Significance
The advent of oxygenic photosynthesis set the stage for the evolution of complex life on an oxygenated planet, but it is unknown when this transformative biochemistry emerged. The existing hydrocarbon biomarker record requires that oxygenic photosynthesis and eukaryotes emerged more than 300 million years before the Great Oxidation Event [∼2.4 billion years ago (Ga)]. We report that hopane and sterane concentrations measured in new ultraclean Archean drill cores from Australia are comparable to blank concentrations, yet their concentrations in the exteriors of conventionally collected cores of stratigraphic equivalence exceed blank concentrations by more than an order of magnitude due to surficial contamination. Consequently, previous hydrocarbon biomarker reports no longer provide valid evidence for the advent of oxygenic photosynthesis and eukaryotes by ∼2.7 Ga.
Keywords: oxygenic photosynthesis, eukaryotes, cyanobacteria, Great Oxidation Event, Pilbara
Abstract
Hopanes and steranes found in Archean rocks have been presented as key evidence supporting the early rise of oxygenic photosynthesis and eukaryotes, but the syngeneity of these hydrocarbon biomarkers is controversial. To resolve this debate, we performed a multilaboratory study of new cores from the Pilbara Craton, Australia, that were drilled and sampled using unprecedented hydrocarbon-clean protocols. Hopanes and steranes in rock extracts and hydropyrolysates from these new cores were typically at or below our femtogram detection limit, but when they were detectable, they had total hopane (<37.9 pg per gram of rock) and total sterane (<32.9 pg per gram of rock) concentrations comparable to those measured in blanks and negative control samples. In contrast, hopanes and steranes measured in the exteriors of conventionally drilled and curated rocks of stratigraphic equivalence reach concentrations of 389.5 pg per gram of rock and 1,039 pg per gram of rock, respectively. Polycyclic aromatic hydrocarbons and diamondoids, which exceed blank concentrations, exhibit individual concentrations up to 80 ng per gram of rock in rock extracts and up to 1,000 ng per gram of rock in hydropyrolysates from the ultraclean cores. These results demonstrate that previously studied Archean samples host mixtures of biomarker contaminants and indigenous overmature hydrocarbons. Therefore, existing lipid biomarker evidence cannot be invoked to support the emergence of oxygenic photosynthesis and eukaryotes by ∼2.7 billion years ago. Although suitable Proterozoic rocks exist, no currently known Archean strata lie within the appropriate thermal maturity window for syngenetic hydrocarbon biomarker preservation, so future exploration for Archean biomarkers should screen for rocks with milder thermal histories.
Elucidating the timing of the advent of oxygenic photosynthesis relative to the rise of atmospheric oxygen represents a key challenge in geobiology. The detection of hopane and sterane hydrocarbons in Archean sedimentary rocks has provided an avenue for examining the evolution of biological oxygen cycling (1–7). These results, in addition to inorganic evidence (e.g., refs. 8 and 9), suggest that atmospheric oxygen accumulation was suppressed for hundreds of millions of years following the emergence of oxygenic photosynthesis. However, the interpretation of the Archean biomarker record has become controversial, with recent arguments for a nonsyngenetic origin of these hydrocarbons (10, 11). Furthermore, the discovery of hopanes and steranes in metamorphosed Archean rocks requires that, if these carbon skeletons are syngenetic, then they have persisted despite experiencing prolonged burial temperatures well above the end of the oil window (∼150 °C), which is the temperature range over which conventional oil is generated from a source rock (12). In addition to clarifying the upper limits for hydrocarbon biomarker preservation, resolving the origin of the hydrocarbon biomarkers hosted in Archean rocks would either recast or solidify the late Archean framework in which we understand one of the most profound biologically mediated transformations of the planet—the Great Oxidation Event [GOE; ∼2.4 billion years ago (Ga)] (13).
Sample Collection
We present results from three Archean drill cores from the Pilbara Craton, Western Australia, that were drilled during the 2012 Agouron Institute Drilling Program (AIDP) (SI Appendix, Figs. S1 and S2). Core AIDP-1 (21°06'38''S, 119°06'4''E) intersected the volcanic Coucal Formation (3.52 Ga) of the Coonterunah Subgroup (14). AIDP-2 and AIDP-3 recovered organic-rich sedimentary rocks from the two zones of lowest regional metamorphism known in the Pilbara (15). According to previous studies of mineral assemblage and organic matter reflectivity, these zones are within the prehnite−pumpellyite metamorphic facies and anthracite coal rank, implying maximum burial temperatures and pressures of 200–300 °C and <7 kbar (1 bar = 100 kPa), respectively (15–18). Core AIDP-2 was drilled in the Ripon Hills region (21°16'51”S, 120°50'2”E) in the same locality as the RHDH2A core, which was recovered in 1985 using conventional drilling methods (19) and reportedly contains syngenetic Archean biomarkers (3, 4). Spatially separated by less than 1 km, AIDP-2 and RHDH2A both intersect the Carawine Dolomite (<2.63 Ga) of the Hamersley Group and the Jeerinah Formation (2.63–2.67 Ga) of the Fortescue Group (20), so they sample the same stratigraphy and have experienced the same regional metamorphic effects (Fig. 1). AIDP-3 (21°46'32''S, 117°34'11''E), which intersected the Marra Mamba Iron Formation and the Jeerinah Formation, was drilled as a time-equivalent core in deeper water facies than AIDP-2. It is stratigraphically equivalent to WRL-1, which was the subject of the first report, to our knowledge, of Archean biomarkers for cyanobacteria and eukaryotes (1).
