Abstract
Background
Evidence on the effect of cardiorespiratory fitness (CRF) on age-related longitudinal changes of lipids and lipoproteins is scarce.
Objectives
This study sought to assess the longitudinal, aging trajectory of lipids and lipoproteins for the life course in adults, and to determine whether CRF modifies the age-associated trajectory of lipids and lipoproteins.
Methods
Data came from 11,418 men, 20 to 90 years of age, without known high cholesterol, high triglycerides, cardiovascular disease, and cancer at baseline and during follow-up from the Aerobics Center Longitudinal Study. There were 43,821 observations spanning 2 to 25 (mean 3.5) health examinations between 1970 and 2006. CRF was quantified by a maximal treadmill exercise test. Marginal models using generalized estimating equations were applied.
Results
Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and non-high-density lipoprotein cholesterol (non-HDL-C) presented similar inverted U-shaped quadratic trajectories with aging: gradual increases were noted until the mid-40s to early 50s, with subsequent declines (all p < 0.0001). Compared to men with higher CRF, those with lower CRF developed abnormal values earlier in life: TC (≥200 mg/dl), LDL-C (≥130 mg/dl), non-HDL-C (≥160 mg/dl), and TG/HDL-C ratio (≥3.0). Notably, abnormal values for TC and LDL-C in men with low CRF were observed around 15 years earlier than in those with high CRF. After adjusting for time-varying covariates, a significant interaction was found between age and CRF in each trajectory, indicating that CRF was more strongly associated with the aging trajectories of lipids and lipoproteins in young to middle-aged men than in older men.
Conclusions
Our investigation reveals a differential trajectory of lipids and lipoproteins with aging according to CRF in healthy men, and suggests that promoting increased CRF levels may help delay the development of dyslipidemia.
Keywords: aging, longitudinal study, lipid profile trajectories
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide (1). Mortality rates from CVD have declined, but its associated burden is persistently high in the United States (2). Dyslipidemia, representing unfavorable blood lipid profiles, plays an important role in the development and progression of coronary heart disease (CHD)(3). Multiple epidemiological studies have demonstrated that high levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), and lower high-density lipoprotein cholesterol (HDL-C) are important lipid risk factors for CHD (4–7). Existing evidence also suggests that elevated triglycerides (TG) are an independent risk factor for CHD (8).
Age-related changes in lipid and lipoprotein concentrations are overall unfavorable. For example, TC, LDL-C, and TG increase up to middle age, then decrease (9–14), whereas the change in HDL-C with aging is not consistent (11–16). Most previous studies had substantial limitations including cross-sectional study design (9,11), restricted age range (14–16), relatively small sample size (10,12,14,16), nonfasting samples (13), and a lack of analysis assessing time-varying covariates (12,15).
Strong evidence suggests that physical activity is a major modifiable lifestyle factor for preventing dyslipidemia (17–20). Meta-analyses and systematic reviews support that aerobic physical activity reduces LDL-C and non-HDL-C, with no consistent effect observed on TG and HDL-C (17). However, an increasing effect on HDL-C with specific amounts of exercise and decreasing effects on TG has been reported in healthy, middle-aged men and in the overweight/obese population (20,21). Previous studies have shown that an improved cardiorespiratory fitness (CRF), an objective indicator of habitual physical activity, resulted in a more favorable lipoprotein-lipid profile, with different effects across age groups (18,19). However, there is little evidence on the impact of CRF on age-related changes in lipids and lipoproteins. Because age-related changes in the lipid profile are mostly unfavorable, the aim of the current study is to identify the age-related trajectory for lipids and lipoproteins and, due to its important public health and clinical implications, to explore factors that might modify the trajectory.
More specifically, using data from the Aerobics Center Longitudinal Study (ACLS), we assessed the longitudinal aging trajectory for TC, LDL-C, TG, HDL-C and non-HDL-C, and determined whether CRF modifies these trajectories in healthy men.
