Significance
mAbs directed to inhibitory immune receptors represent a very promising class of immunotherapeutics. This study suggests a potential mechanism of action of ipilimumab (a fully human anti–cytotoxic T lymphocyte–associated antigen 4), by which FcγRIIIA (CD16)-expressing nonclassical monocytes kill regulatory T cells ex vivo via antibody-dependent cell-mediated cytotoxicity. Notably, at baseline, responder patients display significantly higher peripheral frequencies of nonclassical monocytes and a selective enrichment in tumor-infiltrating CD68+CD16+ macrophages compared with nonresponder patients. If further confirmed, these findings may contribute to the generation of predictive biomarker panels, antibody design, and the development of rational combination therapies to promote antitumor immunity.
Keywords: monocytes, macrophages, Tregs, ipilimumab, ADCC
Abstract
Enhancing immune responses with immune-modulatory monoclonal antibodies directed to inhibitory immune receptors is a promising modality in cancer therapy. Clinical efficacy has been demonstrated with antibodies blocking inhibitory immune checkpoints such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) or PD-1/PD-L1. Treatment with ipilimumab, a fully human CTLA-4–specific mAb, showed durable clinical efficacy in metastatic melanoma; its mechanism of action is, however, only partially understood. This is a study of 29 patients with advanced cutaneous melanoma treated with ipilimumab. We analyzed peripheral blood mononuclear cells (PBMCs) and matched melanoma metastases from 15 patients responding and 14 not responding to ipilimumab by multicolor flow cytometry, antibody-dependent cell-mediated cytotoxicity (ADCC) assay, and immunohistochemistry. PBMCs and matched tumor biopsies were collected 24 h before (i.e., baseline) and up to 4 wk after ipilimumab. Our findings show, to our knowledge for the first time, that ipilimumab can engage ex vivo FcγRIIIA (CD16)-expressing, nonclassical monocytes resulting in ADCC-mediated lysis of regulatory T cells (Tregs). In contrast, classical CD14++CD16− monocytes are unable to do so. Moreover, we show that patients responding to ipilimumab display significantly higher baseline peripheral frequencies of nonclassical monocytes compared with nonresponder patients. In the tumor microenvironment, responders have higher CD68+/CD163+ macrophage ratios at baseline and show decreased Treg infiltration after treatment. Together, our results suggest that anti–CTLA-4 therapy may target Tregs in vivo. Larger translational studies are, however, warranted to substantiate this mechanism of action of ipilimumab in patients.
The clinical benefit of immune checkpoint blockade represents arguably one of the most significant advances of modern oncology, as it has definitively proven to produce substantial clinical responses, not only in tumors traditionally perceived as “immunogenic” but also in a variety of solid tumors (1). Two pivotal phase III clinical trials in patients with advanced melanoma of the fully human, anti–cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) IgG1 monoclonal antibody ipilimumab (Yervoy; previously MDX-010; Medarex/Bristol Myers-Squibb) reported significantly increased overall survival, which led to its approval by the US Food and Drug Administration (2, 3). Ipilimumab represents the first of a new class of cancer therapies that function by enhancing host antitumor immunity. However, clinical benefit following immune checkpoint blockade of CTLA-4 is limited to a fraction (10–15%) of treated patients, and the mechanism(s) of action are still poorly understood. Moreover, no reliable biomarkers of clinical efficacy are available to date. Although CTLA-4 belongs to the family of inhibitory lymphocyte receptors, it does not appear to transmit inhibitory signals into effector T cells (4). Rather, CTLA-4 may control key functions of regulatory T cells (Tregs) and of antigen-presenting cells (5–7). More recently, studies in mice revealed that the antitumor activity of CTLA-4 blockade is mediated by Fc gamma receptor (FcγRIV)-expressing macrophages in the tumor microenvironment (TME) via in trans depletion of tumor-infiltrating Tregs (8–10). We speculated that a similar mechanism might operate in melanoma patients, who show a response to ipilimumab. To investigate this hypothesis, we interrogated peripheral blood mononuclear cells (PBMCs) and matched melanoma metastases from 15 patients responding and 14 nonresponding to ipilimumab.
Results
High Baseline Frequencies of Nonclassical CD14+CD16++ Monocytes in Patients Responding to Ipilimumab.