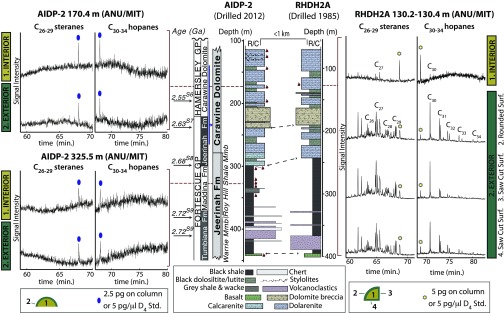
Fig. 1.
Hopanes and steranes in rock extracts from AIDP-2 compared with RHDH2A. Comparison and lithological correlation of the intersected stratigraphy of AIDP-2 (Center Left) and RHDH2A (Center Right) based on previous descriptions of RHDH2A (3, 19). The age references are listed in SI Appendix, and red markers denote the stratigraphic depths of analyzed samples (SI Appendix, Table S1). The MRM analyses of hopanes and steranes monitored the transitions from the molecular ion to the m/z 191 and m/z 217 fragments, respectively. In an RHDH2A sample from the Carawine Dolomite, the C30–34 hopane and C26–29 sterane concentrations are higher in the core surfaces (surf.) compared with the interior (Right). In contrast, hopanes and steranes were not detected in MRM analyses of either interiors or exteriors of a correlative sample from the Carawine Dolomite recovered at 170.4 m in AIDP-2 (Top Left) or from the underlying Roy Hill Member of the Jeerinah Formation at 325.5 m (Bottom Left).
The AIDP cores were drilled and sampled using protocols chosen to minimize hydrocarbon contamination. At the beginning of the AIDP campaign, all core barrels were cleaned with soap and water, and, subsequently, only new drill bits were used. Water without added lubricants was used as the sole drilling fluid for the entirety of the program. AIDP-1, which mainly intersected metamorphosed igneous rocks, provided negative control samples (i.e., nonsedimentary rocks devoid of syngenetic organic matter) and abrasively removed residual organics that may have accumulated on the drill rods during previous projects. Minutes after recovery from depth, untouched portions of selected core material were rinsed with Type-1 ultrapure water in the aluminum core tray before being placed into organic-clean Teflon bags containing several milliliters of ultrapure water to prevent the core material from drying out. After purging with high purity Ar, the sample bags were sealed and frozen at –20 °C in the dark until the whole cores were sawed. In total, the whole-core samples were packaged, purged, and frozen in less than 20 min after recovery from depth.
Methods
Systematic blank analyses using solvent rinses and sawed pieces of a combusted brick demonstrated that the diamond-rimmed blade used with the newly purchased, water-cooled saw constructed entirely of stainless steel and aluminum was a significant vector for hydrocarbon biomarker contamination. After exhausting other methods (see SI Appendix), the saw blade was heated to 300 °C for 1 h and was sonicated in solvent, after which solvent rinses and combusted brick blanks verified that sawing no longer contributed detectable hydrocarbon contaminants. Subsequently, the frozen whole-core samples were sawed into book-matched halves at the Australian National University (ANU), which were distributed to multiple laboratories for independent analyses. The extractable biomarker methods and data described below are specific to the ANU/Massachusetts Institute of Technology (MIT) joint dataset, which analyzed the majority of samples for extractable hydrocarbons (SI Appendix, Table S1). However, all results from all laboratories are consistent with the results described here.
Approximately ∼5- to 10-mm-thick slabs of the rock surface were sawed off from the half-core, such that all exposed surfaces were removed (Fig. 1). These rock pieces were collected as the “exterior” sample, while the remaining rock served as the “interior” sample. The interior and exterior samples were prepared in parallel with a combusted sand blank. Between ∼10 g and 40 g of the rock powder were extracted with solvents (SI Appendix, Table S2). Each extract was concentrated and redissolved in 100 μL of hexane for analyses by gas chromatography mass spectrometry in full scan and metastable reaction monitoring (MRM) modes (see SI Appendix). Each laboratory analyzed either a chert or igneous negative control sample from AIDP-2 or AIDP-1 for extractable hydrocarbons before the analyses of the biomarker target samples. In total, the interiors and exteriors of 10 biomarker target samples across the Carawine Dolomite and Jeerinah Formation of AIDP-2 and 2 biomarker target samples from the Jeerinah Formation of AIDP-3 have been analyzed for extractable hydrocarbons (SI Appendix, Table S1).
