Abstract
Voltage-gated sodium channels are intrinsic plasma membrane proteins that initiate the action potential in electrically excitable cells. They are composed of a pore-forming α-subunit and associated β-subunits. The β1-subunit was the first accessory subunit to be cloned. It can be important for controlling cell excitability and modulating multiple aspects of sodium channel physiology. Mutations of β1 are implicated in a wide variety of inherited pathologies, including epilepsy and cardiac conduction diseases. This review summarizes β1-subunit related channelopathies pointing out the current knowledge concerning their genetic background and their underlying molecular mechanisms.
Keywords: voltage-gated sodium channel, channelopathies, epilepsy, cardiopathies, β1-subunit, GEFS+, Brugada syndrome
Introduction
Action potentials play a central role in most excitable cells, as neurons, skeletal and cardiac muscle, and endocrine cells. Action potential generation and propagation occur through, and are regulated by the function of voltage-gated sodium channels (NaCh), proteins with selective pores for sodium ions that span the cell membrane. In mammals, NaCh are heterotrimeric complexes composed of a pore-forming α-subunit (~260 kDa), a non-covalently associated β1- or β3-subunit and a covalently associated β2- or β4-subunit (Messner and Catterall, 1985; Catterall, 2012).
There are nine NaChs α-subunit pore-forming isoforms encoded by different genes, termed Nav1.1 to Nav1.9, and an atypical non-voltage-dependent one, named NavX (Catterall, 2012). In humans, Nav1.1, Nav1.2, Nav1.3, and Nav1.6 are abundantly expressed in the central nervous system (CNS) and in the peripheral nervous system (PNS); Nav1.1 and Nav1.6 are also expressed in adult ventricular myocytes. Nav1.4 is abundant in adult skeletal muscle while Nav1.5 is expressed predominantly in heart. Nav1.7, Nav1.8, and Nav1.9 are preponderantly located in the PNS (Table 1). The α-subunit isoforms show a high degree of amino-acid sequence identity. Vertebrate α-subunits contain four homologous but non-identical domains (I–IV), each of which contains six transmembrane segments (S1–S6). The residues between S5 and S6 form the channel pore (P-loop) and control ion selectivity and permeation. Positively charged S4 segments act as voltage sensors.
Table 1.
Types of human sodium channels (NaCh) α and β subunits and their tissue distribution.
| Gene | Chromosome | Protein | Uniprot code* | Tissue expression |
|---|---|---|---|---|
| α-subunits | ||||
| SCN1A | 2q24.3 | Nav1.1 or α1.1 | P35498 | Cell bodies of central neurons, T-tubules in myocytes axon initial segments |
| SCN2A | 2q24.3 | Nav1.2 or α1.2 | Q99250 | Central neurons, mainly localizated in unmyelinated and premyelinated axons |
| SCN3A | 2q24.3 | Nav1.3 or α1.3 | Q9NY46 | Cell bodies of central neurons, cardiac myocytes |
| SCN4A | 17q23.3 | Nav1.4 or α1.4 | P35499 | Skeletal muscles |
| SCN5A | 3p21-22 | Nav1.5 or α1.5 | Q86V90 | Cardiac myocytes, immature and denervated skeletal muscles, certain brain neurons |
| SCN8A | 12q13 | Nav1.6 or α1.6 | Q9UQD0 | Somatodendritic distribution in output neurons of cerebellum, cerebral cortex, hippocampus; Purkinje cells in cerebellar granule cell layer, astrocytes, Schwann cells, axon initial segments, dorsal root ganglia, nodes of Ranvier in peripheral and central nervous systems, T-tubules in cardiac myocytes |
| SCN9A | 2q24 | Nav1.7 or α1.6 | Q15858 | Dorsal root ganglia neurons, sympathetic neurons, Schwann cells, neuroendocrine cells |
| SCN10A | 3p22.2 | Nav1.8 or α1.8 | Q9Y5Y9 | Dorsal root ganglia neurons, human heart, intracardiac neurons |
| SCN11A | 3p22.2 | Nav1.9 or α1.9 | Q9UI33 | C-type neurons in dorsal root ganglia |
| SCN7A | 2q24.3 | NavX | Q01118 | Dorsal root ganglia neurons, hippocampus, thalamus, cerebellum, median preoptic nucleus, circumventricular organs, Peripheral nervous system (PNS), heart, skeletal muscle, uterus |
| β -subunits | ||||
| SCN1B | 19q13.1 | SCN1b or β1 | Q07699 | Ubiquitous: central and peripheral neurons, glia, skeletal and cardiac muscles |
| SCN1B | 19q13.1 | SCN1bB or β1B | Cortical neurons, Cerebellar Purkinje cells, Deep cerebellar nuclei, Ventral horn neurons, Dorsal root ganglia neurons, peripheral nerves | |
| SCN2B | 11q23 | SCN2b or β2 | Q5U0K8 | Central and peripheral neurons, glia, cardiac muscles |
| SCN3B | 11q23.3 | SCN3Bor β3 | Q9NY72 | Central and peripheral neurons, adrenal glands, kidney |
| SCN4B | 11q23.3 | SCN4b or β4 | Q8IWT1 | Central and peripheral neurons, glia, skeletal and cardiac muscles |
Modified from Patiño et al. (2009) and Catterall (2012).
To date, five β-subunits have been identified in mammals: β1, its alternative splice variant β1B (previously called β1A), β2, β3, and β4. Each β-subunit is encoded by one of four genes, SCN1B–SCN4B. As well as α-subunits, β-subunits are highly expressed in excitable cells, including central and peripheral neurons, skeletal and cardiac muscle cells. They are also expressed in non-excitable cells such as astrocytes, radial glia, and Bergmann glia (Table 1).
β-subunits (~30–40 kDa) are single pass molecules with an extracellular N-terminus, a transmembrane-spanning segment, and an intracellular C-terminus. The β1B-subunit arising from the retention of a segment of intron 3 (exon 3A) does not include neither the transmembrane nor the intracellular domains, being a soluble protein (Qin et al., 2003; Patiño et al., 2009).