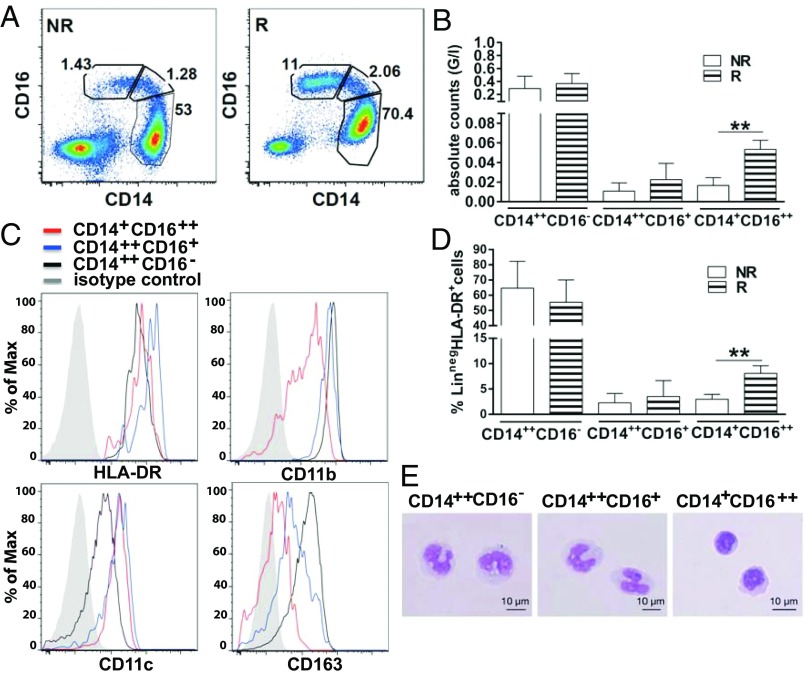
Between September 2010 and July 2013, 29 patients with stage IV cutaneous melanoma, progressing to at least one prior line of therapy, received a maximum of four cycles of 3 mg/kg ipilimumab i.v. every 3 wk. Mean age was 62 y (range, 37–85 y). The majority of patients were male (72%) and had stage IV M1c disease (21 of 29 patients, 72%; Table 1). Median follow-up was 9.41 mo (range, 2–39 mo). Tumor response was assessed 16 or 24 wk after starting ipilimumab by immune-related response criteria (11). To compare data sets, we selected equal numbers of patients who showed objective responses, defined as partial response (PR) or complete response (CR), and of patients who showed progression of disease after treatment with ipilimumab. Objective responses included 2 CRs and 13 PRs. By multicolor flow cytometry, we identified three different monocyte subsets according to their surface expression of HLA-DR, CD14, and CD16 (Fig. S1) (12). Interestingly, patients responding to ipilimumab displayed the highest percentages (Fig. 1 A and D) and absolute counts (Fig. 1B) of circulating nonclassical, FcγRIIIA(CD16)-expressing monocytes at baseline (12–14), whereas there was no difference in the frequency of classical monocytes (CD14++CD16−; Fig. 1 A, B, D, and E) and of CD16-expressing natural killer (NK) cells between responding and nonresponding patients (Fig. S2). The human, nonclassical FcγRIIIA/CD16++ monocyte subset is characterized by high expression levels of surface HLA-DR, CD16, and CD11c and low expression levels of surface CD14, CD11b, and CD163 (Fig. 1C).
Table 1.
Patient characteristics
| Variable | Value (%) |
| Mean age, y | 62 |
| Sex | |
| Male | 21 (72) |
| Female | 8 (28) |
| ECOG performance status | |
| 0 | 17 (59) |
| 1 | 12 (41) |
| M stage | |
| M1a | 2 (7) |
| M1b | 6 (21) |
| M1c | 21 (72) |
| Prior lines of systemic therapy for metastatic disease | |
| 1 | 28 (97) |
| 2 | 1 (3) |
| Ipilimumab cycles | |
| 4 | 23 (79) |
| 3 | 3 (10) |
| 2 | 3 (10) |
| 1 | 0 |
ECOG, Eastern Cooperative Oncology Group.
Fig. 1.
Patients responding to ipilimumab have the highest frequencies of circulating nonclassical CD14+CD16++ monocytes at baseline. Human monocytes are contained within HLA-DR+ cells that do not express B-cell (CD19 or CD20), T-cell (CD3), NK cell (CD56), or granulocyte markers (CD15). One can distinguish three monocyte subsets, namely CD14++CD16−, CD14++CD16+, and CD14+CD16++ monocytes and CD14−CD16− dendritic cells. (A) Representative plots from ipilimumab responder (marked as “R”) and nonresponder (NR) patients with melanoma. (B and D) Pooled data from 15 responding and 14 nonresponding patients with melanoma: percentages and absolute counts of each monocyte subset at baseline. Error bars indicate the mean ± SD (**P < 0.01, unpaired two-tailed Student t test). (C and E) Phenotype and morphology of each monocyte subset.
Selective FcγRIIIA-Dependent Lysis of Tregs ex Vivo Is Mediated by Nonclassical CD14+CD16++ Monocytes.
