ABSTRACT
A subset of basal cells (BCs) in the initial segment (IS) of the mouse epididymis has a slender body projection between adjacent epithelial cells. We show here that these projections occasionally cross the apical tight junctions and are in contact with the luminal environment. Luminal testicular factors are critical for the establishment of the IS epithelium, and we investigated their role in the regulation of this luminal sensing property. Efferent duct ligation (EDL) was performed to block luminal flow from the testis without affecting blood flow. Cytokeratin 5 (KRT5) labeling showed a time-dependent reduction of the percentage of BCs with intercellular projections from 1 to 5 days after EDL, compared to controls. Double labeling for caspase-3 and KRT5 showed that a subset of BCs undergoes apoptosis 1 day after EDL. Ki67/KRT5 double labeling showed a low rate of BC proliferation under basal conditions. However, EDL induced a marked increase in the proliferation rate of a subset of BCs 2 days after EDL. A 2-wk treatment with the androgen receptor antagonist flutamide did not affect the number of BCs with intercellular projections, but reduced BC proliferation. Flutamide treatment also reduced the increase in BC proliferation induced 2 days after EDL. We conclude that, in the adult mouse IS, 1) luminal testicular factors play an important role in the ability of BCs to extend their body projection towards the lumen, and are essential for the survival of a subset of BCs; 2) androgens play an important role in the proliferation of some of the BCs that survive the initial insult induced by EDL; and 3) the formation and elongation of BC intercellular projections do not depend on androgens.
Keywords: androgens, androgen receptor, apoptosis, epididymis, male reproductive tract
INTRODUCTION
The epididymis, located downstream of the testis and efferent ducts, is the site where spermatozoa undergo several maturation steps and are stored. It is formed by a single convoluted tubule and is divided into four distinct regions—the initial segment (IS), caput, corpus, and cauda epididymidis—according to their morphology, physiology, histology, and function [1, 2]. The epididymal epithelium consists of several major cell types (principal cells, narrow cells, clear cells, and basal cells [BCs]), which work in a concerted manner to create the optimum luminal environment for sperm maturation, transport, and storage [1, 3–5]. An elaborate intercellular communication network contributes to the regulation of various transport mechanisms in the epididymis [3, 6–10]. In other pseudostratified epithelia, including the trachea and the prostate, BCs have been shown to self-renew and have the capacity to differentiate into several epithelial cell types [11–14]. However, in the epididymis the potential role of BCs as progenitor cells still remains to be examined. Epididymal BCs have been proposed to participate in transepithelial fluid transport, either directly via aquaporin 3, which is expressed in these cells [15], or indirectly via the paracrine regulation of principal cells [6, 7]. In all epididymal segments, BCs are located at the base of the epithelium, where they nestle underneath other epithelial cell types. However, BCs can also extend a long and narrow projection between adjacent epithelial cells and in the direction of the lumen [8, 16]. We previously showed that in the rat epididymis, these intercellular BC extensions can in fact cross tight junctions (TJs) to sample the luminal environment and regulate proton secretion in neighboring clear cells in a paracrine manner [8]. During postnatal development, we found that BCs undergo significant morphological plasticity, and that in the adult rat BCs have an intercellular projection mainly in the corpus and cauda regions [8, 17]. BCs, therefore, appear to be integral players in the epididymal epithelium, and investigating the maintenance of their phenotype and morphology will increase our understanding of their role in spermatozoa maturation.
We recently reported that, in the mouse epididymis, BCs send an intercellular projection mainly in the IS [18]. Impairment of the IS epithelium's ability to differentiate and function properly leads to the production of dysfunctional spermatozoa, resulting in male infertility [19–25]. The most abundant and studied cell type in this segment is the principal cell, which requires lumicrine testicular factors to differentiate into a fully mature state [26–31]. However, very little is known on the factors that regulate the plasticity and function of BCs. The purpose of the present study was, therefore, to investigate the regulation of BCs in the mouse epididymis. We focused on the IS, where BC morphology is compatible with the luminal sensory role that was previously shown in the rat [8]. Efferent duct ligation (EDL) was performed to examine the role of luminal factors on the maintenance of BC mature characteristics, including elongation of their intercellular extensions, apoptosis, and proliferation. Finally, we examined the role of androgens in the regulation of BCs under normal conditions and after EDL.
MATERIALS AND METHODS
Animals
Adult C57BL/CBAF1 male mice were purchased from Jackson Laboratories. All procedures described were reviewed and approved by the Massachusetts General Hospital (MGH) Subcommittee on Research Animal Care and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were performed on at least five mice for each group.
Efferent Duct Ligation
Unilateral EDL surgeries were performed to prevent lumicrine factors from entering the epididymis, as described previously by Palladino and Hinton [32]. Each mouse was anesthetized with isoflurane (3%–4%, inhaled) in oxygen and was subjected to unilateral EDL using sterile techniques as follows. The testis was accessed through a low midline incision. Efferent ducts were exposed and a needle leading a 4-0 nylon suture was passed around the ducts. Care was taken to avoid damage to nearby blood vessels. The suture was tied tightly around the efferent ducts, and the testes and epididymides were placed back into the abdomen. The surgical site was closed using nylon sutures.
Antiandrogen Treatment of Adult Mice
Adult (10-wk-old) male mice were divided into a control group (n = 5) and a flutamide-treated group (n = 5). For the flutamide-treated group, mice received a dose of 20 mg/kg per day for 2 wk as described previously [33]. One pellet (containing 10 mg), designed to release flutamide continuously for 21 days (Innovative Research), was implanted subcutaneously into the dorsal side of the neck of each mouse. A sham procedure was carried out in control mice. To investigate the combined effects of flutamide and EDL, mice were treated with flutamide for 14 days and EDL was performed with continuous infusion of flutamide for an additional 2 days. The mice were killed and the seminal vesicles, testis, and epididymis were harvested. All organs were weighed to determine the efficacy of the treatment.