β1- and β3-subunits, which share 57% sequence homology, associate non-covalently with the α-subunits (Isom et al., 1992; McCormick et al., 1998; Morgan et al., 2000; Meadows et al., 2001; Spampanato et al., 2004), whereas β2- and β4-subunits, which have a high sequence homology, are covalently bound to the α-subunits by a disulphide bond (McCormick et al., 1998; Yu et al., 2003; Spampanato et al., 2004). All five β-subunits contain an extracellular immunoglobulin-like (Ig) domain homologous to V-type Ig-loop motif present in the Ig superfamily of cell adhesion molecules (CAMs), with a noteworthy homology to the CAM myelin P0 glycoprotein (Isom and Catterall, 1996; Morgan et al., 2000; Yu et al., 2003), a structural feature that enables them to function as CAMs (Isom et al., 1995a; Brackenbury and Isom, 2011; Calhoun and Isom, 2014). This protein motif has been confirmed in the crystallographic studies of the extracellular domain of the β3- and β4-subunits (Gilchrist et al., 2013; Namadurai et al., 2014).
Many studies have tried to demonstrate that β-subunits are able to fine-tune gating and kinetics of α-subunits expressed heterologously. There is no doubt that the classical roles of β-subunits as “conducing” modulators of Na+ current is of paramount importance in regulating ion flux and cell excitability. However, there is a clear trend in literature that underlines the importance of β-subunit “non-conducing” functions, including NaCh cell surface expression regulation, migration and pathfinding, cell adhesion and putative transcriptional modulation (Davis et al., 2004; Brackenbury et al., 2008, 2010; Baroni et al., 2013, 2014; Baroni and Moran, 2015). Furthermore, β-subunits are key players in a variety of pathologies, including epilepsy, cardiac arrhythmia, neuropsychiatric disorders, neuropathic and inflammatory pain, and cancer (Brackenbury and Isom, 2011). Thus, the understanding of the interactions between NaCh α- and β-subunits is of predominant importance, also in view of the exploitation of their therapeutic potential.
In this review we will focus on the multiple roles played by the β1-subunit, which has been the first ancillary subunit to be cloned and to be associated to human diseases. We will describe its mutations and illustrate some hypotheses formulated to attempt the explanation of the mechanisms that lead to β1 mutation-related pathologies.
β1 Functions
From its molecular identification (Isom et al., 1992), the β1-subunit has been proposed to modulate gating and kinetics properties of NaCh, especially inactivation. Co-expression of rat β1-subunit with skeletal muscle or brain rat α-subunits in Xenopus oocytes has been proposed to increase the amplitude of the peak sodium current, accelerate inactivation, and shift the voltage-dependence of inactivation to more negative membrane potentials (Isom et al., 1992; Patton et al., 1994; Moran and Conti, 2001). However, data regarding the heterologous expression of β1 in mammalian cells are contradictory and different results have been described by different groups. It was reported that, in mammalian cells, the β1-subunit is able: to shift the inactivation curve to positive, negative or to not change the potential, to shift the activation curve to negative potentials or to not change it, to hasten the recovery from inactivation or to not change it, to increase or do not modify the density of sodium currents (Bendahhou et al., 1995; Isom et al., 1995b; Hayward et al., 1996; An et al., 1998; Kazen-Gillespie et al., 2000; Tammaro et al., 2002; Moran et al., 2003). It has also been proposed that β1-subunit modulates NaCh gating through the screening of the membrane surface charge (Johnson et al., 2004; Ferrera and Moran, 2006).
Beside the regulation of NaCh gating, it has been proposed that β1-subunit participates in cell–cell and cell–matrix adhesion, contributing to cellular aggregation, ankyrin recruitment, and neurite outgrowth (Srinivasan et al., 1998; Malhotra et al., 2000, 2002; Kazarinova-Noyes et al., 2001; Ratcliffe et al., 2001; Davis et al., 2004; McEwen et al., 2004). Finally, it was demonstrated that in excitable cells the β1-protein acts as a crucial element in the assembly and cell surface expression of the heteromeric complex of the sodium channel, determining the type and the amount of α-subunit to be expressed (Patiño et al., 2009). Indeed, over-expression and silencing of the NaCh accessory subunit, demonstrate that the β1 is able to regulate the NaCh expression, and it is also a key factor in the processes that determine which α-subunit is going to be expressed (Baroni et al., 2013, 2014).
Consistently with these properties, β1-subunit was demonstrated able to rescue trafficking-deficient Nav1.1 channels to the cell surface, thus influencing the disease severity caused by the lack of a properly functional NaCh α-subunit (Rusconi et al., 2007, 2009; Sugiura et al., 2012; Thompson et al., 2012; Bechi et al., 2015). Also in this case, disease severity may be severely influenced by the total or partial lack of the β1-subunit capability to traffic mutant Nav1.1 to the cell surface.
β1-Linked Diseases
One of the most remarkable findings of research on the molecular properties of NaCh β1-subunit was the discovery that its mutations cause inherited diseases that selectively affect the CNS or the heart (Wallace et al., 1998; Antzelevitch, 2003; Fish and Antzelevitch, 2003; Watanabe et al., 2008; Escayg and Goldin, 2010). Unfortunately, the comprehension of the molecular mechanisms underlying the SCN1B mutation physiopathology is limited by the lack of a unique and exhaustive elucidation of the role played by this protein on the regulation of the NaCh. Evidences collected up to now suggest a model in which gene dosage may determine the severity of disease (Moran and Conti, 2001). For example, for SCN1B mutations related to CNS diseases, a single mutant allele may result in the development of a milder disease like generalized epilepsy with febrile seizures plus. In contrast, expression of two non-functional SCN1B alleles may result a more severe epileptic disease like the Dravet Syndrome.