Results and Discussion
Hopanes and steranes in the rock extracts from AIDP-2 and AIDP-3 were typically at or below femtogram detection limits, but if they were detectable, the total sterane and total hopane concentrations were comparable to blank and negative control concentrations (Table 1 and Fig. 1). For comparison, the hopane and sterane concentrations that were detectable and measured in the AIDP samples are approximately one to three orders of magnitude less than the hopane and sterane concentrations reported in earlier Archean biomarker studies (7, 21). Moreover, extractable acyclic isoprenoids and a homologous series of extractable n-alkanes (C10–23+), both of which were prominent in previous Archean biomarker studies (1–3, 5, 7, 22, 23), were also below detection limits or cumulative laboratory blank concentrations in the AIDP samples.
Table 1.
ANU/MIT hopane and sterane concentrations, in picograms per gram of rock
| Sample | Interior ΣC30–34 hopane concentration | Interior ΣC26–29 sterane concentration | Exterior ΣC30–34 hopane concentration | Exterior ΣC26–29 sterane concentration |
| Range for blanks* | nd to 37.9 | nd to 32.9 | nd to 37.9 | nd to 32.9 |
| AIDP-2 441.00 m (negative control) | nd | nd | 31.4 | nd |
| AIDP-2 146.20 m | nd | nd | 10.7 | 11.8 |
| AIDP-2 170.40 m | nd | nd | nd | nd |
| AIDP-2 197.20 m | nd | nd | 50.4 | 49.4 |
| AIDP-2 291.28 m | nd | nd | 7.3 | 16.5 |
| AIDP-2 301.40 m | 5.2 | nd | 8.0 | nd |
| AIDP-2 325.48 m | nd | nd | nd | nd |
| AIDP-2 327.42 m | nd | nd | 38.5 | 24.3 |
| AIDP-3 98.36 m | nd | nd | nd | nd |
| AIDP-3 130.30 m | nd | nd | nd | nd |
| RHDH2A interior† | 3.5 | 21.1 | ||
| RHDH2A sawed surface 1† | 250 | 1,092 | ||
| RHDH2A sawed surface 2† | 259 | 509 | ||
| RHDH2A rounded surface† | 390 | 468 |
Herein, nd denotes “not detected.”
Note that the same blank concentration ranges measured for hopanes and steranes are provided in the interior and exterior columns.
The RHDH2A 130.2−130.4 m sample surfaces were cut according to Fig. 1.
Hydropyrolysates, which are solvent-soluble hydrocarbons released from the kerogen by cracking under high-pressure hydrogen, were analyzed from preextracted rock powders or kerogens (insoluble organic matter) from eight samples from AIDP-2 and AIDP-3. As in the rock extracts, individual hopanes and steranes in the AIDP-2 and AIDP-3 hydropyrolysates were usually at or below detection limits, but when they were detected, neither the total hopane nor the total sterane concentrations exceeded cumulative blank concentrations (<20 pg per gram of rock). These results are consistent with the absence of hopanes and steranes in previous Archean hydropyrolysis (HyPy) studies (24, 25).
However, a series of C10–20 n-alkanes (with a maximum abundance at C13), methylalkanes, and alkylcyclohexanes were detected in hydropyrolysates from two carbonate-rich rocks at 116.09 m and 170.40 m in the Carawine Dolomite of AIDP-2 (SI Appendix, Fig. S6), yet acyclic isoprenoids were not detected in any of the hydropyrolysates. The two samples containing alkanes also hosted the least thermally altered kerogens according to their atomic H/C ratios and pyrolysate polycyclic aromatic hydrocarbon (PAH) ratios. Most samples had H/C ratios of 0.19–0.35 (SI Appendix, Table S5), but these two samples have the highest H/C ratios of 0.54 and 0.46. The same two samples also have the highest abundance of alkylated relative to nonalkylated PAH in the pyrolysates (SI Appendix, Table S10). For example, the methylphenanthrene/phenanthrene (MP/P) ratios for these two samples were 1.0–1.2 compared with the other samples that have MP/P values ranging from 0.2 to 0.5. Similarly, they had methylpyrene/pyrene (MePy/Py) ratios of 0.4–0.6 compared with the other samples that have MePy/Py values close to 0.1. The total n-alkane concentrations in these hydropyrolysates were two orders of magnitude higher than in laboratory blanks (<10 ng per gram of rock), in which methylalkane and alkylcyclohexane series were not detected. The n-alkanes detected in the Carawine Dolomite hydropyrolysate from 116.09 m in AIDP-2 had δ13C values ranging from −38.1‰ to −43.6‰ (SI Appendix, Table S7) compared with the corresponding bulk δ13C of −43.1‰ (SI Appendix, Table S5). This isotopic similarity indicates that these compounds were generated from the kerogen. In contrast, n-alkanes detected in the Jeerinah Formation black shales and more-thermally altered Carawine Dolomite hydropyrolysates were comparable to laboratory blank concentrations (SI Appendix, Table S10).