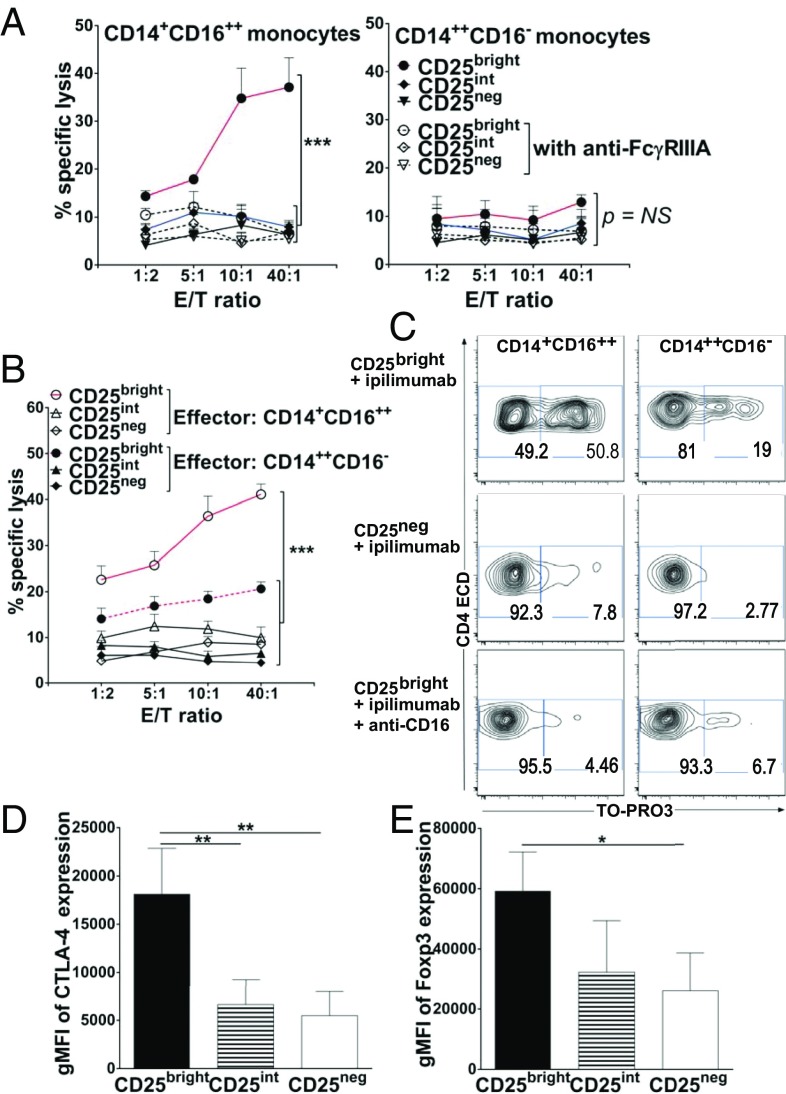
The murine FcγRIV is the ortholog of human FcγRIIIA (15), and its expression pattern on Ly6Clo monocytes is consistent with the corresponding nonclassical CD14+CD16++ monocyte subset in humans, in which it selectively mediates IgG-dependent effector functions in vivo (14, 16–18). We speculated that human, nonclassical monocytes share similar IgG-dependent effector functions and contribute to antitumor responses, whereby depletion of Tregs by ipilimumab may depend on its concomitant ligation to CTLA-4 on Tregs and to FcγRIIIA on CD14+CD16++ monocytes. Therefore, we compared the capacity of CD14+CD16++ and CD14++CD16− monocyte subsets to kill Tregs ex vivo in the presence of ipilimumab via antibody-dependent cell-mediated cytotoxicity (ADCC). Highly purified, healthy donor-derived, CD14+CD16++ and CD14++CD16− monocytes were cocultured at various ratios with FACS-sorted CD3+CD4+ T-cell subsets according to high (CD25bright, i.e., Tregs), intermediate (CD25int), and low (CD25neg) levels of CD25 expression, in the presence or absence of ipilimumab (Fig. S3). As previously shown by Wing et al. and Chung et al. (19, 20), postsort flow cytometric analysis confirmed that the vast majority of CD3+CD4+CD25bright T cells expressed the highest levels of the Treg-associated suppressive markers CTLA-4 and Foxp3, which were reduced by at least half in CD4+CD25int T cells and in CD4+CD25neg T cells (Fig. 2 D and E and Fig. S4). CD3+CD4+CD25bright T cells were also CD127-negative (Fig. S4) (19–22). After a 6-h incubation, CD14+CD16++ cells, but not CD14++CD16− cells purified from the same donor, induced selective lysis of CD3+CD4+CD25bright Tregs (Fig. 2 A and C, Left). Thus, ipilimumab-mediated ADCC correlated with the relative density of CTLA-4 expression on target cells. Importantly, the primary CD14+CD16++ monocyte subset also lysed CD3+CD4+CD25bright T cells from patients with metastatic melanoma (Fig. 2 B and C). The interaction of FcγRIIIA(CD16) with ipilimumab (IgG1) was critical to CD14+CD16++ monocyte-mediated elimination of CD3+CD4+CD25bright Tregs because blocking CD14+CD16++ monocytes with anti-CD16 during the 6-h incubation completely abrogated target cell lysis (Fig. 2 A, Left, and C). In contrast to CD14+CD16++, CD14++CD16− monocytes purified from the same donors did not mediate ADCC-mediated lysis of Tregs (Fig. 2 B, Right, and C).
Fig. 2.