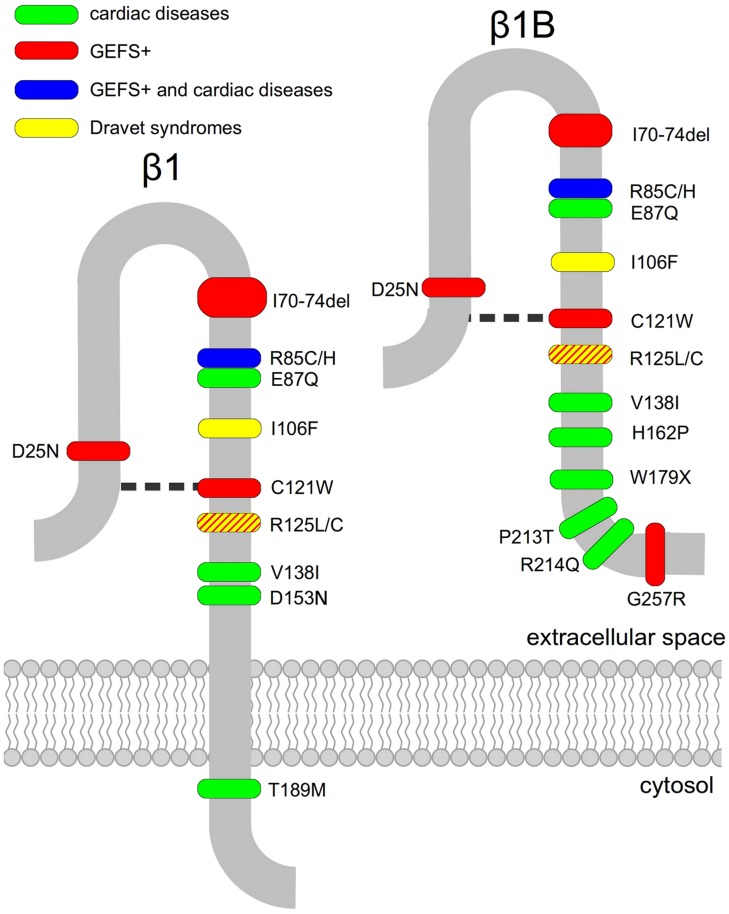
Another peculiarity that distinguishes SCN1B mutations linked either to CNS or to cardiac diseases is that, with the exception of the recently identified mutation G257R (Patiño et al., 2009) which is located in the β1B retained intronic region, all generalized epilepsy with febrile seizures plus (GEFS+) causing mutations are localized in the Ig-loop region (Figure 1), suggesting that the cell adhesion functions mediated by this region are clinically relevant (Brackenbury and Isom, 2011).
FIGURE 1.
Disease-linked mutations of voltage-gated sodium channel β1- and β1B-subunits. Mutated residues associated with epilepsy (green), cardiac diseases (red) or both (blue) are marked. The disulphide bridge involving residue C121 is indicated as a broken line.
The inherited diseases caused by mutations in the NaCh β1-subunit described so far are:
1. Generalized Epilepsy with Febrile Seizures Plus (GEFS+)
Some mutations in SCN1B are linked to GEFS+ (OMIM:604233), an autosomal dominant inherited epilepsy. The first SCN1B mutation identified in GEFS+ was C121W (Wallace et al., 1998, 2002), caused by a 387C-to-G transversion in SCN1B gene. As a consequence, a key disulphide bond involved in maintaining the extracellular Ig-like loop is disrupted (Barbieri et al., 2012).
Functional studies of mutant rat C121W β1-subunit co-expressed with either with brain 1.2 or muscle 1.4 rat α-subunits in Xenopus laevis oocytes showed that the mutated β1-subunit loses its ability to modulate the acceleration of the inactivation rate of the sodium channel compared with wild type (WT) β1-subunit (Wallace et al., 1998; Moran and Conti, 2001). Interestingly, C121W-β1 heterologous expression in mammalian cells yielded contradictory results, depending on the α-subunit co-expressed and on the expression system. When co-expressed with human Nav1.3, human C121W-β1 causes a rightward shift of inactivation compared to the WT-β1, potentially increasing channel excitability (Meadows et al., 2002). On the contrary, when rat C121W-β1 is co-expressed with rat skeletal muscle Nav1.4, sodium channels recover more slowly from fast inactivation (Tammaro et al., 2002).
It has been argued that C121W-β1 acts as a dominant-negative subunit, competing with the WT-β1-subunit in the regulation of the NaCh α-subunit expression and activity (Moran and Conti, 2001; Meadows et al., 2002). In rat neuronal-like cells, the regulatory effect of the over-expression of rat β1-subunit on the α-subunit mRNA, protein and Na+ current levels is abolished by the epileptogenic C121W-β1; conversely, in rat cardiac cells mutation C121W does not alter the β1-subunit modulation of NaCh (Baroni et al., 2013). These findings demonstrate the tissue-specificity of the modulation of NaCh expression.
Successively, six other mutations, I70_E74del, R85C, R85H, G257R, R125L, and D25N were associated to GEFS+, R85C, and R85H are missense mutations of an evolutionary conserved arginine residue in the Ig-loop (Scheffer et al., 2007). When co-expressed with human Nav1.2, human R85H-β1 appeared to modulate the voltage-dependence of NaCh slow inactivation without any effect on other electrophysiological parameters, while co-expression of human Nav1.2 with human R85C-β1 had no detectable effects on any channel property, suggesting a complete loss of function mutant (Xu et al., 2007). Immunohistochemical studies on cells transiently transfected with β1 mutants R85C and R85H failed to detect them at the cell surface, indicating that they are trafficking defective (Xu et al., 2007). Conversely, in surface biotinylation assay, similarly to WT-β1, human β1-R85H was detected at the cell surface of stably transfected Chinese hamster lung 1610 cells (Patiño et al., 2011), pointing out the need for further investigations on the cellular localization of this mutant.
Unlike the other GEFS+- associated SCN1B mutations, that are located in the Ig-domain, the missense mutation G257R is located in the β1B retained intronic region (Patiño et al., 2011). Surface biotinylation assay revealed that differently from Chinese hamster lung 1610 cells stably transfected with human WT-β1, Chinese hamster lung 1610 cells permanently transfected with human G257R fail to show the mutant β1 at the plasma membrane (Patiño et al., 2011).
Mutation I70_E74del is a A-to-C transversion in the splice acceptor site of exon 3 of SCN1B gene, resulting in a deletion of five amino acids within the extracellular Ig-fold (Audenaert et al., 2003). Unfortunately, no functional data are available for this mutation. R125L is a GEFS+-associated mutation, caused by a 374G-to-T transversion in exon 3 of SCN1B gene. It determines the substitution of a highly conserved arginine in the extracellular domain of the protein (Fendri-Kriaa et al., 2011). Even though functional studies on this mutation are still not available, it can be hypothesized that mutation R125L causes electrostatic changes and a loss of hydrogen bonding in the Ig-loop region affecting the structure and stability of the protein. The last GEFS+-associated SCN1B mutation is D25N. This missense mutation is due to a 73G-to-A transversion in exon 2 of SCN1B and causes the neutralization of an charged residue in the Ig-loop (Orrico et al., 2009).
