients with RLS. RLS subjects exhibited a moderate-to-severe disease severity (average IRLS score 24.0) and an average PLMS index of 34.6/h.
Table 1.
Clinical features, sleep architecture, and sleep leg movement activity parameters obtained in the group of patients with RLS.
We detected, on average, 186.6 (SD 97.77) PLMS/patient which were separated from another PLMS event (preceding or following) by ≥ 10 s of EMG inactivity. Among these, we identified a mean of 46.1 (SD 25.55) PLMS/arousal pairs per subject (NREM 41.2 [24.28] and REM 4.5 [3.90]) where the events were separated by ≤ 10 s. In these pairs, the average PLMS duration was 3.2 s (0.65) and the average arousal duration was 6.5 s (0.92).
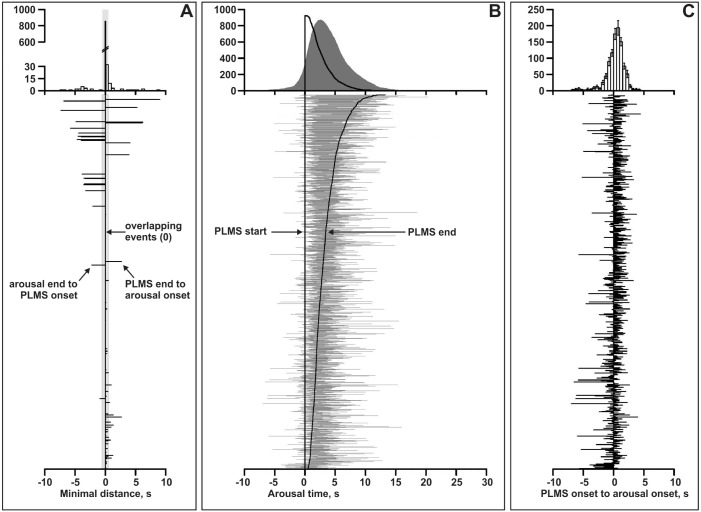
On average, in 98.4% (3.88) of pairs/patient (92.3% of all PLMS), PLMS and arousals overlapped or were separated by < 0.5 s, thus fulfilling the criteria of the current scoring rules for the scoring of PLMS associated to arousals (Figure 1A).7,9
Figure 1.
(A) Offset-to-onset distance in each pair of events (bottom graph) and their statistical distribution (upper graph). (B) Bottom graph – Time relationship between all pairs of PLMS/arousal events; the vertical line indicates the PLMS onset while the “sigmoid” curve over the right side of the figure represents the end of each PLMS event; the horizontal lines represent the extent of the arousal accompanying each PLMS in the pairs. Upper graph – statistical distribution of the time relationship between the two events in the pairs. (C) Onset-to-onset distance in each pair of events (bottom graph) and their statistical distribution (upper graph); negative values indicate arousals preceding PLMS, positive values indicate the opposite combination.
Figure 2 shows a more detailed analysis of the offset-to-onset difference between the 2 events in each pair. With increasing time difference, the corresponding increase in the percentage of associated pairs increased but with a different slope for lags ≤ 0.5 s, compared to lags above this value (4.457 vs. 0.948; t = 19.93, P < 0.0000001). The 2 regression lines calculated for the 2 slope regions (dashed lines) support this observation and further reinforce the current rules for the scoring of PLMS associated to arousals.7,9
Figure 2.