Tissue Fixation and Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (7.5 mg/100 g body weight, i.p.) and the male reproductive organs were harvested, fixed by immersion in periodate-lysine-paraformaldehyde containing 4% paraformaldehyde for 5 h at room temperature, and rinsed three times in PBS, as we described previously [8, 17, 18, 34]. Tissues were then incubated in a solution of 30% sucrose in PBS for at least 24 h. Tissues were embedded in OCT compound (Tissue-Tek; Sakura Finetek), mounted on a cutting block, and frozen. The tissue was then cut at 16-μm thickness using a Leica 3050 cryostat (Leica Microsystems) and sections were placed onto Fisher Superfrost/Plus microscope slides (Fisher Scientific). Sections were hydrated and heated by microwaving in an alkaline buffer (Vector Laboratory) three times for 2 min each time, with 5-min intervals for antigen retrieval. To block nonspecific binding, 1% bovine serum albumin in PBS was applied for 30 min at room temperature. The sections were incubated with primary antibodies in a moist chamber for 90 min at room temperature or overnight at 4°C. The samples were washed in PBS and incubated with secondary antibodies for 60 min at room temperature. The following primary antibodies were used: rabbit monoclonal anti-cytokeratin 5 (KRT5) (1:150; Thermo, Fremont, CA), rat monoclonal anti-Ki67, clone SolA 15, (1:200; ebioscience), and rabbit polyclonal anti-caspase 3 (1:200; Cell Signaling). The anti-ZO-1 rat monoclonal antibody (1:10) was a gift from Dr. Eveline Schneeberger (Department of Pathology, MGH). All secondary antibodies used in this study were affinity purified and were obtained from Jackson ImmunoResearch Laboratories. Secondary antibodies included fluorescein isothiocyanate- and CY3-conjugated donkey or goat anti-rabbit IgG and CY3-conjugated donkey or goat anti-rat IgG. All antibodies were diluted in Dako antibody diluent (Dako).
Quantification of BC Intercellular Projections
BC intercellular projections were quantified in epididymis 16-μm sections immunolabeled for KRT5 from at least five mice in each group. The epididymal region 1, corresponding to the IS (identified by connection with the efferent ducts), was analyzed. EDL-treated mice were analyzed 1, 2, and 5 days after EDL. The projections of BCs were measured and divided into subcategories according to their length, similarly to what we have previously described in the rat epididymis [8]. They were designated as “long” when they reached the apical pole of the epithelium, defined as the region located above the row of adjacent principal cell nuclei. These long projections included those that reached all the way to the luminal border of the epithelium. Projections were designated as “short” when they did not pass the nucleus of adjacent principal cells. Ten-micrometer stacks of z-series wide-field immunofluorescence images were acquired at 2-μm intervals using a 90i Nikon microscope with a 40× objective. Images were analyzed using Volocity software (v.6.3.1; PerkinElmer).
Quantification of BC Proliferation
BC proliferation was quantified in epididymis sections that were double immunolabeled for KRT5 and Ki-67. At least five control and five experimental mice were examined at each time point after EDL (1, 2, and 5 days). Digital images were acquired using a 40× objective on a Nikon 90i microscope and were analyzed using Volocity software. The percentage of proliferating BCs was determined as the number of cells that were positive for both KRT5 and Ki67 compared to the total number of BCs (positive for KRT5) per tubule cross section.
Statistical Analysis
The numeric data were analyzed using GraphPad Prism (version 5; GraphPad Software Inc.) using one-way ANOVA or a two-tailed unpaired t-test. Values are presented as mean ± SEM.
RESULTS
BCs with an Intercellular Projection Are Present in the IS of the Mouse Epididymis
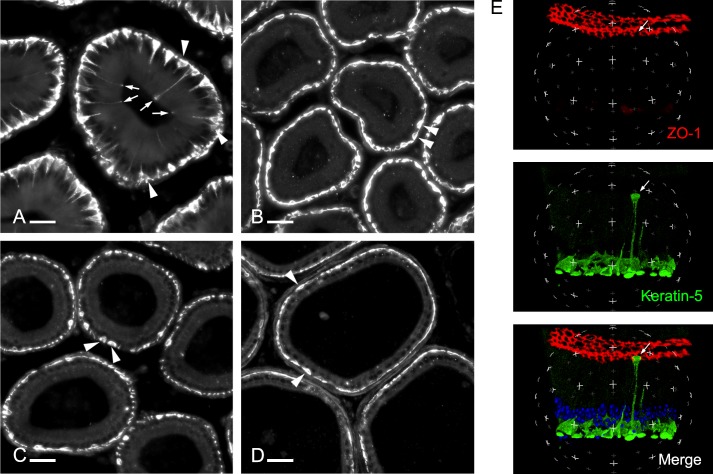
Mouse epididymis sections including the IS, caput, corpus, and cauda epididymidis were labeled for KRT5, a marker of BCs [17, 18]. As we previously showed [18], a subset of BCs located in the IS has a narrow body projection that extends between other epithelial cells toward the lumen (Fig. 1A). These projections have different lengths, and whereas some are long and reach the apical border of the epithelium (arrows), some are short and do not extend above the row of principal cell nuclei (arrowheads). In contrast, almost all BCs in the caput (Fig. 1B), corpus (Fig. 1C), and cauda (Fig. 1D) have a flat appearance and no detectable projection (arrowheads). Double labeling for KRT5 and the TJ marker, ZO-1, showed that some of the long BC projections cross the TJs and are, therefore, in contact with the luminal side of the epithelium (Fig. 1E, arrow; see also Supplemental Movie S1 [Supplemental Data are available online at www.biolreprod.org]). This result shows that BCs have the ability to send a projection that reaches the luminal compartment in the most proximal region of the mouse epididymis.