To further constrain the source of extractable hydrocarbons previously reported in Archean rocks, a Carawine Dolomite quarter core sample from the RHDH2A core (130.2–130.4 m) was analyzed using the same laboratory protocol described for the AIDP samples. Like the AIDP samples, 25–31 g of sample interior and exterior were extracted (SI Appendix, Table S2). The n-alkanes, hopanes, and steranes were enriched on the exterior of the RHDH2A core compared with the interior, where the total hopane and total sterane concentrations in the exterior exceeded the interior and blank concentrations by one to two orders of magnitude (Table 1 and Fig. 1). These patterns substantiate earlier observations of biomarker and bitumen enrichments on the surfaces of conventionally drilled and curated samples relative to the interiors (3, 6, 11). Furthermore, the hopane and sterane concentrations measured in our analysis of the RHDH2A sample are more compatible with the concentrations reported in earlier Archean biomarker studies of the Mount Bruce and Transvaal supergroups (7, 21) than the concentrations measured in the AIDP samples. Comparison of RHDH2A and cumulative blank concentrations indicate that laboratory procedures cannot fully account for the addition of biomarkers to RHDH2A. Clearly, the hydrocarbons must have been present in, or added to, the RHDH2A quarter core before laboratory preparation.
Two hypotheses have been proposed to explain surficial biomarker enrichments in Archean drill core: contamination and the live oil effect (i.e., the outward migration of hydrocarbons due to drill core depressurization during recovery) (11). If the live oil effect generated the surficial biomarker enrichments, then samples from both AIDP-2 and RHDH2A should display surficial hopane and sterane enrichments in excess of cumulative blanks. Instead, contamination is the only explanation for the reproducibility of the detectable surficial hopanes and steranes in conventionally drilled and curated Archean samples (3, 6, 7, 11) and the undetectable or blank level-equivalent hopane and sterane concentrations in samples from the ultraclean AIDP drill cores.
The spatial distribution of AIDP-2 and AIDP-3 geographically brackets and nearly spans the entire stratigraphic section from which all of the other Pilbara biomarkers have been previously reported (1, 3, 4, 21). According to currently available H/C ratios and other indicators of thermal history, the metamorphic grades of AIDP-2 and AIDP-3 are similar to (1–4, 6, 7, 15, 21, 26) or lower than (5, 22, 23) the metamorphic grades of the rocks from which biomarkers have been previously reported. Moreover, known vectors and signatures of contamination (i.e., surface enrichments) are independent of sampling locality (6, 11). For example, the analyses of the saw blade (before it was heat treated) and the sawed surfaces of the RHDH2A quarter-core highlight that sawing can contribute significant but variable hydrocarbon contaminants to the substrate, yet the cumulative blanks reported in previous studies did not record the hydrocarbon contribution from sawing (1, 4, 6, 7). Furthermore, recent studies have illustrated that petroleum-derived chemical fossils are prevalent in aerosols, providing another potential vector for hydrocarbon contamination (27). Evidently, samples that were the subject of earlier Archean biomarker work accumulated contaminants on surfaces during processes that were not recorded by laboratory blanks (e.g., drilling, sampling, sawing, and/or storage). If true, then trace amounts of these unforeseen hydrocarbon contaminants could have migrated inward as the samples dried out, oxidized, and fractured (11). Alternatively, using solvents to clean surface exteriors could have remobilized the hydrocarbon contaminants into the sample interior (6). Therefore, the results and implications from the new AIDP cores logically extend from the Pilbara Craton to other localities (1, 2, 4–7, 21–23), so based on all currently available evidence, we propose moving forward with the null hypothesis that the prior observations are attributable to contamination.
Many AIDP samples, however, host extractable PAHs and diamondoids with concentrations up to 80 ng per gram of rock and 4 ng per gram of rock, respectively (SI Appendix, Fig. S4). PAHs and diamondoids were identified by comparison with published mass spectra, published relative retention times, and parallel analysis with standards. PAHs and diamondoids were also detected in the hydropyrolysates, where individual PAH concentrations were up to 1039 ng per gram of rock, and their concentrations were significantly higher than their corresponding HyPy blank concentrations of less than 1 ng per gram of rock for phenanthrene and pyrene (SI Appendix, Table S10). For both rock extracts and hydropyrolysates, the Carawine Dolomite hosted significantly higher concentrations of PAHs and diamondoids than the underlying Jeerinah Formation black shale samples. Like the PAHs and diamondoids that previous studies reported in Archean rock extracts and hydropyrolysates (3, 21, 24, 28), the low molecular mass aromatics (≤C16) predominate over high molecular mass PAHs (>C16), and nonalkylated parent PAHs are in greater abundance compared with alkylated PAH (SI Appendix, Figs. S4 and S5 and Tables S8 and S10).