Selective FcγRIIIA-dependent lysis ex vivo of Tregs (CD3+CD4+CD25brightCD127−Foxp3++CTLA-4++ Tregs) by nonclassical CD14+CD16++ monocytes. (A) ADCC: killing of sorted CD3+CD4+CD25bright, CD25int, or CD25neg T cells (target) labeled with anti-CD4ECD from healthy donors by purified CD14+CD16++ (Left) or CD14++CD16− (Right) autologous monocytes (effector) at the indicated E:T ratios in the presence of ipilimumab with (open symbols) or without (filled symbols) anti-CD16 blocking Ab. (B) Similar assessment for ADCC of CD3+CD4+CD25bright, CD25int, or CD25neg T cells from patients with melanoma by purified CD14+CD16++ (open symbols) or CD14++CD16− (filled symbols) autologous monocytes. (A–C) ADCC assessments used a flow cytometry-based assay whereby lysed target cells took up an otherwise membrane-impermeable DNA stain, TO-PRO3. Specific lysis was based on the frequency of CD4ECD+ TO-PRO3+ relative to CD4ECD+ TO-PRO3− events. With colorimetric labeling, specific lysis was plotted against the y axes with respect to the E:T ratio shown along the x axes. Data points are the averages ± SEM of triplicate means from three or four independent experiments in three different healthy donors and four different patients with melanoma (***P < 0.001 for pairwise comparisons between CD25bright vs. CD25int or CD25neg T cells targeted by CD14+CD16++ monocytes blocked or not with anti-CD16 for all E:T ratios tested; P value not significant for pairwise comparisons between CD25bright vs. CD25int or CD25neg T cells targeted by CD14++CD16− monocytes blocked or not with anti-CD16 for all E:T ratios tested; ANOVA). (C) Representative ADCC plots at the top E:T ratio for CD25bright and CD25neg T cells from one patient with melanoma and for CD25bright T cells from one normal donor with or without anti-CD16 blocking Ab. (D and E) gMFI of CTLA-4 and Foxp3 expression by sorted CD3+CD4+CD25bright, CD25int, or CD25neg T cells from healthy donors. Mean gMFI measurements of CTLA-4 are 18,100 ± 4,700, 6,600 ± 2,600, and 5,400 ± 2,500 in CD3+CD4+CD25bright, CD3+CD4+CD25int, and CD3+CD4+CD25neg T cells, respectively. Mean gMFI measurements of Foxp3 are 59,300 ± 13,000, 32,300 ± 17,000, and 26,100 ± 12,400 in CD3+CD4+CD25bright, CD3+CD4+CD25int, and in CD3+CD4+CD25neg T cells, respectively. Data represent the averages ± SD from three or four independent experiments (**P < 0.01 and ***P < 0.001, unpaired two-tailed Student t test).
Baseline Enrichment of CD68+CD16+ Macrophages in the TME of Patients Responding to Ipilimumab.
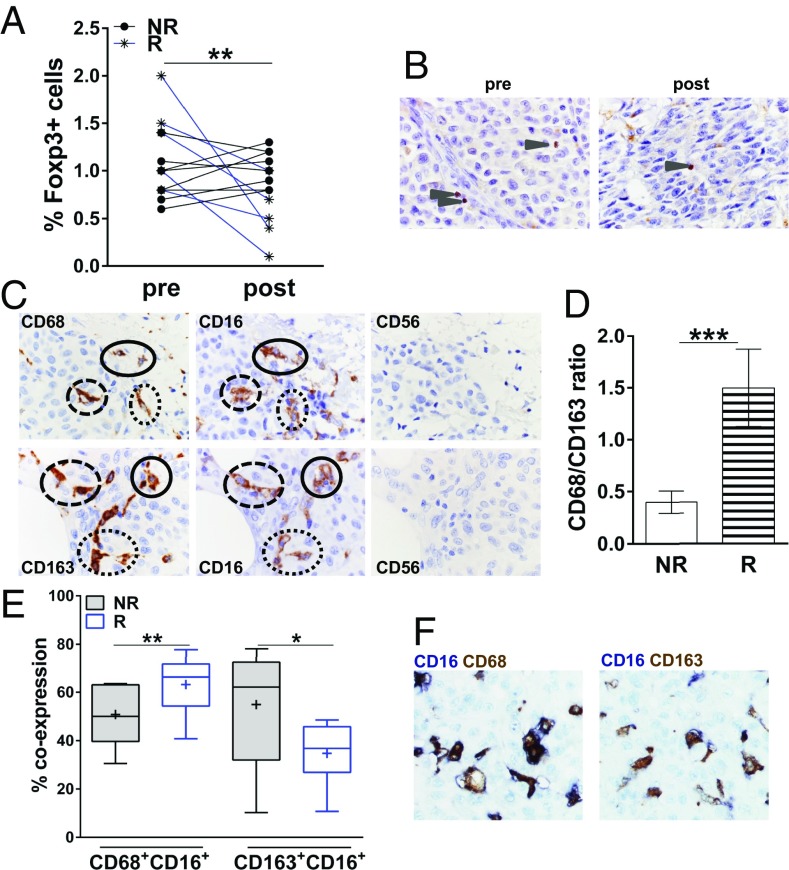
To investigate the TME, we studied matched pre- and postipilimumab metastatic lesions from 13 patients with melanoma and assessed by immunohistochemistry (IHC) the presence of CD8+ T cells, CD56+ NK cells, Foxp3+ Tregs, and CD68+ or CD163+ macrophages in the tumor nests. Tissue from melanoma metastases was obtained according to the study protocol at baseline and 3–4 wk after the last ipilimumab dose from shrinking lesions from responding patients or progressing lesions from nonresponding patients, respectively. Whereas, at baseline, Foxp3+ tumor-infiltrating Treg counts were similar in responding vs. nonresponding patients (Fig. 3A), they were significantly lower in postipilimumab lesions from responding patients than in those from nonresponding patients (Fig. 3 A and B). Tregs are a major component of the immunosuppressive microenvironment of melanoma-infiltrated lymph nodes, and their depletion may favor an immune-promoting TME (23). We evaluated macrophage infiltration of melanoma lesions by assessing the distribution of CD68+ and CD163+ cells. We initially evaluated the distribution of CD68, a well-established macrophage marker. Melanoma-infiltrating CD68+ macrophages were present in melanoma metastases (Fig. 3C). As tumor-associated macrophages represent a heterogeneous population with distinct immune properties, we set out to further evaluate the distribution of CD163+ macrophages (Fig. 3C). CD68+ and CD163+ macrophages from our patients expressed CD16 (FcγRIIIA; Fig. 3C). At baseline, responding patients had increased CD68+/CD163+ ratios compared with nonresponding patients (Fig. 3D). Next, to quantify more precisely the colocalization of CD16- and CD68- or CD163-expressing cells on the same tissue section at baseline, we used an automated staining and a standardized acquisition technique by assessing the coexpression of each of two markers in three representative tumor regions (Fig. 3 E and F). Consistent with our previous observations, responding patients had significantly higher intratumoral CD16+CD68+ cell densities than nonresponding patients. In contrast, the latter displayed higher CD16+CD163+ densities (Fig. 3E). Finally, we did not find any significant differences in CD8+ T-cell infiltrate in responding vs. nonresponding patients, and CD56+ NK cell infiltrates were negligible for both patient groups and time points (Fig. 3C).