FIG. 1.
Mouse epididymis cryosections labeled for the BC marker, keratin 5 (KRT5), showing the IS (A), caput (B), corpus (C), and cauda (D) regions. Arrows indicate BCs that extend a narrow body projection toward the lumen. Arrowheads indicate BCs with no intercellular projections. BC projections are present in the IS. Bars = 30 μm. E) A 3D reconstruction of a stack of z-series optical sections acquired by laser scanning microscopy, showing a BC whose projection crossed the TJs and is in contact with the luminal content (arrow). Other BCs are mainly located beneath adjacent epithelial cells. TJs are labeled for ZO-1 in red, and BCs are labeled for KRT5 (green). Nuclei and spermatozoa are labeled with DAPI (blue). (See also Supplemental Movie S1.)
EDL Reduces the Number of BCs with an Intercellular Projection
Unilateral EDL was performed to determine whether luminal factors regulate the formation and extension of BC projections in the IS. Care was taken to avoid blockage of the blood vessels that are closely associated with the efferent ducts. EDL induced the retraction of BC long (arrows) and short (arrowheads) projections in a time-dependent manner (Fig. 2, A–D). Quantification analysis (Fig. 2E and Table 1) showed that EDL induced a progressive reduction in the percentage of BCs with intercellular projections (including short and long; open bars) and in the percentages of BCs that had long (gray bars) and short projections (black bars). Hatched bars indicate the nonprojecting BCs. These results indicate that luminal factors regulate the intercellular projections observed in BCs in the IS.
FIG. 2.
Reduction of BC projections in the IS after EDL. Control (A, A′) and 1 (B, B′), 2 (C, C′), and 5 days (D, D′) after EDL. BCs were labeled for KRT5. In A and B, the arrows indicate BCs with long projections that reach the luminal border of the epithelium. The arrowheads in A indicate BCs with short projections that do not reach the apical border of the epithelium. In B, C, and D, the arrowheads indicate BCs with no projections. In A′, B′, C′, and D′, BCs are shown in green, and nuclei and spermatozoa are labeled with DAPI (blue). Bars = 30 μm. E) White bars: percentage of all BCs with projections. Gray bars: percentage of BCs with long projections detected at the epithelial apical border. Black bars: percentage of BCs with short projections. Hatched bars: percentage of BCs with no projections. The numbers of BCs counted/total number of BCs (including cells with no projection) are indicated above bars. Data are represented as mean ± SEM.
TABLE 1.
The retraction of the BC projection after EDL.
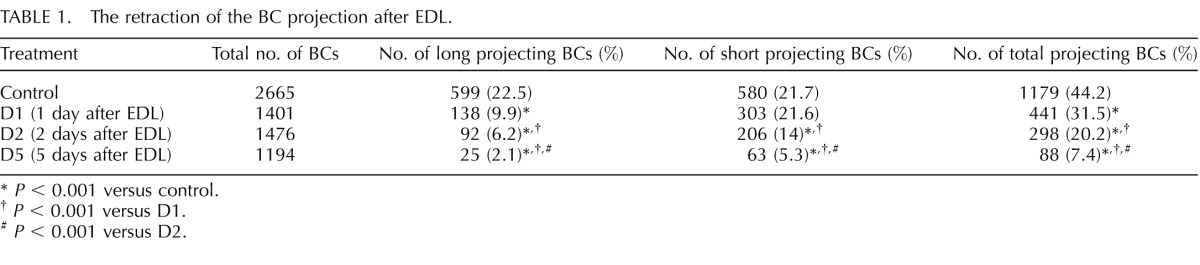
P < 0.001 versus control.
P < 0.001 versus D1.
P < 0.001 versus D2.
Modulation of BC Death and Proliferation after EDL
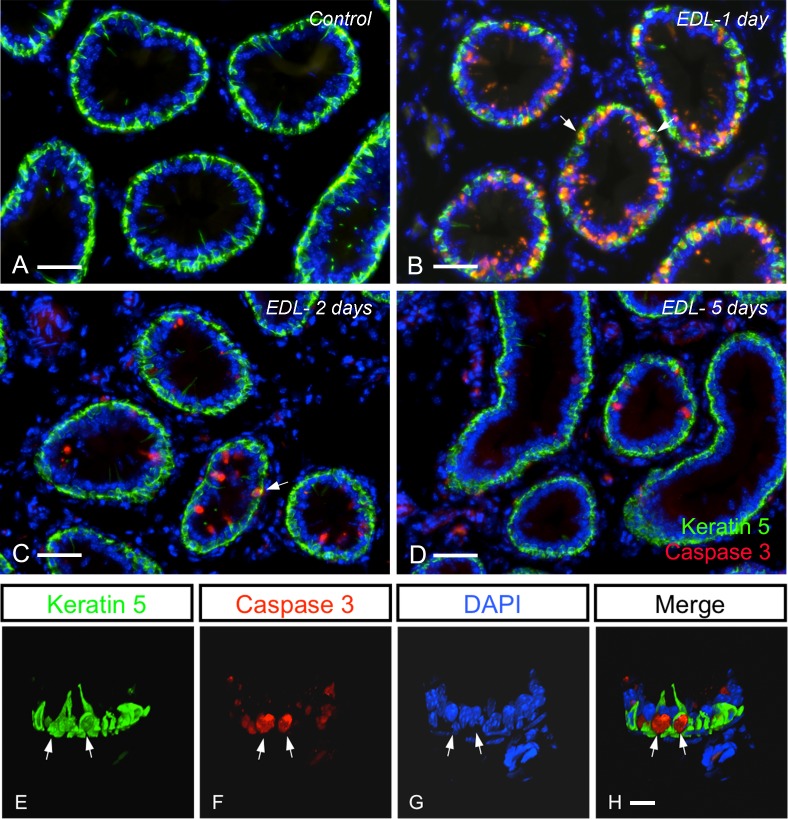
Previous studies have shown that EDL induces significant alterations of principal cells, including apoptosis [26–31]. We examined here whether EDL affected apoptosis and proliferation in BCs by performing double labeling for KRT5 and caspase 3 or Ki67, respectively. In control mice, overall very rare caspase 3-positive epithelial cells were detected (Fig. 3A shows no apoptotic cells). The number of apoptotic cells dramatically increased 1 day after EDL (Fig. 3B), and then progressively decreased 2 and 5 days after EDL (Fig. 3, C and D). Although several apoptotic cells were principal cells (KRT5-negative), as previously reported [26, 35], some BCs were also positive for caspase 3 (Fig. 3B, arrows). Higher-magnification 3D reconstruction clearly showed two caspase 3-positive BCs 1 day after EDL (Fig. 3, E–H; see also Supplemental Movie S2).