The PAHs and diamondoids are indigenous (i.e., native to the rock before drilling) based on several lines of evidence. First, their concentrations in the sample material significantly exceed laboratory blank PAH and diamondoid concentrations. For PAHs that are less sensitive to evaporation, the exterior/interior distribution ratios were close to 1. PAHs and diamondoids were detected in both rock extracts and hydropyrolysates. Finally, the PAH and diamondoid distribution and alkylation patterns impart high thermal signatures that match the regional metamorphic grade. More specifically, a predominance of unsubstituted, condensed PAHs is characteristic of hydrothermal petroleums (29, 30), ancient mineral deposits (31, 32), pyrolysis and combustion products (33), and high-maturity organic matter (34). Based on extractable PAHs, the MPI-1 parameter yields calculated vitrinite reflectances (Rc) between 2.63–3.00% (SI Appendix, Table S9) (21, 35) [, where P is phenanthrene and MeP is methyl phenanthrene]. These Rc values are compatible with the measured H/C ratios, which ranged from 0.2 to 0.5 (SI Appendix, Table S5). At a vitrinite reflectance of 2% and H/C ratio of 0.5, catagenesis transitions into metagenesis, which corresponds to the dry gas zone where only methane is generated (12, 36). Although duration of heating and type of organic matter complicate direct conversions between Rc, H/C ratio, and burial temperature, the PAH and diamondoid distributions in tandem with the H/C ratios confirm that these rocks are overmature and are within the prehnite−pumpellyite metamorphic facies (12, 15, 17, 36–38).
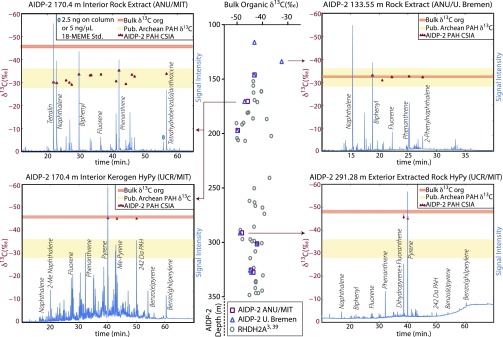
Stable carbon isotopic data can be assessed to test whether the indigenous PAHs and diamondoids were generated from the coexisting kerogen and have the same age as the host rock (i.e., syngenetic) or, conversely, migrated from underlying or overlying strata. Consistent with earlier work (1, 10, 39, 40), the bulk organic carbon was found to be anomalously 13C-depleted, with δ13C values ranging from −32‰ to −50‰ (Fig. 2 and SI Appendix, Table S5). The extractable PAHs from the Carawine Dolomite were typically 13C-enriched by 10–20‰ compared with the host rock organic matter (Fig. 2 and SI Appendix, Table S6), with two exceptions. First, the sample at 133.55 m exhibited an isotopic match (Δ < 0.8‰) between the extractable PAHs (−32.3‰ to −33.2‰) and the associated kerogen (−32.4‰), which was enriched in 13C by >10‰ compared with the bulk δ13C of the other AIDP samples. This anomously 13C-enriched sample was a 10-cm pyrobituminous stylocumulate occurring in stylolitic to brecciated dolarenite of the upper Carawine where dark insoluble matter concentrated as a result of pressure solution and carbonate dissolution (SI Appendix, Fig. S3). Notably, the δ13C values of the PAHs from this sample and the PAHs from the other AIDP Carawine Dolomite samples are equivalent, suggesting that they could share the same allochthonous source. Second, three high molecular mass PAHs (228–252 Da), identified as benzo-naphtho-thiophene, benz[a]anthracene, and benzo[e]pyrene, from the AIDP-2 sample at 116.09 m had isotopic values ranging from −38.9‰ to −41.3‰, yielding an offset from the host rock kerogen of 1.8–4.3‰. These three compounds are the most 13C-depleted PAHs that have been reported from Archean rock extracts (21, 24, 25). Only a single compound-specific isotopic measurement was possible for this sample, so the analytical uncertainty and reproducibility are not well constrained. In contrast to the extractable PAHs, the δ13C of the hydropyrolysate PAHs match the bulk organic matter within a range of 0.1‰ to 2.9‰ (Fig. 2), and they are the most 13C-depleted organic molecules reported from Archean rocks, ranging from −42.6‰ to −45.9‰ (SI Appendix, Table S7). The HyPy hydrocarbon δ13C values reported here are, to our knowledge, the first compound-specific isotopic measurements that are in agreement with the strongly 13C-depleted kerogens (δ13Cbulk < −39‰) of the Neoarchean (1, 10, 39, 40).
Fig. 2.
Compound specific and bulk organic δ13C data. Stable carbon isotopic measurements of bulk organic matter from AIDP-2 and RHDH2A (3, 39) are compared (Center). The RHDH2A depth was converted to the AIDP-2 depth scale by a correction factor of 45 m due to the stratigraphic offset (see Fig. 1). Rock extract and HyPy full scan chromatograms are plotted with compound-specific isotopic analysis (CSIA) of PAH δ13C. AIDP-2 133.55 m (SI Appendix, Fig. S3) is the only sample that displayed an isotopic match between the extractable PAHs and the bulk organic matter (Top Right). The extractable PAH δ13C from the other Carawine Dolomite samples diverged from the corresponding bulk organic matter δ13C (Top Left). The hydropyrolysate PAHs from the Carawine Dolomite and the Jeerinah Formation match the bulk organic carbon isotopic composition (Bottom Left and Bottom Right). The range of previously published (Pub.) Archean PAH δ13C is plotted for reference (21, 24, 25). The analytical uncertainty is plotted for the PAH δ13C measurements, but it is smaller than the symbol in most cases. The different GC methods yield different retention times.