Fig. 3.
Treg reduction in melanoma metastases from responder patients (marked as “R”) after ipilimumab dosing. (A) Quantitative IHC analysis of Foxp3+ cells per 100 melanoma cells in matched pre- and postipilimumab melanoma lesions from eight nonresponding patients (NR) and five responding patients, respectively (**P < 0.01, paired two-tailed Student t test). At baseline (pre), infiltration with Foxp3+ Tregs was similar in responders vs. nonresponders (P = 0.074, not significant, paired two-tailed Student t test). (B) IHC analysis from a representative responding patient (case G) with decreased tumor infiltration of Foxp3+ cells (arrowhead) after ipilimumab dosing (post) compared with baseline (pre). Foxp3 was developed by using DAB (DAB chromogen). (Magnification, 1,000×.) (C) Representative IHC staining of CD68, CD163, CD16, and CD56 on serial sections from patients B (Top) and F (Bottom) at baseline. Circles represent marker-expressing cells across subsequent sections. (Magnification, 1,000×.) (D) Quantitative IHC analysis of CD68+/CD163+ macrophage ratio in melanoma lesions at baseline from eight nonresponding patients and five responding patients, respectively. Error bars indicate mean ± SD (***P < 0.001, paired two-tailed Student t test). (E) Baseline density of colocalization, defined as percentage of CD16 and CD68 or CD163 expression of each of two markers in three representative tumor regions per sample. Box plots show the medians and interquartile ranges (25th to 75th percentiles), with whiskers approximating 95% of the data. The line in the middle of the box is plotted at the median; the plus sign represents the mean (Tukey method; **P < 0.01 and *P < 0.05 for CD16+CD68+ or CD16+CD163+ cellular density in responders vs. nonresponders, respectively, paired two-tailed Student t test). (F) Representative images of colocalization of CD16 with CD68 (Left) or CD163 (Right) on the same tissue section from case E at baseline. (Magnification, 1,000×.)
Discussion
Immune checkpoint blockade arguably represents a milestone in modern medicine, and will likely become a major treatment option for patients with cancer, with increased long-term efficacy at reduced toxicity. However, immune checkpoint blockade works well in only a fraction of treated patients. Possible cause(s) for the lack of response to checkpoint blockade remain still largely unknown, as do the appropriate biomarkers for patient selection. Preclinical studies highlight macrophages as key innate effector cells mediating ADCC in mice, although this remains unresolved in humans (8–10).
Our results show that, as opposed to nonresponders, patients responding to ipilimumab treatment display the highest peripheral counts of nonclassical monocytes at baseline. This monocyte subset has been associated to the patrolling of blood vessels’ endothelium and the sensing of nucleic acids, and it is characterized for the secretion of Th1-polarizing cytokines (12). The murine FcγRIV is the ortholog of human FcγRIIIA (15) and its expression pattern on Ly6Clo murine monocytes is consistent with the corresponding nonclassical CD14+CD16++ monocyte subset in humans, and it selectively mediates IgG-dependent effector functions in vivo (14, 16–18). In monocyte/T-cell cocultures, ipilimumab depletes Tregs ex vivo via an ADCC-dependent mechanism, selectively mediated by FcγRIIIA (CD16)-expressing, nonclassical monocytes. This is in contrast with classical CD14++CD16− monocytes, which lack FcγRIIIA expression and are unable to lyse Tregs. Functional FcγR polymorphisms have been reported as novel pharmacogenetic biomarkers that could be used to better target the use of mAbs in patients with cancer. The FcγR coding polymorphism of FCGR3AV158F is associated with differential affinity of the receptor for IgG1. However, currently there is no consistent effect of FcγR genotype on the clinical antitumor activity of therapeutic mAbs of IgG1 isotype (24).