FIG. 3.
Mouse IS double labeled for caspase 3 (red) and KRT5 (green) showing that EDL causes a wave of apoptosis. Control (A) and 1 (B), 2 (C), and 5 days (D) after EDL. Apoptotic principal cells and apoptotic BCs (arrows; see also Supplemental Movie S2) are detected 1 day after EDL (B). Some apoptotic principal cells and BCs are seen 2 days after EDL (C). A few apoptotic principal cells are still detected 5 days after EDL. Bars = 50 μm. E–H) A 3D reconstruction of BCs double labeled for caspase 3 and KRT5, 1 day after EDL. Arrows show two apoptotic BCs. Bars = 10 μm. Nuclei and spermatozoa are labeled with DAPI (blue).
A different pattern was observed for the rate of proliferation of epithelial cells induced after EDL. Although a few proliferating epithelial cells, including Ki67-positive BCs (Fig. 4A, arrows) were shown in control mice, the number of Ki67-positive cells significantly decreased 1 day after EDL (Fig. 4B). Interestingly, a dramatic increase in the number of proliferating BCs was detected 2 days after EDL (Fig. 4C, arrows), but only rare proliferating principal cells were detected at this time point. Very few proliferating cells were detected 5 days after EDL (Fig. 4D). Figure 4, E–H, shows a high-magnification 3D reconstruction of a proliferating BC detected 2 days after EDL (see also Supplemental Movie S3). Quantification confirmed the marked increase in the number of proliferating BCs from 2% under control conditions to 12% 2 days after EDL and the return back to a low proliferative state (0.4%) 5 days after EDL (Fig. 4I). These data show modulation of apoptotic cell death and proliferation patterns of BCs after EDL.
FIG. 4.
Effect of EDL on BC proliferation. IS double labeled for Ki67 (red) and KRT5 (green) under control conditions (A) and 1 (B), 2 (C), and 5 days (D) after EDL. Arrows indicate proliferating BCs (positive for both KRT5 and Ki67). An increase of proliferating BCs is seen 2 days after EDL. Bars = 50 μm. E–H) A 3D reconstruction of BCs double labeled for Ki67 and KRT5, 2 days after EDL (arrow shows a proliferating BC). Bars = 10 μm (see also Supplemental Movie S3). Nuclei and spermatozoa are labeled with DAPI (blue). I) Percentage of proliferating BCs in the IS. A significant decrease of BC proliferation was detected 1 and 5 days after EDL compared to control. In contrast, a dramatic increase in the percentage of proliferating BCs was observed 2 days after EDL compared to control. Data are represented as means ± SEM. *P < 0.05 and **P < 0.001 versus control.
Androgens Are Essential for BC Proliferation but Not for the Formation of Intercellular Projections
The effect of androgens on the maintenance of BC characteristics was examined in the steady-state epididymis. Treatment of adult mice with the antiandrogen drug flutamide for a period of 2 wk significantly reduced the weight of the seminal vesicles and epididymides (Table 2), as previously published [17, 36–38], thus validating the treatment. The number of BCs with long and short projections (Fig. 5, A–C) was not affected by flutamide, nor was the percentage of BCs with no projections (48.6% in controls and 49.2% after flutamide treatment). BCs with an intercellular projection were absent at birth (not shown) and started to appear during Postnatal Week 3 (Fig. 5D). Their number progressively increased during Postnatal Weeks 4 (Fig. 5E) and 5 (Fig. 5F).
TABLE 2.
Seminal vesicle weight, testis weight, epididymis weight, and body weight (BW) in adult control and flutamide-treated mice.*
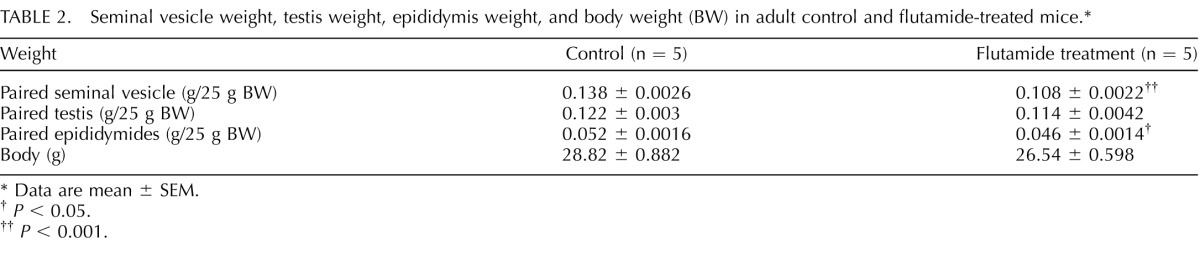
Data are mean ± SEM.