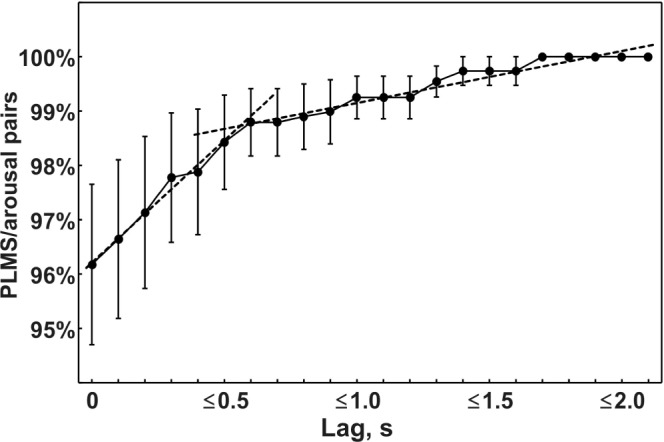
Analysis of the percentage of event pairs (PLMS/arousal) separated by different time lags. Data are shown as mean (filled circles) and standard error (whiskers). Dotted lines represent the regression lines obtained for lags from 0 to 0.5 s and from 0.6 to > 2 s.
Figure 1C depicts the distribution of onset-to-onset intervals between PLMS and arousals in the whole group of patients; in 41.2% of cases, the arousal onset preceded the PLMS onset, while the opposite was true for the remaining 58.8% of pairs. However, in 45.9% of cases, the arousal onset occurred in the second surrounding the PLMS onset (from 0.5 s before to 0.5 s after the PLMS event onset). The overall distribution appeared to be normal; however, it did not pass the Shapiro-Wilk W test of normality (W = 0.902, P = 0.0001). In particular, it should be noted that the left tail (arousal preceding PLMS) was significantly longer than the right tail.
Finally, Figure 1B shows the time relationship between all pairs of PLMS/arousal events in the lower part, while the upper graph shows the statistical distribution of the time relationship between the 2 events in the pairs. This graphical representation suggested a possible association between the duration of the PLMS and the arousal. We therefore analyzed the correlation between PLMS duration and arousal duration in all pairs of events included in this study. Figure 3 shows this analysis for which a significant correlation coefficient of 0.447 (P < 0.000001) was found; this was futher confirmed by the same analysis run for each patient which showed a mean correlation coefficient of 0.398 (SD 0.166).
Figure 3.
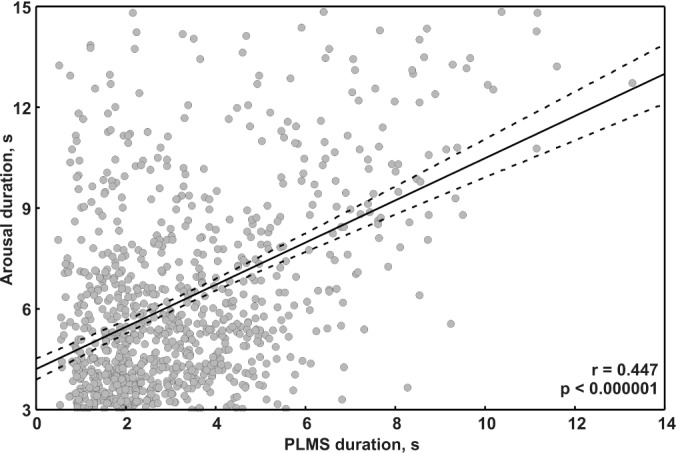
Analysis of the correlation between PLMS duration and arousal duration in all event pairs used in this study. The regression line is drawn as a continuous black line. Dotted lines represent the 95% confidence intervals of the regression line.
DISCUSSION
The results of this study show that the great majority of PLMS/arousal pairs not separated by more than 10 s are indeed correctly identified by the current scoring rules that define the association between these two events.7,9 Even if this was not a physiological study, this finding lends statistical support to the rule and justifies its retention in future revisions of the overall criteria for scoring PLMS.
Due to the criteria used in this study for the detection of PLMS/arousal pairs, we analyzed approximately 25% of PLMS contained in the recordings. In RLS, the portion of PLMS accompanied by arousals reported in the literature is consistently below 50% and ranges between 17% and 55%,16–21 the variability possibly depending on the different rules used for the definition of the association and on the high interscorer variability in the detection of arousals.22 Thus, the portion of PLMS analyzed in the present study appears to be within the expected range. This also supports the choice of the time window (10 s) which was based on the need to avoid uncertainties in the assignment of pairs within sequences of repeated events, since it allowed identification of the majority of events of interest.