Effective tumor control may be achieved by shifting the balance from immune tolerance to protective immune responses that eliminate cancer cells. Compared with baseline, only responding patients have decreased levels of intratumoral Tregs in postipilimumab tumor lesions, and, in addition, they display the highest CD68+/CD163+ macrophage ratio, indicative of a TME enriched with inflammatory macrophages. CD163 is a scavenger receptor for the hemoglobin–haptoglobin complex and is typically expressed by immune-suppressive macrophages (known as M2-like) (25), which have been associated with poor prognosis in patients with early-stage melanoma (26). CD163+ macrophages are known for secreting immune-suppressive mediators such as IL-10 and TGF-β, low tumoricidal activity, and promotion of tissue remodeling and angiogenesis (27). On the contrary, CD68+CD16+ are classically activated, M1-like macrophages that secrete inflammatory mediators such as IL-12, TNF-α, and inducible nitric oxide synthase; exhibit antitumor activity; and elicit tumor tissue disruption (28). Interestingly, both CD68+ and CD163+ macrophages from our patients express CD16 (FcγRIIIA; Fig. 3C). The higher baseline CD68+/CD163+ ratio and density of CD16+CD68+, as opposed to CD16+CD163+ cells in the melanoma lesions from responding patients, may reflect an immune-inflammatory TME enriched with a beneficial macrophage population. However, we cannot conclusively prove that the macrophages identified in the TME are ultimately responsible for the intratumoral depletion of Tregs in vivo. Furthermore, whether the observed reduction in Tregs is mechanistically significant remains an unresolved puzzle. Nevertheless, our data are hypothesis-generating and suggest that the increased CD8+/Treg ratio in responding lesions may result from ADCC of intratumoral Tregs. Studies of combined GVAX/CTLA-4 blockade in the B16 murine melanoma model and patients with melanoma previously highlighted the importance of CD8 T cells in the TME (29, 30). We did not find, however, any significant differences in CD8+ T-cell infiltrate in responding vs. nonresponding patients.
We recognize the inherent limitations of the present study caused by its small sample size. Studies on larger datasets are required to validate prospectively baseline classical and nonclassical blood monocytes, CD68+/CD163+ macrophage ratios, and CD16+CD68+ cellular density in the TME as potential biomarkers of response to ipilimumab treatment. Critical mechanistic questions remain still open, particularly whether the observed reductions in Tregs are mechanistically significant, and whether the macrophages identified in the TME are ultimately responsible of such reduction in vivo. Moreover, from a clinical standpoint, it is unclear whether the depletion of Tregs in the TME is required for efficacy of ipilimumab; based on preclinical work, there is indeed uncertainty whether the mechanism of action of anti–CTLA-4 treatment is caused by effector T-cell expansion and/or Treg depletion.
Future novel techniques will likely contribute to improve the phenotypic and functional characterization of melanoma-associated macrophages and other potential effector cells, hopefully enabling the investigation of cell subsets with selective IgG-dependent effector properties in vivo. The present study provides insight for a potential mechanism of action of ipilimumab treatment, highlighting the contribution of multiple host-dependent factors. If confirmed in larger patient datasets, our results may contribute to the generation of much-needed predictive biomarker panels, antibody design, and the development of rational, synergistic combination therapies that mobilize relevant immune effector cells to promote anticancer immunity.
Methods
Human Cells, Patient Population, and Study Design.
Human sample collection and use adhered to the study protocols approved by the institutional review and privacy boards of the University Hospital of Lausanne, Switzerland (protocol N: 400/11-IPI-Biology) and the University Medical Center, Tübingen, Germany (protocol N: 43/2008BO1), and the local ethics committee in accordance with the Helsinki Declaration. Patients gave informed consent before study inclusion. Healthy volunteers or patients provided peripheral blood withdrawn by using tubes containing Li-heparin–coated beads (Sarstedt). Blood was centrifuged for 10 min at 210 × g for plasma preservation, followed by PBMC preparation by gradient centrifugation using Lymphoprep (Ficoll equivalent; Axis-Shield). All cells were used fresh or after cryopreservation. Viable cell recovery was consistently 85–100%. Patients in this study were diagnosed with metastatic melanoma and received a maximum of four cycles of 3 mg/kg ipilimumab i.v. every 3 wk upon disease progression with at least one prior treatment. Blood samples were withdrawn at baseline, during treatment, 20 d after treatment, and then monthly for as long as 14 mo after the last ipilimumab dose.
Cell Sorting.