2. Dravet Syndrome (DS)
Dravet syndrome or severe myoclonic epilepsy of infancy (OMIM:607208) is a severe form of generalized epilepsy with febrile seizures, characterized by generalized tonic, clonic, and tonic–clonic seizures triggered at first by fever, arising shortly after birth. Cognitive development is normal until ~2 years of age, when it slows or stagnates (Dravet, 1978; Wolff et al., 2006). Classically, DS is considered to be a SCN1A-linked disease (Oguni et al., 2005; Korff and Nordli, 2006). However, a small but growing number of DS patients affected by mutations in SCN1B has been described. Differently from GEFS+, all the SCN1B mutations causing DS have been found in homozygosis.
The first SCN1B mutation identified in DS is R125C, which prevents normal trafficking of β1 to the cell surface and thus results in a functional null phenotype (Patiño et al., 2009). Chinese hamster lung 1610 cells stably transfected with the rat Nav1.2 subunit as well HEK cells permanently transfected with human Nav1.1 were further stably transfected with human WT- or R125C-β1. Western-blot analysis of cell fractions unequivocally demonstrated that human R125C is inefficiently expressed at the cell surface at physiological temperatures, but the overcome of this trafficking defect at a lower temperature permits the mutant β1-subunit to be fully capable of modulating sodium current (Patiño et al., 2009). Another SCN1B mutation linked to DS, I106F, is caused by a 316A>T nucleotide change resulting in residue substitution in the Ig-loop (Ogiwara et al., 2012). No functional data are available for this mutant protein.
Mouse models support the link between SCN1B and epilepsy. Scn1b-null mice have frequent spontaneous generalized seizures, display aberrant neuronal excitability, and have defects in neuronal development (Chen et al., 2004; Brackenbury et al., 2013). Importantly, abnormalities in brain development are observed at P5, prior to seizure onset, suggesting that structural alterations, aberrant cell adhesive interactions, and abnormal excitability early in development may be causative factors in epileptogenesis (Brackenbury et al., 2013). A knock-in mouse model of C121W-mediated GEFS+ displays hyper-excitability in specific sub-populations of central neurons, reduced dendritic arborisation of subicular pyramidal neurons, and increased susceptibility to febrile seizures (Wimmer et al., 2010; Hatch et al., 2014).
3. Brugada Syndrome (BrS)
Brugada syndrome (BrS) is a condition characterized by a distinct ST-segment elevation in the right precordial leads of the electrocardiogram and by an increased risk of cardiac arrhythmia and sudden death (Brugada and Brugada, 1992). The condition predominantly exhibits an autosomal dominant pattern of inheritance and incomplete penetrance. It has an average prevalence of 5:10000 worldwide, and is much more common in men than in women (Priori et al., 2002; Smits et al., 2002; Antzelevitch, 2003). The mean age of BrS clinical debut is 40 years; however, the first occurrence of symptoms may occur in early childhood or old age (Antzelevitch, 2003). Currently, BrS is associated to more than 100 mutations in seven genes (SCN5A, GPD1L, CACNA1C, CACNB2, KCNE3, SCN3B), including SCN1B.
E87Q is the first β1 mutation linked to BrS (BrS5, OMIM:612838), caused by a 259G-C transversion in exon 3 of the human SCN1B gene. It results in a substitution of the neutralization of a highly conserved glutamic acid within the Ig-loop, which is common to both the β1 and β1B transcripts. Functional studies of the E87Q mutation in transiently transfected CHO cells show that the co-expression of mutant human β1 or β1B with human cardiac Nav1.5 neither increases the sodium current nor produces a negative shift in the voltage dependence of the activation curve with respect to cells transfected with WT-β1 or -β1B and Nav 1.5. Mutant E87Q-β1 or -β1B shifted only the voltage dependence of inactivation to negative potentials (Watanabe et al., 2008).
Another mutation linked to BrS is W179X that has been found in β1B. It is a non-sense mutation caused by a 536G-A transition in exon 3A of the SCN1B gene (Watanabe et al., 2008). A variant of this mutation, produced by a 537G-A transition, also causes the W179X mutation. This variant has been correlated with cardiac conduction defects without any BrS symptom (Watanabe et al., 2008). It is conceivable that the lack of a β1 protein causes a disease by simply haploinsufficiency. Functional studies of W179X mutation showed that the co-expression of human W179X-β1B with human cardiac Nav1.5 failed to increase sodium currents and did not modulate the activation and inactivation (Watanabe et al., 2008).
The BrS linked-mutation, R214Q, has been found in exon 3A of β1B-subunit (Hu et al., 2012) It is due to a 641G-to-A transversion. Sodium currents of cells transfected with human SCN5A and SCN1Bb-R214Q resulted 56.5% smaller than that of SCN5A plus SCN1Bb-WT and 33.05% smaller than that of cells transfected with the sole SCN5A. Furthermore, R214Q caused no significant shift in steady-state inactivation and activation, but slowed recovery from inactivation (Hu et al., 2012).
SCN1B BrS-linked H162P mutation was found in a Danish patient by Holst et al. (2012). As the patient did not completely fulfill the diagnostic criteria for BrS and no functional data are available, further investigations would be mandatory to confirm the clinical relevance of this mutant.
Finally two SCN1B mutations, V138I and T189M, have been related to sudden unexplained nocturnal death syndrome (SUNDS), a disorder whose electrocardiogram (ECG) characteristics and clinical phenotype are very similar to BrS (Liu et al., 2014).
4. Atrial Arrhythmias
R85H and D153N are SCN1B mutations that have been associated with familiar atrial fibrillation (ATFB13 OMIM:615377). R85H is located in the Ig-loop, and thus affects both β1 and β1B. Conversely, D153N is located in exon 4 of SCN1B and thus can only affect β1. Both mutations result in a reduction of sodium currents in heterologous expression systems. In comparison with human WT-β1 co-expressed with human SCN5A in CHO cells, D153N does not affect the sodium channel activation or inactivation. However, R85H resulted in a positive shift of voltage-dependence of both, activation and inactivation (Watanabe et al., 2009).