P < 0.05.
P < 0.001.
FIG. 5.
Androgens do not affect BC projections in the mouse epididymis. IS showing BCs labeled for KRT5 under control conditions (A) and after a 2-wk flutamide treatment (B). Both groups show a normal number of BCs with an intercellular projection. Bars = 50 μm. C) Percentage of BCs with short and long projections in the IS in controls versus flutamide-treated mice. The numbers of BCs counted/total number of BCs are indicated above each bar. Both long and short projection patterns appear normal after flutamide treatment. Data are represented as means ± SEM. D–F) IS of a 3- (D), 4- (E) and 5-wk-old (F) mouse showing a progressive increase in the number of BCs that have an intercellular projection (arrows). The hashed lines delineate the luminal border of the epithelium. ED, efferent ducts.
Flutamide induced a significant decrease in the number of proliferating BCs compared to control (Fig. 6, A–C). Higher magnification showed that proliferation of BCs was detected in both projecting (Fig. 6a′) and nonprojecting (Fig. 6a″) BCs. These data indicate that androgens do not appear to be essential for the regulation of BC projections, but are necessary for their proliferation.
FIG. 6.
Androgens are essential for BC proliferation. Epididymides stained for KRT5 (green) and Ki67 (red) from control (A, a′, a″) and flutamide-treated mice (B). Arrows in A indicate Ki67-positive BCs. The arrow in a′ indicates a projecting Ki67-positive BC and the arrow in a″ indicates a nonprojecting Ki67-positive BC. No Ki67-positive BCs were detected in B. Bars = 150 μm. C) Percentage of proliferating BCs in the IS. The percentage of proliferating BCs was determined as the number of cells that were positive for both KRT5 and Ki67 compared to the total number of BCs (positive for KRT5) per tubule cross section. Flutamide treatment induces a significant reduction of BC proliferation compared to control. *P < 0.001 versus control.
Androgens Regulate the Increase in BC Proliferation Observed 2 Days after EDL
We then investigated the role of androgens in the increase in BC proliferation induced by EDL. Mice were treated with flutamide for 2 wk and were then subjected to EDL in the continued presence of flutamide for an additional 2 days. These mice were compared to either mice that were treated with flutamide for 2 wk + 2 days with no EDL, control mice, or mice subjected to EDL for 2 days without flutamide treatment. The weight of the seminal vesicles was significantly reduced but the epididymis weight was not affected in the flutamide + EDL group compared to the group subjected to EDL only (Table 3). As shown above, a marked increase in BC proliferation was observed 2 days after EDL in the absence of flutamide (Fig. 7, A, A′, B, B′, and E), and flutamide treatment significantly lowered the percentage of proliferating BCs observed under basal conditions (Fig. 7, C, C′, and E). When EDL was performed in the presence of flutamide, an increase in BC proliferation was observed, but this increase was much lower than that observed in the absence of flutamide (Fig. 7, D, D′, and E). These results indicate that androgens regulate the induction of BC proliferation observed 2 days after EDL.
TABLE 3.
Seminal vesicle weight, testis weight, epididymis weight, and body weight (BW) in EDL and flutamide + EDL mice.*

Data are mean ± SEM.
P < 0.002.
FIG. 7.
Androgens are necessary for the increase in BC proliferation induced 2 days after EDL. IS double labeled for Ki67 (red) and KRT5 (green). A–D show Ki67 staining only and A′–D′ are merge panels showing both Ki67 and KRT5 labeling for epididymides from mice under control (Ctl) conditions (A, A′), after EDL (B, B′), after flutamide (Flu) treatment (C, C′), and after both EDL and Flu treatment (D, D′). Arrows indicate Ki67-positive BCs. E) Percentage of proliferating BCs in the IS. The percentage of proliferating BCs was determined as the number of cells that were positive for both KRT5 and Ki67 compared to the total number of BCs (positive for KRT5) per tubule cross section. A higher percentage of BC proliferation was detected in the EDL-only group, and in the combined Flu/EDL group (Flu + EDL) compared to Ctl. A lower percentage of proliferating BCs was detected in the Flu-treated group compared to Ctl. The percentage of proliferating BCs detected in the EDL group was significantly higher compared to the Flu + EDL group. Bars = 30 μm. Data are represented as means ± SEM. *P < 0.01 versus control. †P < 0.01 versus EDL.
DISCUSSION
We recently reported that epididymal BCs send an intercellular projection in a region-specific manner depending on the species examined. In the mouse, BC projections are present in the IS, whereas in the rat they are present in the corpus and cauda regions [8, 16–18]. The present study shows that, similarly to the rat, BC projections in the mouse epididymis occasionally cross the TJ barrier and are in direct contact with the luminal content. We found a marked decrease in the number of BCs with intercellular projection after EDL, showing that testicular lumicrine factors are essential regulators of the maintenance of their projections. In addition, EDL induced a significant remodeling of the IS epithelium, including a wave of apoptosis in several BCs 1 day after EDL, followed by an increase in the proliferation rate of a subset of BCs after 2 days.