Previous work suggests that the Jeerinah Formation and other organic-rich Archean shales generated and expelled petroleum (17) and that the Pilbara Craton, including the Ripon Hills region, has experienced multiple episodes of hydrothermal fluid flow, particularly around the time of peak regional metamorphism at ∼2.1 Ga (18, 41). The isotopic match between the AIDP bulk organic matter and the hydropyrolysate PAHs supports a syngenetic origin for the hydropyrolysate PAHs generated by thermochemical cleavage of covalent bonds within the host kerogens. In contrast, the δ13C values of extractable PAHs do not covary with the AIDP bulk organic δ13C through the carbon isotopic anomaly at 2.6–2.7 Ga, suggesting that the kerogens of the Jeerinah Formation and Carawine Dolomite may not necessarily be the primary source of the cooccurring extractable PAHs, despite strong evidence of indigeneity. The 13C enrichment of the bulk organic matter hosted in the stylolitic sample at 133.55 m in tandem with PAHs of equivalent isotopic composition throughout the Carawine Dolomite provides further evidence for emplacement of migrated allochthonous organic matter that was rich in PAHs. Supercritical hydrothermal fluids rich in CO2 or CH4 are efficient solvents for organic compounds, and unsubstituted, condensed PAHs are particularly abundant in hydrothermal fluids (29, 30, 42). Therefore, hydrothermal fluids could account for PAH migration from its original source, where this second generation of migrated overmature hydrothermal PAHs would have diluted or replaced the primary bitumens. Alternatively, lateral oil migration before peak metamorphism could have introduced the extractable organic matter with isotopic compositions distinct from the associated kerogen. The extractable high molecular mass PAHs from 116.09 m that have isotopic compositions approaching the bulk organic carbon isotopic composition hint at mixing between syngenetic high molecular mass extractable PAHs and migrated PAHs of low molecular mass containing one to three fused aromatic rings, but future studies are required to test this observation. Lastly, the extractable PAHs could be syngenetic but heavily altered by reactions between hydrothermal fluids and the bitumen (28), but this mechanism may not be able to account for the full >10‰ 13C enrichment. Regardless of the source of the extractable PAHs, the biomolecular precursors of the PAHs and diamondoids are unknown.
We have demonstrated the feasibility of collecting organic-lean and/or overmature drill core samples without the addition of contaminant biomarker hydrocarbons. Our results verify that hydrocarbon concentration gradients through a sample can be used to distinguish contaminants from indigenous hydrocarbons (11), which is a critical proof of concept for Precambrian organic geochemistry that had never been directly demonstrated before this work, to our knowledge. Furthermore, HyPy analyses of kerogen-bound organic matter are another critical self-consistency check for syngeneity of hydrocarbon biomarkers (43). In agreement with previous HyPy studies of Archean rocks (24), hopanes and steranes were not detected in hydropyrolysates from AIDP-2 or AIDP-3. Together, these results from the Pilbara Craton illustrate that previously studied Archean samples host a mixture of indigenous PAHs and diamondoids in addition to hydrocarbon contaminants, including alkanes, steranes, and hopanes that accumulated during sample collection and curation. The Archean sedimentary rocks that are presently known and characterized are too mature to allow for the preservation of syngenetic hopane and sterane molecules, if they had been generated during this time. Future studies should explore less mature archives (if they exist) and leverage other techniques such as Rock-Eval pyrolysis, elemental analysis, and Raman spectroscopy to identify promising targets for hydrocarbon biomarker analysis. In light of the findings described here, previously reported Archean biomarkers should no longer be invoked as evidence supporting the rise of cyanobacteria and eukaryotes before the GOE.
Supplementary Material
Acknowledgments
We thank Paul van Loenhout, Lou Lequerica, Jamie Hamilton, and the crew from Mt. Magnet Drilling for their exceptional commitment to drilling cleanliness. We would also like to thank the Yindjibarndi people for granting access to their land, Martin Van Kranendonk for organizational and logistical support, Les Bonser for his field support, Kliti Grice and Paul Greenwood for providing assistance and laboratory space that made drilling preparation possible, and John Hayes, Ann Pearson, and two anonymous reviewers for constructive comments and insightful discussion. We thank Mark Williams for assistance with HyPy experiments at University of California, Riverside; Geoscience Australia, particularly Junhong Chen, for providing compound-specific isotopic analysis (CSIA) data for the ANU/MIT team; and Enno Schefuß for providing access to CSIA measurements for C.H. We thank the Agouron Institute for funding the AIDP drilling and sample analysis. We thank the Fallon laboratory at ANU and Ines Hilke and Heike Geilmann at the Max Planck Institute for Biogeochemistry for assistance with TOC and bulk δ13C measurements. Finally, we acknowledge the Max Planck Society and the German Research Foundation (HA 7218/2-1) for support provided to C.H., Macquarie University for PhD scholarships awarded to Y.H. and C.A.P., the National Science Foundation (NSF) graduate research fellowship program for funding support to K.L.F., and the NSF Frontiers in Earth System Dynamics program for additional funding support to R.E.S. and G.D.L.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419563112/-/DCSupplemental.