R85H, has been also reported as an epilepsy mutation in patients from two families without history of seizure disorders (Scheffer et al., 2007). Further functional studies would be mandatory to confirm the clinical relevance of this mutant, that represents an exception among SCN1B mutations. In fact all the SCN1B mutations identified so far have demonstrated to selectively affect the CNS or the heart.
5. Long QT- Syndrome (LQTS)
Long QT- syndrome (LQTS) is a cardiovascular disorder associated with syncopal episodes, torsades de pointes, ventricular fibrillation, and sudden death. This syndrome is characterized by prolonged QT-interval in the ECG because of an abnormality in cardiac repolarization. At least 15 forms of LQTS have been identified, each with specific associated genes, variations in penetrance, allele dominance, and co-morbidities.
A recent report identified mutation P213T of β1B to cause LQTS (OMIM: 611819; Giudicessi and Ackerman, 2013). When heterologously co-expresed in HEK cells, both human β1B-WT and β1B-P213T increased sodium currents with respect to expression of human Nav1.5 alone. The activation voltage dependence curve was significantly shifted to the left in cells co-expressing Nav1.5 and β1B-P213T compared with Nav1.5 β1B-WT, while the inactivation voltage dependence curve was not affected by the mutation. P213T of β1B significantly accelerates the recovery from inactivation. Furthermore, the probability of having more channels in the slow inactivated state resulted significantly lower for Nav1.5β1B-P213T than for Nav1.5 β1B-WT. This change could lead to higher channel availability, unbalancing the currents that determine the duration of action potentials, and determining the condition for LQTS onset (Riuró et al., 2014).
Evidence in transgenic mice suggests that β1 subunit is involved in normal cardiac function and that mutations of SCN1B can result in disease. Consistent with LQTS, Scn1b-null mice have abnormal cardiac action potentials evidenced by prolonged QT intervals that persist after pharmacological autonomic blockade (Lopez-Santiago et al., 2007). Scn1b-null ventricular myocytes also display increased peak and persistent Na+ current relative to WT cells (Lopez-Santiago et al., 2007).
Concluding Remarks
A growing list of SCN1B mutations linked to inherited diseases reveals the important roles that the β1-subunit plays in the NaCh-function. β1-subunit channelopathies belong to two categories: epileptic syndromes and cardiac arrhythmias. Each SCN1B mutation seems to have a tissue-selectivity whose molecular mechanism is far to be elucidated. Another peculiarity is the wide spectrum of phenotypes and clinical manifestations that can be observed in patients affected by the same SCN1B mutation.
The comprehension of the pathophysiology of diseases caused by mutations of β1-subunit is severely limited by the understanding of the functional role of the β1-subunit. The β1-subunit was recognized as a part of the NaCh complex since the early attempts to identify the molecules that compose this channel. However, even though the molecular identification of the β1-subunit, and the possibility to express it in heterologous systems, the role of this protein is still controversial. The β1-subunit could play three different roles. It has been claimed that β1-subunit is involved in the fine tuning of the NaCh gating. This proposal comes from the heterologous expression of β1-subunit in Xenopus oocytes, where it dramatically regulates the NaCh inactivation. However, when the β1-subunit is heterologously expressed in mammalian cells results are contradictory, and, in general, its role seems to be correlated to an indirect effect by charge surface modifications and not to a specific NaCh modulation. The second possible role of the β1-subunit is associated to the interactions of the NaCh with the cytoskeleton and the extracellular matrix, determining the correct docking of the NaCh in specific regions of the plasma membrane. A further role in modulating the gene expression, and therefore the amount and quality of NaCh α-subunits has been also recently illustrated. All three possible roles could be implicated in the genesis of the diseases caused by β1-subunit mutations but none of these hypotheses has been incontrovertibly demonstrated yet. As occurs with any biological mechanism with a high degree of complexity, one could hypothesize that other genes – for example, genes encoding some of the β1-subunit interacting proteins, may likely exert their influence on the severity of the diseases linked to β1-subunit mutations or determine the tissue-specificity. The disclosure of this specific genetic relationships will not only shed new light on the biology of NaCh heteromeric complex but also provide critical information to design more appropriate pharmacological therapies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by Fondazione Compagnia di San Paolo.
References
- An R., Wang X., Kerem B., Benhorin J., Medina A., Goldmit M., et al. (1998). Novel LQT-3 mutation affects Na+ channel activity throught interactions between α- and β1-subunits. Circ. Res. 83 141–146 10.1161/01.RES.83.2.141 [DOI] [PubMed] [Google Scholar]
- Antzelevitch C. (2003). Brugada syndrome: clinical, genetic, molecular, cellular and ionic aspects. Expert Rev. Cardiovasc. Ther. 1 177–185 10.1586/14779072.1.2.177 [DOI] [PubMed] [Google Scholar]
- Audenaert D., Claes L., Ceulemans B., Löfgren A., Van Broeckhoven C., De Jonghe P. (2003). A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology 61 854–856 10.1212/01.WNL.0000080362.55784.1C [DOI] [PubMed] [Google Scholar]
- Barbieri R., Baroni D., Moran O. (2012). Identification of an intra-molecular disulfide bond in the sodium channel β1-subunit. Biochem. Biophys. Res. Commun. 420 364–367 10.1016/j.bbrc.2012.02.163 [DOI] [PubMed] [Google Scholar]