Luminal-Reaching Property
The absence of effect of flutamide on the number of BCs with intercellular projections in adult mice suggests that androgens are not directly involved in the formation of these projections. This is supported by our result showing that BC intercellular projections appear early after birth, before the increase in androgens that accompanies puberty. The reduction in the number of BCs with intercellular projections that we observed after EDL suggests that luminal factors maintain the luminal-sensing property of BCs in the mouse IS. In contrast, we previously showed a significant decrease of BC projections in the corpus epididymidis of rats treated with flutamide for 2 wk [17], indicating that the more distal regions might rely on androgens for the maintenance of epithelial differentiation. In agreement with this notion, treatment of rats with inhibitors of 5a-reductase to prevent DHT formation resulted in reduced expression of IGF1 and FGF10 in the corpus and cauda regions specifically [39], indicating that these receptors might be involved in the region-specific androgen-dependent plasticity of BCs. BCs in the epididymis and prostate express EGF and FGF10 receptors [40, 41] indicating that they might be involved in the region-specific androgen-dependent plasticity of BCs. In addition, it will be interesting to test whether the extracellular signal-regulated kinase (ERK) pathway is involved in the regulation of intercellular projections in BCs, as EDL results in the inhibition of this pathway [31, 42]. Whether or not ROS1, which is essential for differentiation of the IS epithelium [25, 43], also regulates the formation and elongation of intercellular projections in BCs is currently being investigated in our laboratory. Activation of this pathway coincides with the arrival of testicular fluid in the IS, indicating that one of the lumicrine factors might be a ligand for ROS1 [31, 42].
Luminal factors might regulate the formation of BC projections either via a direct effect on BCs or indirectly via cross talk with principal cells. We found that not all BCs are in contact with the luminal compartment at a specific time point, and we might ask how these cells can be directly modulated by lumicrine factors. BCs form a dense network at the basolateral side of the epithelium and are connected with each other via gap junctions [16, 44, 45]. It is possible that a few luminal-sampling BCs could serve as sentinel cells that would initiate signaling that could propagate to adjacent BCs, therefore sustaining a more generalized response. Alternatively, we previously postulated that BCs use claudin 1 as a molecular ladder to extend their intercellular projection along the side of adjacent principal cells [8]. Principal cells also express claudin 1 [46] and they are highly affected by EDL. Under these conditions, they might, therefore, no longer provide the support that is required for BCs to send their intercellular projections.
Another unanswered question is, “Why do BCs send their intercellular projections in a region-specific manner and why do these regions differ in the rat versus the mouse epididymis?” Interestingly, a detailed analysis of gene expression in the different segments of the mouse and rat epididymis showed that the mouse IS and the rat corpus and cauda epididymides express a subset of common genes [47]. Additional studies will be required to determine which of these genes (if any) might participate in the regulation of the luminal-sensing property of BCs.
Proliferation and Apoptosis
In agreement with previous studies, we showed a low level of proliferation in all cell types of the IS under control conditions, confirming the stable nature of this epithelium [48–50]. In this segment, epithelial cell proliferation is regulated by androgens and growth factors [42]. Interestingly, we observed an even lower proliferative activity in BCs after flutamide treatment, showing the contribution of androgens in the maintenance of this cell type in the steady-state epididymis. Very few cells showed apoptotic activity in the control tissue, and flutamide alone did not affect apoptosis (data not shown). Consistent with previous reports [26, 29, 35, 51–53], we observed a wave of apoptosis in principal cells of the IS, which reached a maximum level 1 day after EDL. A wave of apoptosis in principal cells has previously been observed following orchidectomy, although in that study a maximum level was observed 2 days postorchidectomy [27]. In contrast, we found that EDL did not induce apoptosis or proliferation in narrow cells (data not shown), in agreement with a previous study showing no change in their ultrastructure after castration [54]. Interestingly, a subset of the BCs that survived the initial insult caused by EDL showed increased proliferative activity, with a maximum level reached after 2 days. Undifferentiation of BCs after EDL might have triggered an increase in their proliferation rate. However, the remaining BCs continued to express the differentiation marker KRT5, indicating that they did not regress to a completely undifferentiated state. The retraction of their intercellular projections together with their increased proliferation rate indicates that they might have regressed to an intermediate state. Although flutamide treatment reduced the proliferative effect of EDL in BCs, an increase in proliferation was still observed compared to the control (no EDL, no flutamide) group and the flutamide-only group. These results indicate that androgens may have a proproliferative effect on BCs, similarly to what was observed previously for principal cells [55]. Conversely, luminal factors may have an overall antiproliferative action on BCs.
Lumicrine factors keep the ERK1/2 pathway in a highly activated state in this segment [31, 42], and it was, therefore, surprising that proliferative activity was maintained low despite activation of this “prosurvival” pathway [56]. A balance between the proproliferative ERK pathway and the antiproliferative action of the tumor suppressors PTEN (phosphatase and TENSIN homolog) and RB1 (retinoblastoma 1), which are also highly expressed in the IS, was proposed to maintain the low proliferative activity in this segment [42, 57]. The increase in apoptosis, followed by an increase in principal cell and BC proliferation that was observed after EDL, in the present study and others, indicates dynamic changes in this balance. This is consistent with the rapid reduction of ERK1/2 phosphorylation (phospho-ERK1/2) and increase in PTEN expression that was observed after EDL, which would further tip the balance towards an antiproliferative state shortly after EDL [31]. Interestingly, androgen replacement cannot prevent EDL-induced apoptosis in principal cells, indicating the participation of other factors in this response or that androgen replacement does not replace luminal androgen [52]. Among the lumicrine factors that are secreted by the testis, the fibroblast growth factor (FGF) h shown to play an important role in IS principal cell survival by regulating phospho-ERK1/2 and dual-specificity phosphatase 6 (Dusp6) [31, 42]. In addition, the orphan receptor tyrosine kinase ROS1 was proposed to play a key role in the differentiation and maintenance of the IS epithelium. ROS1 KO male mice have an undifferentiated IS epithelium and produce immotile spermatozoa [20, 25]. We recently showed that deletion of ROS1 kinase activity in ROS1KM/KM mice inhibits the ERK1/2 pathway, leading to failure of the IS to differentiate and male infertility [43]. Interestingly, we observed similar proliferation indexes in IS epithelial cells in both ROS1KM/KM and WT mice during postnatal development and in adults, indicating that the ROS1 pathway does not have a direct role in cell proliferation. All these studies focused on principal cells in the IS, and future research will be required to determine whether or not these hypothesis also apply to BCs.