CD3+CD4+ T cells were enriched by using Dynabeads FlowComp Human CD4 kit (Invitrogen/Molecular Probes). The following antibodies were used to stain cells for subsequent FACS sorting: anti–CD3-APC-H7 (isotype IgG1κ; BD Biosciences), anti–CD4-ECD (isotype IgG1; Beckman Coulter), and anti–CD25-PE (isotype IgG2a; BD Biosciences), and AmCyan was used as a live/dead marker (Invitrogen-Molecular Probes). CD3+CD4+ T cells with high (CD25bright, i.e., Tregs), intermediate (CD25int), and low (CD25neg) levels of CD25 expression were sorted by using a BD FASCAria cell sorter. The fraction purity was 97% on average.
Purification of CD16+ and CD14+ Monocytes.
Adherent cells, after 1 h of PBMC incubation in a tissue culture dish with a 20-mm grid (Plasma 150 × 20 Style; Becton Dickinson) in RPMI 1640 with 2% (vol/vol) FCS, 1% penicillin/streptomycin, and 2 mM AAG (Arg, Asp, Glu), were gently trypsinized. CD16+ and CD14+ monocytes were separated by magnetic-activated cell sorting (human CD16 and CD14 microbeads; Miltenyi Biotec) according to manufacturer instructions. The purity of these cell subsets was checked with the following antibodies: anti–HLA-DR-APC (isotype IgG2aκ; BD Biosciences), anti–CD14-FITC (isotype IgG2a; Beckman Coulter), and anti–CD16-ECD (isotype IgG1; Beckman Coulter), and Vivid-Red (Invitrogen-Molecular Probes) was used as a live/dead marker. A Gallios flow cytometer was used for the measurement. Cell purity was ∼90% for CD14++CD16− and 80% for CD14+CD16++ subsets.
ADCC Assay.
CD3+CD4+ T cells with different expression levels of CD25 (CD25bright, CD25int, CD25neg) obtained and sorted from healthy donors or patients with melanoma were cocultured with autologous CD14++CD16− or CD14+CD16++ monocytes at the effector:target cell (E:T) ratios 40:1, 10:1, 5:1, and 1:2. Cells were cocultured in the absence or presence of ipilimumab (10 µg/mL; isotype IgG1κ; Bristol-Myers Squibb), anti-CD16 blocking antibody, clone 3G8 (10 µg/mL; isotype IgG1κ; BD Biosciences), or isotypic control anti-CD16 antibody (10 µg/mL; isotype IgG1κ; Beckman Coulter). After 6 h of incubation at 37 °C and 5% CO2, TO-PRO-3 iodide (642/661; Invitrogen/Molecular Probes) was added, and the level of cell death was measured by using a Gallios flow cytometer.
Flow Cytometry Analysis.
Expression of FoxP3 (cytoplasmic) and CTLA-4 (cytoplasmic and surface) was analyzed in cells with different expression levels of CD25 by flow cytometry. CTLA-4 and CD127 surface staining was performed by incubation with anti–CTLA-4-APC (isotype IgG2aκ; BD Biosciences) and anti–CD127-PE-Cy7 (isotype IgG1κ; eBioscience) for 20 min at 4 °C, with 1 × 106 cells per sample. FoxP3 and CTLA-4 cytoplasmic staining was performed on fixed (Cytofix/Cytoperm Solution; BD Biosciences) and permeabilized (0.1% saponin) samples at 1 × 106 cells per sample. For FoxP3 staining, anti–FoxP3-APC (isotype IgG2aκ; eBioscience) was used. For the characterization of different subpopulations of monocytes, 1 × 106 cells per sample were stained for 20 min at 4 °C with the following antibodies: anti–CD16-ECD (isotype IgG1; Beckman Coulter), anti–HLA-DR-PerCP-Cy5.5 (isotype IgG2aκ; BioLegend), anti–CD11c-PE-Cy7 (isotype IgG1κ; eBioscience), anti–PD-L1 (isotype IgG1, κ; BD Biosciences), anti–CD163-APC (isotype IgG1κ; R&D Systems), anti–PD-L2-APC (isotype IgG1κ; BD Biosciences); anti–CD11b-Alexa700 (isotype IgG1κ; BD Biosciences), anti–CD14-Pacific Blue (isotype IgG2aκ; BD Biosciences); anti–CD3-FITC (isotype IgG1; Beckman Coulter), anti–CD19-FITC (isotype IgG1; Beckman Coulter), anti–CD20-FITC (isotype IgG2bκ; BioLegend), anti–CD56-FITC, and anti–NKp46-FITC (isotype IgG1κ; BioLegend), and Vivid-Green (Invitrogen-Molecular Probes) was used as a live/dead marker. Negative controls included directly labeled and unlabeled isotype-matched irrelevant mAbs. Results were calculated as geometric mean of fluorescence intensity (gMFI). All results were analyzed by using FlowJo software, version 9.6.4.
IHC Staining and Evaluation.