- Baroni D., Barbieri R., Picco C., Moran O. (2013). Functional modulation of voltage-dependent sodium channel expression by wild type and mutated C121W-β1 subunit. J. Bioenerg. Biomembr. 45 353–368 10.1007/s10863-013-9510-3 [DOI] [PubMed] [Google Scholar]
- Baroni D., Moran O. (2015). Differential gene expression profiles of two excitable rat cell lines after over-expression of WT- and C121W-β1 sodium channel subunits. J. Neurosc. 297 105–117 10.1016/j.neuroscience.2015.03.052 [DOI] [PubMed] [Google Scholar]
- Baroni D., Picco C., Barbieri R., Moran O. (2014). Antisense-mediated post-transcriptional silencing of SCN1B gene modulates sodium channel functional expression. Biol. Cell 106 13–29 10.1111/boc.201300040 [DOI] [PubMed] [Google Scholar]
- Bechi G., Rusconi R., Cestèle S., Striano P., Franceschetti S., Mantegazza M. (2015). Rescuable folding defective NaV1.1 (SCN1A) mutants in epilepsy: properties, occurrence, and novel rescuing strategy with peptides targeted to the endoplasmic reticulum. Neurobiol. Dis. 75 100–114 10.1016/j.nbd.2014.12.028 [DOI] [PubMed] [Google Scholar]
- Bendahhou S., Cummins T., Potts J., Tong J., Agnew W. (1995). Serine-1321-independent regulation of the μ1 adult skeletal muscle Na+ channel by protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 92 12003–12007 10.1073/pnas.92.26.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury W. J., Calhoun J. D., Chen C., Miyazaki H., Nukina N., Oyama F., et al. (2010). Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc. Natl. Acad. Sci. U.S.A. 107 2283–2288 10.1073/pnas.0909434107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury W. J., Djamgoz M. B. A., Isom L. L. (2008). An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist 14 571–583 10.1177/1073858408320293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury W. J., Isom L. L. (2011). Na channel β subunits: overachievers of the ion channel family. Front. Pharmacol. 2:53 10.3389/fphar.2011.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury W. J., Yuan Y., O’Malley H. A., Parent J. M., Isom L. L. (2013). Abnormal neuronal patterning occurs during early postnatal brain development of Scn1b-null mice and precedes hyperexcitability. Proc. Natl. Acad. Sci. U.S.A. 110 1089–1094 10.1073/pnas.1208767110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugada P., Brugada J. (1992). Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 20 1391–1396 10.1016/0735-1097(92)90253-J [DOI] [PubMed] [Google Scholar]
- Calhoun J. D., Isom L. L. (2014). The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels. Handb. Exp. Pharmacol. 221 51–89 10.1007/978-3-642-41588-3_4 [DOI] [PubMed] [Google Scholar]
- Catterall W. A. (2012). Voltage-gated sodium channels at 60: structure, function and pathophysiology. J. Physiol. 590 2577–2589 10.1113/jphysiol.2011.224204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Westenbroek R. E., Xu X., Edwards C. A., Sorenson D. R., Chen Y., et al. (2004). Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J. Neurosci. 24 4030–4042 10.1523/JNEUROSCI.4139-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. H., Chen C., Isom L. L. (2004). Sodium channel beta1 subunits promote neurite outgrowth in cerebellar granule neurons. J. Biol. Chem. 279 51424–51432 10.1074/jbc.M410830200 [DOI] [PubMed] [Google Scholar]
- Dravet C. (1978). Les epilepsies graves de l’enfant. Vie Med. 8 543–548. [Google Scholar]
- Escayg A., Goldin A. L. (2010). Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51 1650–1658 10.1111/j.1528-1167.2010.02640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendri-Kriaa N., Kammoun F., Salem I. H., Kifagi C., Mkaouar-Rebai E., Hsairi I., et al. (2011). New mutation c.374C>T and a putative disease-associated haplotype within SCN1B gene in Tunisian families with febrile seizures. Eur. J. Neurol. 18 695–702 10.1111/j.1468-1331.2010.03216.x [DOI] [PubMed] [Google Scholar]
- Ferrera L., Moran O. (2006). Beta1-subunit modulates the Nav1.4 sodium channel by changing the surface charge. Exp. Brain Res. 172 139–150 10.1007/s00221-005-0323-4 [DOI] [PubMed] [Google Scholar]
- Fish J. M., Antzelevitch C. (2003). Cellular and ionic basis for the sex-related difference in the manifestation of the Brugada syndrome and progressive conduction disease phenotypes. J. Electrocardiol. 36(Suppl), 173–179 10.1016/j.jelectrocard.2003.09.054 [DOI] [PubMed] [Google Scholar]
- Gilchrist J., Das S., Van Petegem F., Bosmans F. (2013). Crystallographic insights into sodium-channel modulation by the β4 subunit. Proc. Natl. Acad. Sci. U.S.A. 110 E5016–E5024 10.1073/pnas.1314557110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicessi J. R., Ackerman M. J. (2013). Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl. Res. 161 1–14 10.1016/j.trsl.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch R. J., Reid C. A., Petrou S. (2014). Enhanced in vitro CA1 network activity in a sodium channel β1(C121W) subunit model of genetic epilepsy. Epilepsia 55 601–608 10.1111/epi.12568 [DOI] [PubMed] [Google Scholar]
- Hayward L., Brown R., Jr., Cannon S. (1996). Inactivation defects caused by myotonia-associated mutations in the sodium channel III-IV linker. J. Gen. Physiol. 107 559–576 10.1085/jgp.107.5.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst A. G., Saber S., Houshmand M., Zaklyazminskaya E. V., Wang Y., Jensen H. K., et al. (2012). Sodium current and potassium transient outward current genes in Brugada syndrome: screening and bioinformatics. Can. J. Cardiol. 28 196–200 10.1016/j.cjca.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Hu D., Barajas-Martínez H., Medeiros-Domingo A., Crotti L., Veltmann C., Schimpf R., et al. (2012). A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Na(v)1.5 and K(v)4.3 channel currents. Heart Rhythm 9 760–769 10.1016/j.hrthm.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom L., Catterall W. (1996). Na+ channel subunits and ig domains. Nature 383 307–308 10.1038/383307b0 [DOI] [PubMed] [Google Scholar]