References
- 1.Brocks JJ, Logan GA, Buick R, Summons RE. Archean molecular fossils and the early rise of eukaryotes. Science. 1999;285(5430):1033–1036. doi: 10.1126/science.285.5430.1033. [DOI] [PubMed] [Google Scholar]
- 2.Brocks JJ, Buick R, Summons RE, Logan G. A reconstruction of Archean biological diversity based on molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Hamersley Basin, Western Australia. Geochim Cosmochim Acta. 2003;67(22):4321–4335. [Google Scholar]
- 3.Eigenbrode JL. 2004. Late Archean microbial ecology: An integration of molecular, isotopic, and lithological studies. PhD thesis (The Pennsylvania State University, State College)
- 4.Eigenbrode JL, Freeman K, Summons RE. Methylhopane biomarker hydrocarbons in Hamersley Province sediments provide evidence for Neoarchean aerobiosis. Earth Planet Sci Lett. 2008;273(3):323–331. [Google Scholar]
- 5.Dutkiewicz A, Volk H, George SC, Ridley J, Buick R. Biomarkers from Huronian oil-bearing fluid inclusions: An uncontaminated record of life before the Great Oxidation Event. Geology. 2006;34(6):437–440. [Google Scholar]
- 6.Sherman LS, Waldbauer JR, Summons RE. Improved methods for isolating and validating indigenous biomarkers in Precambrian rocks. Org Geochem. 2007;38(12):1987–2000. [Google Scholar]
- 7.Waldbauer JR, Sherman LS, Sumner DY, Summons RE. Late Archean molecular fossils from the Transvaal Supergroup record the antiquity of microbial diversity and aerobiosis. Precambrian Res. 2009;169(1):28–47. [Google Scholar]
- 8.Anbar AD, et al. A whiff of oxygen before the great oxidation event? Science. 2007;317(5846):1903–1906. doi: 10.1126/science.1140325. [DOI] [PubMed] [Google Scholar]
- 9.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506(7488):307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature. 2008;455(7216):1101–1104. doi: 10.1038/nature07381. [DOI] [PubMed] [Google Scholar]
- 11.Brocks JJ. Millimeter-scale concentration gradients of hydrocarbons in Archean shales: Live-oil escape or fingerprint of contamination? Geochim Cosmochim Acta. 2011;75(11):3196–3213. [Google Scholar]
- 12.Tissot BP, Welte DH. Petroleum Formation and Occurrence. Springer; Berlin: 1984. pp. 69–253. [Google Scholar]
- 13.Bekker A, et al. Dating the rise of atmospheric oxygen. Nature. 2004;427(6970):117–120. doi: 10.1038/nature02260. [DOI] [PubMed] [Google Scholar]
- 14.Buick R, et al. Record of emergent continental crust ∼3.5 billion years ago in the Pilbara craton of Australia. Nature. 1995;375(6532):574–577. [Google Scholar]
- 15.Smith RE, Perdrix JL, Parks TC. Burial metamorphism in the Hamersley Basin, Western Australia. J Petrol. 1982;23(1):75–102. [Google Scholar]
- 16.Frey M, Capitani CD, Liou JG. A new petrogenetic grid for low-grade metabasites. J Metamorph Geol. 1991;9(4):497–509. [Google Scholar]
- 17.Rasmussen B. Evidence for pervasive petroleum generation and migration in 3.2 and 2.63 Ga shales. Geology. 2005;33(6):497–500. [Google Scholar]
- 18.Rasmussen B, Fletcher IR, Sheppard S. Isotopic dating of the migration of a low-grade metamorphic front during orogenesis. Geology. 2005;33(10):773–776. [Google Scholar]
- 19.Richards MN. 1985. CRA Exploration Pty. Ltd.: Final report on exploration licenses Ripon Hills North 45/63, Ripon Hills South 45/64, and Gingarrigan Creek 45/65, Nullagine, S F 51-5, Western Australia (Geol Surv Western Australia, Perth, WA, Australia) Rep 13140.