IHC was performed on 4-μm paraffin sections from complete excisions of progressing or partially regressing melanoma lesions and from core biopsy specimens of completely regressing lesions. The following primary mouse mAbs were used: anti-CD68 (clone PG-M1, 1:200 dilution; Dako), anti-CD163 (clone 10D6, 1:200 dilution; Novocastra), anti-CD8 (clone C8/144B, 1:50 dilution; Dako), anti-CD56 (clone 1B6, 1:50 dilution; Novocastra), anti-CD16 (clone 2H7, 1:80 dilution; Novocastra), and anti-Foxp3 (clone 150D, 1:50 dilution; BioLegend). Slides were placed on a BenchMark XT IHC/ISH staining module (Ventana; Roche), for deparaffinization, endogenous peroxidase quenching, and epitope retrieval. For CD68, CD163, CD8, and CD56, the ultraView Universal DAB Detection Kit (Ventana) was used, whereas, for CD16 and Foxp3, the Envision+ System HRP-labeled Polymer with Liquid DAB+ Substrate Chromogen System (both from Dako) was used. The ratio of CD68+ cells and CD163+ cells was quantified in serial sections from all samples analyzed. All stains included a negative control with a matched Ab isotype, whereas staining of tonsillar sections served as positive controls for all antibodies. The percentage of tumor-infiltrated lymphoid populations was scored by visual inspection by using a 40× objective. (Magnification, 400×.) Areas of maximum infiltrates from the periphery of the tumors were selected and counted blindly. For double CD16/CD68 and CD16/CD163 immunostaining (Discovery Ultra staining module; Ventana), the following primary antibodies were used: ready-to-use rabbit mAb, clone SP175 (Ventana-Roche); mouse mAb, clone PG-M1; and mouse mAb, clone 10D6 (both at 1:200 dilution). For CD16 detection, the UltraMap anti–Rb-alkaline phosphatase multimer detection kit (Ventana) was used, and a blue-color reaction was obtained with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP). For CD68 and CD163 detection, the OmniMap anti–Ms-HRP multimer detection kit (Ventana) was used and a brown color was obtained with DAB. Stained slides were scanned in a batch format by using the Vectra multispectral imaging system (Perkin-Elmer), and the same regions of the acquired 4× images of CD16/CD68- and CD16/CD163-stained slides were collected. Then, 20× multispectral images were acquired from the collected areas and processed by using inForm tissue finder software (PerkinElmer). For unmixing of the images and analysis, the spectral libraries of hematoxylin as well as of both chromogens were generated. The percentages of CD68 and CD163 that colocalized with CD16 within the tumor areas were calculated through a thresholded colocalization analysis. Thresholds used to determine the cutoff for all three antibody stains were set visually.
Tumor Response Assessment.
Tumor response was assessed at weeks 12, 16, and 24 after treatment with ipilimumab by using the immune-related response criteria (11) Patients achieving CR, PR, or stable disease at week 16/24 after ipilimumab treatment were considered responders. Patients with progressive disease at week 16/24 after ipilimumab treatment were considered nonresponders.
Monolayer of Cells.
In the sample chamber, 200 µL of sorted cell suspension (containing ∼40,000 cells) was placed, and smears were carried out at 28 × g for 5 min by using Shandon Cytospin 4 (Thermo Scientific). After centrifugation, the sample was dried with cold air and stained with Giemsa (Fluka) by using Romanowsky staining.
Statistical Analysis.
Statistical analyses were made by Stata 13.0 (StataCorp) or GraphPad Prism, version 6. Error bars in figures represent the SEM or SD calculated by using GraphPad Prism. Specific statistical tests used were paired or unpaired two-tailed Student t test and ANOVA.
Supplementary Material
Acknowledgments
We appreciate the support and assistance of the Centre Hospitalier Universitaire Vaudois physicians, nurses, and staff of the Medical Oncology Service, Institute of Pathology, and Blood Bank Donor Room. We thank Mrs. H. Bichat and E. Fortis for technical support; Mrs. S. Abed-Maillard, L. Leyvraz, and Dr. L. Cagnon for assistance with our clinical protocols; and the clinical trial participants and healthy volunteers who provided samples for research. This work was supported by grants from Fondation Leenaards (to E.R.), Fondation Contre le Cancer (to E.R.), Fondation Nuovo-Soldati (to E.R.), a grant from Fondation Emma Muschamp (to P.G.F.), and Swiss National Science Foundation grant Sinergia CRSII3_141879 (to P.R. and D.E.S.).
Footnotes
Conflict of interest statement: P.R. holds a consultant or advisory role with Immatics Biotechnologies and Formune. O.M. holds a consultant or advisory role with Bristol-Myers Squibb, Hoffmann-La Roche, MSD, and Glaxo-SmithKline. B.W. receives honoraria and research funding from Bristol-Myers Squibb.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417320112/-/DCSupplemental.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. Immunology beats cancer: A blueprint for successful translation. Nat Immunol. 2012;13(12):1129–1132. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann MF, Köhler G, Ecabert B, Mak TW, Kopf M. Cutting edge: Lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163(3):1128–1131. [PubMed] [Google Scholar]
- 6.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 7.Wang CJ, et al. Cutting edge: Cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J Immunol. 2012;189(3):1118–1122. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulliard Y, et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210(9):1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selby MJ, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 10.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 12.Cros J, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81(3):584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 14.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 15.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biburger M, et al. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35(6):932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 20.Chung DJ, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viguier M, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173(2):1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 24.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 29.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105(8):3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.