- Isom L. L., De Jongh K. S., Patton D. E., Reber B. F., Offord J., Charbonneau H., et al. (1992). Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 256 839–842 10.1126/science.1375395 [DOI] [PubMed] [Google Scholar]
- Isom L., Ragsdale D., De Jongh K., Westenbroek R., Reber B., Scheuer T., et al. (1995a). Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with CA motif. Cell 83 433–442 10.1016/0092-8674(95)90121-3 [DOI] [PubMed] [Google Scholar]
- Isom L., Scheuer T., Brownstein A., Ragsdale D., Murphy B., Catterall W. (1995b). Functional coexpression of the β1 and type IIA α subunits of sodium channels in mammalian cell line. J. Biol. Chem. 270 3306–3312 10.1074/jbc.270.7.3306 [DOI] [PubMed] [Google Scholar]
- Johnson D., Montpetit M. L., Stocker P. J., Bennett E. S. (2004). The sialic acid component of the beta1 subunit modulates voltage-gated sodium channel function. J. Biol. Chem. 279 44303–44310 10.1074/jbc.M408900200 [DOI] [PubMed] [Google Scholar]
- Kazarinova-Noyes K., Malhotra J. D., McEwen D. P., Mattei L. N., Berglund E. O., Ranscht B., et al. (2001). Contactin associates with Na+ channels and increases their functional expression. J. Neurosci. 21 7517–7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazen-Gillespie K., Ragsdale D., D’Andrea M., Mattei L., Rogers K., Isom L. (2000). Cloning, localization, and functional expression of sodium channel beta1A subunits. J. Biol. Chem. 275 1079–1088 10.1074/jbc.275.2.1079 [DOI] [PubMed] [Google Scholar]
- Korff C. M., Nordli D. R. J. (2006). Epilepsy syndromes undetermined whether focal or generalized in infants. Epilepsy Res. 70(Suppl. 1), S105–S109 10.1016/j.eplepsyres.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Liu C., Tester D. J., Hou Y., Wang W., Lv G., Ackerman M. J., et al. (2014). Is sudden unexplained nocturnal death syndrome in Southern China a cardiac sodium channel dysfunction disorder? Forensic Sci. Int. 236 38–45 10.1016/j.forsciint.2013.12.033 [DOI] [PubMed] [Google Scholar]
- Lopez-Santiago L. F., Meadows L. S., Ernst S. J., Chen C., Malhotra J. D., McEwen D. P., et al. (2007). Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J. Mol. Cell Cardiol. 43 636–647 10.1016/j.yjmcc.2007.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J. D., Kazen-Gillespie K., Hortsch M., Isom L. L. (2000). Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 275 11383–11388 10.1074/jbc.275.15.11383 [DOI] [PubMed] [Google Scholar]
- Malhotra J. D., Koopmann M. C., Kazen-Gillespie K. A., Fettman N., Hortsch M., Isom L. L. (2002). Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. J. Biol. Chem. 277 26681–26688 10.1074/jbc.M202354200 [DOI] [PubMed] [Google Scholar]
- McCormick K., Isom L., Ragsdale D., Smith D., Scheuer T., Catterall W. (1998). Molecular determinants of the Na+ channel function in the extracellular domain of the b1 subunit. J. Biol. Chem. 273 3954–3962 10.1074/jbc.273.7.3954 [DOI] [PubMed] [Google Scholar]
- McEwen D. P., Meadows L. S., Chen C., Thyagarajan V., Isom L. L. (2004). Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J. Biol. Chem. 279 16044–16049 10.1074/jbc.M400856200 [DOI] [PubMed] [Google Scholar]
- Meadows L. S., Malhotra J., Loukas A., Thyagarajan V., Kazen-Gillespie K. A., Koopman M. C., et al. (2002). Functional and biochemical analysis of a sodium channel beta1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J. Neurosci. 22 10699–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows L., Malhotra J. D., Stetzer A., Isom L. L., Ragsdale D. S. (2001). The intracellular segment of the sodium channel beta 1 subunit is required for its efficient association with the channel alpha subunit. J. Neurochem. 76 1871–1878 10.1046/j.1471-4159.2001.00192.x [DOI] [PubMed] [Google Scholar]
- Messner D., Catterall W. (1985). The sodium channel from rat brain. Separation and characterisation of subunits. J. Biol. Chem. 260 10597–10604. [PubMed] [Google Scholar]
- Moran O., Conti F. (2001). Skeletal muscle sodium channel is affected by an epileptogenic β1 subunit mutation. Biochem. Biophys. Res. Commun. 282 55–59 10.1006/bbrc.2001.4502 [DOI] [PubMed] [Google Scholar]
- Moran O., Conti F., Tammaro P. (2003). Sodium channel heterologous expression in mammalian cells and the role of the endogenous β1-subunits. Neurosci. Lett. 336 175–179 10.1016/S0304-3940(02)01284-3 [DOI] [PubMed] [Google Scholar]
- Morgan K., Stevens E., Shah B., Cox P., Dixon A., Lee K., et al. (2000). β3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. U.S.A. 97 2308–2313 10.1073/pnas.030362197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namadurai S., Balasuriya D., Rajappa R., Wiemhöfer M., Stott K., Klingauf J., et al. (2014). Crystal structure and molecular imaging of the Nav channel β3 subunit indicates a trimeric assembly. J. Biol. Chem. 289 10797–10811 10.1074/jbc.M113.527994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I., Nakayama T., Yamagata T., Ohtani H., Mazaki E., Tsuchiya S., et al. (2012). A homozygous mutation of voltage-gated sodium channel β(I) gene SCN1B in a patient with Dravet syndrome. Epilepsia 53 e200–e203 10.1111/epi.12040 [DOI] [PubMed] [Google Scholar]
- Oguni H., Hayashi K., Osawa M., Awaya Y., Fukuyama Y., Fukuma G., et al. (2005). Severe myoclonic epilepsy in infancy: clinical analysis and relation to SCN1A mutations in a Japanese cohort. Adv. Neurol. 95 103–117. [PubMed] [Google Scholar]
- Orrico A., Galli L., Grosso S., Buoni S., Pianigiani R., Balestri P., et al. (2009). Mutational analysis of the SCN1A, SCN1B and GABRG2 genes in 150 Italian patients with idiopathic childhood epilepsies. Clin. Genet. 75 579–581 10.1111/j.1399-0004.2009.01155.x [DOI] [PubMed] [Google Scholar]