Methods
Study population
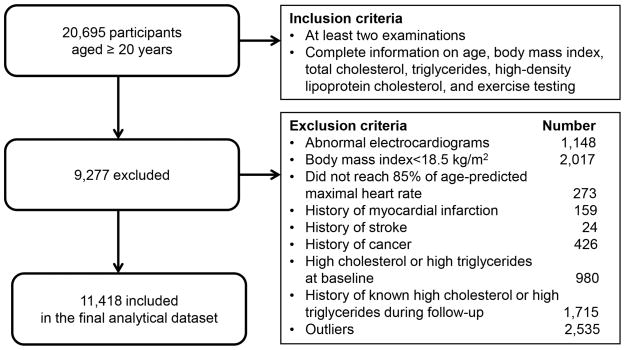
The ACLS is a prospective study of adults who received extensive preventive medical examinations at the Cooper Clinic in Dallas, Texas. The study participants are mainly non-Hispanic whites (>95%) and college graduates from middle-to-upper socioeconomic strata. Our analyses included male participants who received at least 2 medical examinations (2 to 25 visits; mean number of visits = 3.5) between 1970 and 2006. All study participants had complete data on TC, HDL-C, and TG at baseline, normal resting and exercising electrocardiograms, a body mass index (BMI) ≥18.5 kg/m2, and were able to reach 85% of age-predicted maximal heart rate during the treadmill tests at each visit. We excluded participants who had a history of myocardial infarction, stroke, and cancer at baseline and during the follow-up. In addition, participants with self-reported high cholesterol or high triglycerides during any visit were excluded to remove potential treatment effects on lipid and lipoprotein levels. Finally, a total of 11,418 individuals with 43,821 observations were analyzed (Figure 1). The study protocol was approved annually by the Cooper Institute Institutional Review Board and all participants gave their informed consent for the baseline examination and follow-up study.
Figure 1.
Participant Flow Diagram
Assessment of CRF
CRF was measured using a modified Balke protocol (22), and details of the measurements are described elsewhere (23). Exercise treadmill duration on this protocol is highly correlated with measured peak oxygen uptake (VO2max) in men (24). Treadmill time, expressed in metabolic equivalents (METs), corresponds to maximal aerobic power and is considered an objective laboratory measure of CRF. In a previous study using ACLS data, CRF was demonstrated to decline at a nonlinear rate with aging (23). Thus, CRF was standardized for age, and the study subjects were further categorized into low (lower than 33.3th percentile), middle (those within 33.3th to 66.7th percentile) and high CRF categories (higher than 66.7th percentile) according to the distribution of age-standardized CRF at baseline.
Assessment of clinical and life-style related variables
Details of the clinical examination in ACLS are described elsewhere (23,25,26). Examinations were conducted after an overnight fast of at least 12 h. Serum TC, TG, HDL-C, and fasting plasma glucose (FPG) were analyzed by automated laboratory techniques in the Cooper Clinic in accordance with quality control standards of Centers for Disease Control and Prevention lipid standardization program. Blood pressure (BP) was measured with mercury manometers after at least 5 min of quiet sitting. Body mass index (BMI) was calculated from measured weight and height in accordance with standard procedures. Waist circumference (WC) was measured at the level of the umbilicus. Underwater weighing, sum of skin fold-thickness, or both were used to estimate percent body fat (% BF). When available, hydrostatically estimated % BF was chosen (26,27). A standardized questionnaire was used to obtain information about age, smoking, drinking alcohol, physical activity habit, and past and family history of numerous health indicators.
Statistical analysis
Statistical analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, North Carolina) and R software (R Development Core Team). Data were expressed as means ± standard deviations for continuous variables and as proportions for categorical variables. Marginal models were fitted for the longitudinal data and the generalized estimating equations (GEE) approach was applied, which has the main advantage of providing a robust inference of the regression coefficients, which is valid regardless of whether or not the correlation structure is correctly specified (28). An autoregressive moving average (ARMA)(1,1) model was chosen after careful comparison of popular correlation structures on the basis of log likelihood ratio tests (29). Dependent variables for each marginal model were TC, LDL-C, TG, HDL-C, and non-HDL-C. Gamma distribution was applied for TG levels due to its positive skewness. Using the EMPIRICAL option in the PROC GLIMMIX statement, the classical sandwich estimators were obtained as covariance estimators for fixed effects to ensure a consistent estimator.