It is interesting to note that, despite the fact that arousals are commonly believed to be caused by PLMS, their onset precedes the onset of PLMS in more than 40% of cases; thus at least in a large percentage of cases, it is difficult to claim a cause/effect relationship. This finding is not surprising and is in agreement with a previous report that analyzed in a less detailed way this relationship23; however, it should also be considered that arousals, characterized by a shift towards higher frequencies, are often preceded by slow waves that are not considered by the AASM rules for scoring arousals9 but are integrated in a single and more complex event by the rules coding the so-called cyclic alternating pattern (CAP), and usually indicated as A2 or A3 CAP subtypes.24,25 Thus, if we consider also slow waves, we would expect that the distribution depicted in the upper graph of Figure 1C should change significantly and shift towards the left side of the graph, similar to the distribution already reported in 1999 by Parrino et al., who analyzed the relationship between CAP events and PLMS in NREM sleep.25 This was not done in the current study, as there are no rules established regarding the association between PLMS and CAP events. This is further supported by previous studies that analyzed the EEG spectral content surrounding PLMS and where, after a careful synchronization between the motor events and the EEG spectral analysis, an increase in delta waves has been found that precedes the increase in fast activities accompanying PLMS.26,27
An interesting (and to our knowledge, new) finding of this study was the significant correlation between PLMS duration and arousal duration. The duration of these events might be interpreted as one feature describing their “intensity.” Currently, there is no definition of “intensity” available; however, it appears sensible to believe that these events have different “intensities” correlated with different degrees of sleep disruption, possibly reflected in differences in associated EEG, motor, and autonomic changes.26,27 For motor events, a possible candidate for an index of “intensity” might be the area under the curve that integrates both duration and amplitude into a single value14; however, since the EMG is an essentially non-calibrated signal, this would be difficult to implement. Moreover, in this study we used the whole duration of the motor event that was determined by the two legs; however, it might be speculated that bilateral movements might produce longer duration than monolateral movements, thus contributing to the correlation found between the length of arousals and PLMS. We did not control for bilaterality or monolaterality of movements in this study; however, it has already been reported that bilateral PLMS are accompanied by larger heart rate and spectral EEG changes than monolateral movements.27
The tight time relationship between PLMS and arousals and their correlated durations seem to indicate that both events might be regulated by a complex mechanism including them and other sleep events,28 such as heart rate and blood pressure,29–31 rather than being connected by a simple reciprocal cause/effect relationship.
We did not include patients with a significant number of apnea episodes. Nevertheless, some few remaining respiratory events or variations from one polysomnographic study to the next cannot be excluded. Although recent findings show that the association between PLMS and apnea events is temporally much wider than that found in the present study,32 the contemporary presence of arousal might have introduced a small bias into the results.
In conclusion, this study supports the current definition for the association between PLMS and arousals. In general, there is a need to perform this type of analysis (or more complex analyses if necessary) for all criteria currently used for scoring PLMS and to incorporate their results into the decision process regarding an eventual improvement of the same criteria. In line with this concept, a recent evidence-based analysis of the association between PLMS and sleep-related breathing events has shown the need to modify the current rules.32 We also believe that this type of approach would be of benefit in several other fields of sleep scoring, especially those involving the quantification of different transient sleep motor events (chin EMG and other muscles events, bruxism, etc.).