- Patiño G. A., Brackenbury W. J., Bao Y., Lopez-Santiago L. F., O’Malley H. A., Chen C., et al. (2011). Voltage-gated Na+ channel β1B: a secreted cell adhesion molecule involved in human epilepsy. J. Neurosci. 31 14577–14591 10.1523/JNEUROSCI.0361-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patiño G. A., Claes L. R. F., Lopez-Santiago L. F., Slat E. A., Dondeti R. S. R., Chen C., et al. (2009). A functional null mutation of SCN1B in a patient with Dravet syndrome. J. Neurosci. 29 10764–10778 10.1523/JNEUROSCI.2475-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D., Isom L., Catterall W., Goldin A. (1994). The adult rat brain b1 subunit modifies activation and inactivation gating of multiple sodium channel α subunits. J. Biol. Chem. 269 17640–17655. [PubMed] [Google Scholar]
- Priori S. G., Napolitano C., Gasparini M., Pappone C., Della Bella P., Giordano U., et al. (2002). Natural history of Brugada syndrome: insights for risk stratification and management. Circulation 105 1342–1347 10.1161/hc1102.105288 [DOI] [PubMed] [Google Scholar]
- Qin N., D’Andrea M. R., Lubin M., Shafaee N., Codd E. E., Correa A. M. (2003). Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit. Eur. J. Biochem. 270 4762–4770 10.1046/j.1432-1033.2003.03878.x [DOI] [PubMed] [Google Scholar]
- Ratcliffe C. F., Westenbroek R. E., Curtis R., Catterall W. A. (2001). Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154 427–434 10.1083/jcb.200102086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riuró H., Campuzano O., Arbelo E., Iglesias A., Batlle M., Pérez-Villa F., et al. (2014). A missense mutation in the sodium channel β1b subunit reveals SCN1B as a susceptibility gene underlying long QT syndrome. Heart Rhythm 11 1202–1209 10.1016/j.hrthm.2014.03.044 [DOI] [PubMed] [Google Scholar]
- Rusconi R., Combi R., Cestèle S., Grioni D., Franceschetti S., Dalprà L., et al. (2009). A rescuable folding defective Nav1.1 (SCN1A) sodium channel mutant causes GEFS+: common mechanism in Nav1.1 related epilepsies? Hum. Mutat. 30 E747–E760 10.1002/humu.21041 [DOI] [PubMed] [Google Scholar]
- Rusconi R., Scalmani P., Cassulini R. R., Giunti G., Gambardella A., Franceschetti S., et al. (2007). Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J. Neurosci. 27 11037–11046 10.1523/JNEUROSCI.3515-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer I. E., Harkin L. A., Grinton B. E., Dibbens L. M., Turner S. J., Zielinski M. A., et al. (2007). Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain 130 100–109 10.1093/brain/awl272 [DOI] [PubMed] [Google Scholar]
- Smits J. P. P., Eckardt L., Probst V., Bezzina C. R., Schott J. J., Remme C. A., et al. (2002). Genotype-phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A-related patients from non-SCN5A-related patients. J. Am. Coll. Cardiol. 40 350–356 10.1016/S0735-1097(02)01962-9 [DOI] [PubMed] [Google Scholar]
- Spampanato J., Aradi I., Soltesz I., Goldin A. L. (2004). Increased neuronal firing in computer simulations of sodium channel mutations that cause generalized epilepsy with febrile seizures plus. J. Neurophysiol. 91 2040–2050 10.1152/jn.00982.2003 [DOI] [PubMed] [Google Scholar]
- Srinivasan J., Schachner M., Catterall W. (1998). Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl. Acad. Sci. U.S.A. 95 15753–15757 10.1073/pnas.95.26.15753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y., Ogiwara I., Hoshi A., Yamakawa K., Ugawa Y. (2012). Different degrees of loss of function between GEFS+ and SMEI Nav 1.1 missense mutants at the same residue induced by rescuable folding defects. Epilepsia. 53 e111–e114 10.1111/j.1528-1167.2012.03467.x [DOI] [PubMed] [Google Scholar]
- Tammaro P., Conti F., Moran O. (2002). Modulation of sodium current in mammalian cells by an epilepsy-correlated β1-subunit mutation. Biochem. Biophys. Res. Commun. 291 1095–1101 10.1006/bbrc.2002.6570 [DOI] [PubMed] [Google Scholar]
- Thompson C. H., Porter J. C., Kahlig K. M., Daniels M. A., George A. L. J. (2012). Nontruncating SCN1A mutations associated with severe myoclonic epilepsy of infancy impair cell surface expression. J. Biol. Chem. 287 42001–42008 10.1074/jbc.M112.421883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. H., Scheffer I. E., Parasivam G., Barnett S., Wallace G. B., Sutherland G. R., et al. (2002). Generalized epilepsy with febrile seizures plus: mutation of the sodium channel subunit SCN1B. Neurology 58 1426–1429 10.1212/WNL.58.9.1426 [DOI] [PubMed] [Google Scholar]
- Wallace R., Wang D., Singh R., Scheffer I., George A., Phillips H., et al. (1998). Febrile seizures and generalized epilepsy associated with mutation of the Na+-channels β1-subunit gene SCN1B. Nat. Genet. 19 366–370 10.1038/448 [DOI] [PubMed] [Google Scholar]
- Watanabe H., Darbar D., Kaiser D. W., Jiramongkolchai K., Chopra S., Donahue B. S., et al. (2009). Mutations in sodium channel β1- and β2-subunits associated with atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2 268–275 10.1161/CIRCEP.108.779181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Koopmann T. T., Le Scouarnec S., Yang T., Ingram C. R., Schott J., et al. (2008). Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Invest. 118 2260–2268 10.1172/JCI33891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer V. C., Reid C. A., Mitchell S., Richards K. L., Scaf B. B., Leaw B. T., et al. (2010). Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. J. Clin. Invest. 120 2661–2671 10.1172/JCI42219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M., Cassé-Perrot C., Dravet C. (2006). Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia 47(Suppl. 2), 45–48 10.1111/j.1528-1167.2006.00688.x [DOI] [PubMed] [Google Scholar]
- Xu R., Thomas E. A., Gazina E. V., Richards K. L., Quick M., Wallace R. H., et al. (2007). Generalized epilepsy with febrile seizures plus-associated sodium channel beta1 subunit mutations severely reduce beta subunit-mediated modulation of sodium channel function. Neuroscience 148 164–174 10.1016/j.neuroscience.2007.05.038 [DOI] [PubMed] [Google Scholar]
- Yu F. H., Westenbroek R. E., Silos-Santiago I., McCormick K. A., Lawson D., Ge P., et al. (2003). Sodium channel beta4 a new disulfide-linked auxiliary subunit with similarity to beta2. J. Neurosci. 23 7577–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]