In model 1, we evaluated the age-related longitudinal change in TC, LDL-C, TG, HDL-C, and non-HDL-C. Linear and quadratic models were investigated altogether. In model 2, the influence of CRF was also examined. The models were further adjusted for time-varying covariates including WC, % BF, FPG, systolic and diastolic BP, smoking status, drinking alcohol, and physical inactivity. Under the estimated correlation matrix, CRF was found to be highly correlated with WC and % BF, respectively, which may indicate collinearity. This may influence the association between CRF and the age-related trajectories of lipids and lipoproteins. To reduce possible collinearity issues, all marginal models were fitted with WC and % BF as responses, and with CRF as a predictor. Then, the residuals of these 2 variables with variations unexplained by CRF were included in the multivariable models with other covariates. In addition, all models were fitted after adjusting for the baseline exam time as a proxy variable for the birth cohort.
We applied a log-likelihood ratio test to assess whether the model improved the fit. In order to compare models, we also used the Bayesian information criterion (BIC) and Akaike information criterion (AIC) to determine the balance between obtaining a good fit of the model to the data and yielding precise parameter estimates. All statistical tests were 2-sided, where p < 0.05 was accepted as statistical significance.
For the graphical presentation of age-related trajectories for lipids and lipoproteins, the crude overall trajectories with aging under model 1 were examined. To compare these trajectories among low, middle, and high CRF categories, WC representing abdominal obesity was also considered with a constant WC residual (−0.179, mean of the residual), along with age, age-squared, baseline exam date, CRF, and an age·CRF interaction term. Cutoffs for abnormal lipids and lipoproteins were set at: ≥200 mg/dl for TC; ≥130 mg/dl for LDL-C; ≥160 mg/dl for non-HDL-C; and ≥3.0 for TG/HDL-C ratio (3,30,31).
Sensitivity analyses
In order to eliminate the possible influence of abnormal metabolic status or potential behavioral changes on the trajectories, a sensitivity analysis was performed among a subgroup of 5,554 participants, after excluding subjects with abnormal lipid and lipoprotein profiles at baseline (those having ≥240 mg/dl TC, ≥160 mg/dl LDL-C, ≥200 mg/dl TG, or <40 mg/dl HDL-C), or known diabetes or hypertension at baseline and during follow-ups. To investigate the effects of diet on the trajectories for lipids and lipoproteins, another subgroup analysis was conducted among 2,361 participants who had records of low-fat or low-cholesterol dietary habits.
Results
Study participants
Baseline characteristics stratified according to 3 CRF categories are presented in Table 1. A higher CRF at baseline was inversely associated with TC, LDL-C, TG, non-HDL-C, TG/HDL-C ratio, TC/HDL-C ratio, and LDL-C/HDL-C ratio; and was positively associated with HDL-C. The lower CRF category had higher BMI, WC, % BF, FPG, systolic and diastolic BP; and increased prevalence of diabetes, hypertension, current smoking, physical inactivity, and parental history of cardiovascular disease (CVD). Table 2 presents the number of participants, number of visits, and follow-up time by age group. Compared to younger individuals, older individuals tended to have longer durations of follow-up (p < 0.0001).
Table 1.