- 20.Rasmussen B, Fletcher IR. Dating sedimentary rocks using in situ U-Pb geochronology of syneruptive zircon in ash-fall tuffs <1 mm thick. Geology. 2010;38(4):299–302. [Google Scholar]
- 21.Brocks JJ, Buick R, Logan GA, Summons RE. Composition and syngeneity of molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Pilbara Craton, Western Australia. Geochim Cosmochim Acta. 2003;67(22):4289–4319. [Google Scholar]
- 22.George SC, Volk H, Dutkiewicz A, Ridley J, Buick R. Preservation of hydrocarbons and biomarkers in oil trapped inside fluid inclusions for >2 billion years. Geochim Cosmochim Acta. 2008;72(3):844–870. [Google Scholar]
- 23.Ventura GT, et al. Molecular evidence of Late Archean archaea and the presence of a subsurface hydrothermal biosphere. Proc Natl Acad Sci USA. 2007;104(36):14260–14265. doi: 10.1073/pnas.0610903104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brocks JJ, et al. Release of bound aromatic hydrocarbons from late Archean and Mesoproterozoic kerogens via hydropyrolysis. Geochim Cosmochim Acta. 2003;67(8):1521–1530. [Google Scholar]
- 25.Marshall CP, et al. Structural characterization of kerogen in 3.4 Ga Archaean cherts from the Pilbara Craton, Western Australia. Precambrian Res. 2007;155(1):1–23. [Google Scholar]
- 26.de Kock MO, et al. Paleomagnetism of a Neoarchean-Paleoproterozoic carbonate ramp and carbonate platform succession (Transvaal Supergroup) from surface outcrop and drill core, Griqualand West region, South Africa. Precambrian Res. 2009;169(1):80–99. [Google Scholar]
- 27.Illing CJ, Hallmann C, Miller KE, Summons RE, Strauss H. Airborne hydrocarbon contamination from laboratory atmospheres. Org Geochem. 2014;76:26–38. [Google Scholar]
- 28.Brocks JJ, Summons RE, Buick R, Logan GA. Origin and significance of aromatic hydrocarbons in giant iron ore deposits of the late Archean Hamersley Basin, Western Australia. Org Geochem. 2003;34(8):1161–1175. [Google Scholar]
- 29.Kawka OE, Simoneit BRT. Polycyclic aromatic hydrocarbons in hydrothermal petroleums from the Guaymas Basin spreading center. Appl Geochem. 1990;5(1):17–27. [Google Scholar]
- 30.Kvenvolden KA, Simoneit BRT. Hydrothermally derived petroleum: Examples from Guaymas Basin, Gulf of California, and Escanaba Trough, northeast Pacific Ocean. AAPG Bull. 1990;74(3):223–237. [Google Scholar]
- 31.Püttmann W, Hagemann HW, Merz C, Speczik S. Influence of organic material on mineralization processes in the Permian Kupferschiefer Formation, Poland. Org Geochem. 1988;13(1):357–363. [Google Scholar]
- 32.Chen J, Walter MR, Logan GA, Hinman MC. The Paleoproterozoic McArthur River (HYC) Pb/Zn/Ag deposit of northern Australia: Organic geochemistry and ore genesis. Earth Planet Sci Lett. 2003;210(3):467–479. [Google Scholar]
- 33.Blumer M, Youngblood WW. Polycyclic aromatic hydrocarbons in soils and recent sediments. Science. 1975;188(4183):53–55. doi: 10.1126/science.188.4183.53. [DOI] [PubMed] [Google Scholar]
- 34.Renzi L, Peirong W. PAH in fossil fuels and their geochemical significance. J Southeast Asian Earth Sci. 1991;5(1):257–262. [Google Scholar]
- 35.Boreham CJ, Crick IH, Powell TG. Alternative calibration of the Methylphenanthrene Index against vitrinite reflectance: Application to maturity measurements on oils and sediments. Org Geochem. 1988;12(3):289–294. [Google Scholar]
- 36.Lewan MD, et al. Evaluation of petroleum generation by hydrous pyrolysis experimentation. Philos Trans R Soc A. 1985;315(1531):123–134. [Google Scholar]
- 37.Waples DW. Time and temperature in petroleum formation: Application of Lopatin's method to petroleum exploration. AAPG Bull. 1980;64(6):916–926. [Google Scholar]
- 38.Burnham AK, Sweeney JJ. A chemical kinetic model of vitrinite maturation and reflectance. Geochim Cosmochim Acta. 1989;53(10):2649–2657. [Google Scholar]
- 39.Eigenbrode JL, Freeman KH. Late Archean rise of aerobic microbial ecosystems. Proc Natl Acad Sci USA. 2006;103(43):15759–15764. doi: 10.1073/pnas.0607540103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Des Marais DJ. Isotopic evolution of the biogeochemical carbon cycle during the Proterozoic Eon. Org Geochem. 1997;27(5):185–193. [Google Scholar]
- 41.Blake TS, et al. Two episodes of regional-scale Precambrian hydrothermal alteration in the eastern Pilbara, Western Australia. Precambrian Res. 2011;188(1):73–103. [Google Scholar]
- 42.Simoneit BRT. In: Aqueous Organic Geochemistry at High Temperature/High Pressure. Marine Hydrothermal Systems and the Origin of Life. Holm NG, editor. Kluwer; Dordrecht, The Netherlands: 1992. pp. 43–66. [Google Scholar]
- 43.Love GD, Snape CE, Carr AD, Houghton RC. Release of covalently-bound alkane biomarkers in high yields from kerogen via catalytic hydropyrolysis. Org Geochem. 1995;23(10):981–986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.