In other epithelia, including the airways and the prostate, BCs are progenitor cells involved in the renewal of other epithelial cell populations [11–13, 58–61]. Lineage-tracing studies will be required to determine whether BCs have a similar function in the epididymis. In particular, it will be interesting to determine whether the BCs that survive the initial damage caused by EDL represent a subpopulation of BCs that are necessary for the maintenance of the epithelium, and to identify the factors that stimulate their proliferation.
In conclusion, the present study suggests that luminal testicular factors might have an antiproliferative action, and androgens have a proliferative effect on BCs in the IS of the mouse epididymis. In addition, we showed that testicular lumicrine factors sustain the formation and elongation of BC intercellular projections, which do not appear to require the action of androgens.
Footnotes
Current address: ShanghaiTech University School of Life Science and Technology, Pudong Zhang-Jiang Hi-Tech Park, Haike Road No. 100 Research Center, Shanghai 201210, China.
Supported by NIH grants RO1HD040793 and RO1DK097124 (to S.B.). N.D.S is supported by NIH grant RO1HD069623. The Microscopy Core facility of the MGH Program in Membrane Biology receives support from the Boston Area Diabetes and Endocrinology Research Center (DK57521) and the Center for the Study of Inflammatory Bowel Disease (DK43351). S.B. is a recipient of the Charles and Ann Sanders Research Scholar Award at MGH.
REFERENCES
- Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis In Neill JD. (ed.), Knobil and Neill's Physiology of Reproduction, 3rd ed New York: Elsevier; 2006. 1071 1148 [Google Scholar]
- Jones RC. Evolution of the vertebrate epididymis. J Reprod Fertil Suppl. 1998;53:163–181. [PubMed] [Google Scholar]
- Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. J Androl. 2011;32:576–586. doi: 10.2164/jandrol.111.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell Tissue Res. 2012;349:717–731. doi: 10.1007/s00441-012-1381-0. [DOI] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Leung GP, Leung MC, Shum WW, Zhou WL, Wong PY. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol. 2005;125:443–454. doi: 10.1085/jgp.200409205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung GP, Cheung KH, Leung CT, Tsang MW, Wong PY. Regulation of epididymal principal cell functions by basal cells: role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1) Mol Cell Endocrinol. 2004;216:5–13. doi: 10.1016/j.mce.2003.10.077. [DOI] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–1117. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleannee C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to the apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. 2010;298:C817–C830. doi: 10.1152/ajpcell.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl. 2004;25:494–505. doi: 10.1002/j.1939-4640.2004.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl. 1993;14:23–44. [PubMed] [Google Scholar]
- Shum WW, Hill E, Brown D, Breton S. Plasticity of basal cells during postnatal development in the rat epididymis. Reproduction. 2013;146:455–469. doi: 10.1530/REP-12-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod. 2014 doi: 10.1095/biolreprod.113.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Gödecke S, Birchmeier C. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–1193. doi: 10.1101/gad.10.10.1184. [DOI] [PubMed] [Google Scholar]
- Yeung C, Sonnenberg-Riethmacher E, Cooper T. Receptor tyrosine kinase c-ros knockout mice as a model for the study of epididymal regulation of sperm function J Reprod Fertil Suppl 1998. 53 137. [PubMed] [Google Scholar]
- Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, Huhtaniemi I. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology. 2011;152:689–696. doi: 10.1210/en.2010-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, Sipila P. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling PLoS One 2012. 7 e38457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara L, Welsh M, Saunders PT, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152:718–729. doi: 10.1210/en.2010-0928. [DOI] [PubMed] [Google Scholar]
- Sipila P, Cooper TG, Yeung CH, Mustonen M, Penttinen J, Drevet J, Huhtaniemi I, Poutanen M. Epididymal dysfunction initiated by the expression of simian virus 40 T-antigen leads to angulated sperm flagella and infertility in transgenic mice. Mol Endocrinol. 2002;16:2603–2617. doi: 10.1210/me.2002-0100. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Breton S, Setiawan I, Xu Y, Lang F, Cooper TG. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H+-ATPase. Mol Reprod Dev. 2004;68:159–168. doi: 10.1002/mrd.20067. [DOI] [PubMed] [Google Scholar]
- Abe K, Takano H. Early degeneration of the epithelial cells in the initial segment of the epididymal duct in mice after efferent duct cutting. Arch Histol Cytol. 1989;52:299–310. doi: 10.1679/aohc.52.299. [DOI] [PubMed] [Google Scholar]
- Fan X, Robaire B. Orchidectomy induces a wave of apoptotic cell death in the epididymis. Endocrinology. 1998;139:2128–2136. doi: 10.1210/endo.139.4.5888. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- Nicander L, Osman DI, Ploen L, Bugge HP, Kvisgaard KN. Early effects of efferent ductule ligation on the proximal segment of the rat epididymis. Int J Androl. 1983;6:91–102. doi: 10.1111/j.1365-2605.1983.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Turner TT, Johnston DS, Finger JN, Jelinsky SA. Differential gene expression among the proximal segments of the rat epididymis is lost after efferent duct ligation Biol Reprod 2007. 77 165 171 Published online ahead of print March 21, 2007. [DOI] [PubMed] [Google Scholar]