Descriptive Baseline Characteristics for the Subjects According to the Categories of CRF
| Variables | Overall (n = 11,418) | Categories of CRF
|
p for Linear Trend | ||
|---|---|---|---|---|---|
| Low (n = 5,130) | Middle (n = 3,625) | High (n = 2,663) | |||
| Age, yrs | 43.8 ± 9.0 | 44.2 ± 8.7 | 43.9 ± 9.0 | 43.0 ± 9.5 | <0.0001 |
| Total cholesterol, mg/dl | 202.6 ± 36.1 | 207.6 ± 36.8 | 201.6 ± 34.9 | 194.4 ± 34.6 | <0.0001 |
| LDL-C, mg/dl | 132.3 ± 32.5 | 136.2 ± 32.8 | 132.0 ± 31.7 | 125.1 ± 31.6 | <0.0001 |
| TG, mg/dl | 117.7 ± 79.0 | 140.9 ± 94.3 | 107.6 ± 64.2 | 86.8 ± 43.6 | <0.0001 |
| HDL-C, mg/dl | 47.1 ± 12.0 | 43.9 ± 11.0 | 48.0 ± 11.7 | 52.1 ± 12.4 | <0.0001 |
| Non-HDL-C, mg/dl | 155.5 ± 37.1 | 163.7 ± 37.5 | 153.6 ± 35.5 | 142.3 ± 34.4 | <0.0001 |
| TG/HDL-C ratio | 2.88 ± 3.28 | 3.67 ± 4.02 | 2.53 ± 2.83 | 1.84 ± 1.28 | <0.0001 |
| TC/HDL-C ratio | 4.57 ± 1.43 | 5.00 ± 1.51 | 4.43 ± 1.32 | 3.91 ± 1.07 | <0.0001 |
| LDL-C/HDL-C ratio | 2.99 ± 1.09 | 3.27 ± 1.13 | 2.92 ± 1.03 | 2.54 ± 0.92 | <0.0001 |
| Maximal METS | 12.4 ± 2.3 | 10.5 ± 1.2 | 12.8 ± 0.8 | 15.4 ± 1.6 | <0.0001 |
| Treadmill time duration, min | 19.5 ± 4.6 | 15.5 ± 2.6 | 20.6 ± 1.7 | 25.4 ± 2.4 | <0.0001 |
| Height, cm | 179.4 ± 6.5 | 179.1 ± 6.6 | 179.6 ± 6.4 | 179.4 ± 6.4 | 0.0028 |
| Weight, kg | 83.4 ± 12.2 | 87.6 ± 13.6 | 82.0 ± 10.2 | 77.4 ± 8.5 | <0.0001 |
| BMI, kg/m2 | 25.9 ± 3.3 | 27.3 ± 3.7 | 25.4 ± 2.6 | 24.0 ± 2.0 | <0.0001 |
| Waist circumference, cm | 92.3 ± 9.7 | 96.9 ± 10.0 | 90.8 ± 7.8 | 85.7 ± 6.6 | <0.0001 |
| Percent body fat | 20.3 ± 6.1 | 23.4 ± 5.6 | 19.5 ± 5.0 | 15.5 ± 5.0 | <0.0001 |
| Fasting plasma glucose, mg/dl | 98.5 ± 13.3 | 100.1 ± 15.6 | 97.7 ± 12.0 | 96.6 ± 9.2 | <0.0001 |
| Systolic blood pressure (mm Hg) | 119.5 ± 12.6 | 120.3 ± 12.6 | 118.8 ± 12.3 | 119.1 ± 12.7 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 80.0 ± 9.1 | 81.5 ± 9.4 | 79.2 ± 8.9 | 78.2 ± 8.4 | <0.0001 |
| Diabetes | 314 (2.8) | 209 (4.1) | 69 (1.9) | 36 (1.4) | <0.0001 |
| Hypertension | 2,789 (24.4) | 1,540 (30.0) | 785 (21.7) | 464 (17.4) | <0.0001 |
| Current smoker | 1,652 (14.5) | 1,035 (20.2) | 428 (11.8) | 189 (7.1) | <0.0001 |
| Heavy drinker | 762 (6.7) | 321 (6.3) | 239 (6.6) | 202 (7.6) | 0.0319 |
| Physical inactivity | 2,226 (19.5) | 1,764 (34.4) | 374 (10.3) | 88 (3.3) | <0.0001 |
| Parental history of CVD | 3,034 (26.6) | 1,423 (27.7) | 933 (25.7) | 678 (25.5) | 0.0175 |
Data are presented as means ± SD or number (proportions). Linear trend for TG and TG/HDL-C ratio was tested after log-transformation due to excessive skewness. CRF was categorized into low (lower than 33.3th percentile), middle (those within 33.3th to 66.7th percentile) and high CRF categories (higher than 66.7th percentile) according to the distribution of age-standardized CRF at baseline.
BMI = body mass index; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; MET = metabolic equivalent; TC = total cholesterol; TG = triglycerides.
Table 2.
Distribution of Number of Visits and Follow-Up Time by Age Group
| Variables | Overall (n = 11,418) | Age group (years)
|
p for Linear Trend | |||
|---|---|---|---|---|---|---|
| 20–39 (n = 1,711) | 40–49 (n = 4,077) | 50–59 (n = 3,680) | ≥60 (n =1,950) | |||
| Number of visits | 3.8 ± 2.8 | 2.6 ± 1.0 | 3.1 ± 1.7 | 4.1 ± 2.8 | 6.0 ± 4.3 | <0.0001 |
| Follow-up time (yrs) | 5.9 ± 5.4 | 3.1 ± 2.7 | 4.6 ± 3.9 | 6.6 ± 5.4 | 9.9 ± 6.9 | <0.0001 |
Data are presented as means ± SD.