DISCLOSURE STATEMENT
This was not an industry supported study. This work was performed at the Sleep Research Centre, Department of Neurology IC, Oasi Institute (IRCCS), Troina, Italy. This study (Drs. Ferri and Rundo) was partially supported by a grant of the Italian Ministry of Health (“Ricerca Corrente”). Drs. Manconi and Fulda are supported by the Swiss National Science Foundations (Grant No.:320030_144007). The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Lugaresi E, Coccagna G, Tassinari CA, Ambrosetto C. Rilievi poligrafici sui fenomeni motori nella sindrome delle gambe senza riposo. Riv Neurol. 1965;35:550–61. [PubMed] [Google Scholar]
- 2.Lugaresi E, Tate L, Coccagna G, Ambrosetto C. Particularités cliniques et polygraphiques du syndrome d'impatience des membres inferieurs. Rev Neurol (Paris) 1965;113:545–55. [Google Scholar]
- 3.Coleman RM. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park: Addison-Wesley; 1982. pp. 265–95. [Google Scholar]
- 4.American Sleep Disorders Association. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 5.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 6.Wetter TC, Dirlich G, Streit J, Trenkwalder C, Schuld A, Pollmacher T. An automatic method for scoring leg movements in polygraphic sleep recordings and its validity in comparison to visual scoring. Sleep. 2004;27:324–8. doi: 10.1093/sleep/27.2.324. [DOI] [PubMed] [Google Scholar]
- 7.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chesson AL, Quan SF, editors. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 9.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV, editors. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.0. Darien, IL: American Academy of Sleep Medicine; 2012. www.aasmnet.org. [Google Scholar]
- 10.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 11.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Med. 2014;15:860–73. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington, DC: Public Health Service; US Government Printing Office; 1968. [Google Scholar]
- 14.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 15.Ferri R. The time structure of leg movement activity during sleep: the theory behind the practice. Sleep Med. 2012;13:433–41. doi: 10.1016/j.sleep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Jama L, Hirvonen K, Partinen M, et al. A dose-ranging study of pramipexole for the symptomatic treatment of restless legs syndrome: polysomnographic evaluation of periodic leg movements and sleep disturbance. Sleep Med. 2009;10:630–6. doi: 10.1016/j.sleep.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Oertel WH, Benes H, Garcia-Borreguero D, et al. Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: a randomized, placebo-controlled polysomnographic study. Sleep Med. 2010;11:848–56. doi: 10.1016/j.sleep.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Boehm G, Wetter TC, Trenkwalder C. Periodic leg movements in RLS patients as compared to controls: are there differences beyond the PLM index? Sleep Med. 2009;10:566–71. doi: 10.1016/j.sleep.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Hornyak M, Hundemer HP, Quail D, Riemann D, Voderholzer U, Trenkwalder C. Relationship of periodic leg movements and severity of restless legs syndrome: a study in unmedicated and medicated patients. Clin Neurophysiol. 2007;118:1532–7. doi: 10.1016/j.clinph.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Ferri R, Gschliesser V, Frauscher B, Poewe W, Hogl B. Periodic leg movements during sleep and periodic limb movement disorder in patients presenting with unexplained insomnia. Clin Neurophysiol. 2009;120:257–63. doi: 10.1016/j.clinph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med. 2007;3:133–45. [PubMed] [Google Scholar]
- 23.Karadeniz D, Ondze B, Besset A, Billiard M. EEG arousals and awakenings in relation with periodic leg movements during sleep. J Sleep Res. 2000;9:273–7. doi: 10.1046/j.1365-2869.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16:27–45. doi: 10.1016/j.smrv.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di Giovanni G, Terzano MG. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13:314–23. doi: 10.1097/00004691-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ferrillo F, Beelke M, Canovaro P, et al. Changes in cerebral and autonomic activity heralding periodic limb movements in sleep. Sleep Med. 2004;5:407–12. doi: 10.1016/j.sleep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Ferri R. Two legs, one heart, one sleeping brain. Sleep Med. 2006;7:299–300. doi: 10.1016/j.sleep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Walters AS, Rye DB. Evidence continues to mount on the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep. 2010;33:287. doi: 10.1093/sleep/33.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 32.Manconi M, Zavalko I, Fanfulla F, Winkelman JW, Fulda S. An evidence-based recommendation for a new definition of respiratory-related leg movements. Sleep. 2015;38:295–304. doi: 10.5665/sleep.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]