- Xu B, Abdel-Fattah R, Yang L, Crenshaw SA, Black MB, Hinton BT. Testicular lumicrine factors regulate ERK, STAT, and NFKB pathways in the initial segment of the rat epididymis to prevent apoptosis. Biol Reprod. 2011;84:1282–1291. doi: 10.1095/biolreprod.110.090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MA, Hinton BT. Expression of multiple gamma-glutamyl transpeptidase messenger ribonucleic acid transcripts in the adult rat epididymis is differentially regulated by androgens and testicular factors in a region-specific manner. Endocrinology. 1994;135:1146–1156. doi: 10.1210/endo.135.3.7915228. [DOI] [PubMed] [Google Scholar]
- Ilagan R, Zhang LJ, Pottratz J, Le K, Salas S, Iyer M, Wu L, Gambhir SS, Carey M. Imaging androgen receptor function during flutamide treatment in the LAPC9 xenograft model. Mol Cancer Ther. 2005;4:1662–1669. doi: 10.1158/1535-7163.MCT-05-0197. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker H-C, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–663. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T, Riley T. p53 independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Mol Reprod Dev. 1999;53:188–197. doi: 10.1002/(SICI)1098-2795(199906)53:2<188::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Isnard-Bagnis C, Herak-Kramberger C, Sabolic I, Van Hoek A, Brown D, Breton S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol Reprod. 2002;66:1716–1722. doi: 10.1095/biolreprod66.6.1716. [DOI] [PubMed] [Google Scholar]
- Caflisch CR. Effect of a nonsteroidal antiandrogen, flutamide on intraluminal acidification in rat testis and epididymis. Andrologia. 1993;25:363–367. doi: 10.1111/j.1439-0272.1993.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Paris F, Weinbauer GF, Blum V, Nieschlag E. The effect of androgens and antiandrogens on the immunohistochemical localization of the androgen receptor in accessory reproductive organs of male rats. J Steroid Biochem Mol Biol. 1994;48:129–137. doi: 10.1016/0960-0760(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Henderson NA, Cooke GM, Robaire B. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5alpha-reductase inhibition. J Endocrinol. 2006;190:779–791. doi: 10.1677/joe.1.06862. [DOI] [PubMed] [Google Scholar]
- Cotton LM, O'Bryan MK, Hinton BT. Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev. 2008;29:193–216. doi: 10.1210/er.2007-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube E, Dufresne J, Chan PT, Cyr DG. Epidermal growth factor regulates connexin 43 in the human epididymis: role of gap junctions in azoospermia. Hum Reprod. 2012;27:2285–2296. doi: 10.1093/humrep/des164. [DOI] [PubMed] [Google Scholar]
- Xu B, Yang L, Lye RJ. Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biol Reprod. 2010;83:807–817. doi: 10.1095/biolreprod.110.085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun HJ, Roy J, Smith TB, Wood LB, Lane K, Woolfenden S, Punko D, Bronson RT, Haigis KM, Breton S, Charest A. ROS1 signaling regulates epithelial differentiation in the epididymis. Endocrinology. 2014;155:3661–3673. doi: 10.1210/en.2014-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG. Connexins and pannexins. Spermatogenesis. 2011;1:325–338. doi: 10.4161/spmg.1.4.18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C, Nashan D, Sorg C, Oberpenning F, Schulze H, Nieschlag E, Cooper T. Basal cells of the human epididymis—antigenic and ultrastructural similarities to tissue-fixed macrophages. Biol Reprod. 1994;50:917–926. doi: 10.1095/biolreprod50.4.917. [DOI] [PubMed] [Google Scholar]
- Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–863. doi: 10.1210/endo.142.2.7975. [DOI] [PubMed] [Google Scholar]
- Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, Wilson E, Brown EL, Kopf GS, Johnston DS. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–570. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Flannery J. Mitotic activity in the epithelium of the epididymis in young and old adult rats. Biol Reprod. 1970;3:283–292. doi: 10.1093/biolreprod/3.3.283. [DOI] [PubMed] [Google Scholar]
- Majumder G, Turkington R. Regulation by testosterone and serum protein of DNA synthesis in the developing epididymis of the rat. J Endocrinol. 1976;70:105–115. doi: 10.1677/joe.0.0700105. [DOI] [PubMed] [Google Scholar]
- Nagy F, Edmonds RH. Cellular proliferation and renewal in the various zones of the hamster epididymis after colchicine administration. Fertil Steril. 1975;26:460–468. doi: 10.1016/s0015-0282(16)41118-0. [DOI] [PubMed] [Google Scholar]
- Robaire B, Fan X. Regulation of apoptotic cell death in the rat epididymis J Reprod Fertil Suppl 1998. 53 211. [PubMed] [Google Scholar]
- Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod. 1979;20:162–181. doi: 10.1095/biolreprod20.2.162. [DOI] [PubMed] [Google Scholar]
- Smith TB, Cortez-Retamozo V, Grigoryeva LS, Hill E, Pittet MJ, Da Silva N. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology. 2014;2:755–762. doi: 10.1111/j.2047-2927.2014.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Bedford J. Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat Rec. 1979;193:293–311. doi: 10.1002/ar.1091930209. [DOI] [PubMed] [Google Scholar]
- Hamzeh M, Robaire B. Effect of testosterone on epithelial cell proliferation in the regressed rat epididymis. J Androl. 2009;30:200–212. doi: 10.2164/jandrol.108.006171. [DOI] [PubMed] [Google Scholar]
- Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805–817. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci U S A. 2013;110:8105–8110. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]