Marginal models for longitudinal change in lipids and lipoproteins with aging
Model 1 was defined as the age-related trajectories of TC, LDL-C, log TG, HDL-C, and non-HDL-C (Table 3). The age-squared term was significant for all lipids and lipoproteins, indicating that longitudinal aging trajectories for lipids and lipoproteins are consistently nonlinear. Age-squared was inversely associated with TC, LDL-C, log TG, and non-HDL-C (all p < 0.0001) and showed a positive relation to HDL-C (p = 0.002). In other words, TC, LDL-C, log TG, and non-HDL-C have inverted U-shaped quadratic trends with aging, meaning that they all tend to increase up to a certain age and then subsequently decrease. In contrast, HDL-C has a U-shaped quadratic trend with aging. When CRF and an interaction term of age·CRF were added to model 2, the model fits were improved on the basis of each lipid and lipoprotein’s AIC and BIC values. In model 2, CRF was inversely associated with TC, LDL-C, TG, and non-HDL-C (all p < 0.0001), and was positively associated with HDL-C (p < 0.0001). The age·CRF interaction was significant (all p < 0.01), indicating that the associations of CRF with lipids and lipoproteins are not consistent across different ages.
Table 3.
Population-Averaged Coefficients and Standard Errors for Age and CRF for Lipids and Lipoproteins From Generalized Estimating Equation Models
| Variable | TC
|
LDL-C
|
Log TG
|
HDL-C
|
Non-HDL-C
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||
| β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | |
| Intercept | 206.6* | 0.3 | 205.9* | 0.3 | 134.6* | 0.3 | 134.1* | 0.3 | 4.80* | 0.01 | 4.76* | 0.01 | 48.07* | 0.12 | 48.40* | 0.11 | 158.5* | 0.4 | 157.5* | 0.3 |
| Age | 0.116* | 0.025 | 0.124* | 0.025 | −0.108* | 0.023 | −0.101* | 0.023 | 0.00306* | 0.00039 | 0.00310* | 0.00037 | 0.1989* | 0.0083 | 0.1965* | 0.0080 | −0.086† | 0.026 | −0.070‡ | 0.025 |
| Age2 | −0.044* | 0.002 | −0.043* | 0.002 | −0.037* | 0.002 | −0.037* | 0.002 | −0.00034* | 0.00003 | −0.00033* | 0.00003 | 0.0015‡ | 0.0005 | 0.0015‡ | 0.0005 | −0.045* | 0.002 | −0.045* | 0.002 |
| Baseline exam date | −0.783* | 0.036 | −0.815* | 0.036 | −0.935* | 0.032 | −0.966* | 0.032 | 0.00126 | 0.00066 | 0.00011 | 0.00059 | 0.1163* | 0.0142 | 0.1404* | 0.0135 | −0.897* | 0.038 | −0.953* | 0.036 |
| CRF | −2.296* | 0.100 | −2.063* | 0.090 | −0.07742* | 0.00174 | 1.3377* | 0.0325 | −3.713* | 0.100 | ||||||||||
| Age × CRF | 0.071* | 0.009 | 0.044* | 0.008 | 0.00063* | 0.00016 | 0.0173* | 0.0030 | 0.052* | 0.009 | ||||||||||
In Table 4, TC, LDL-C, log TG, and non-HDL-C showed persistently inverse associations with age-squared (all p < 0.0001), whereas the significant association with age-squared in HDL-C disappeared after adjusting for all time-varying covariates such as WC, % BF, FPG, systolic and diastolic BP, smoking status, alcohol drinking status, and physical activity habit. CRF also consistently exhibited an inverse association with TC, LDL-C, TG, and non-HDL-C (all p < 0.0001) and a positive association with HDL-C (p <0.0001). The age·CRF interaction was also significant (all p < 0.01).
Table 4.
Population-Averaged Coefficients and Standard Errors for All Predictors for Lipids and Lipoproteins From Generalized Estimating Equation Models
| Variable | TC
|
LDL-C
|
Log TG
|
HDL-C
|
Non-HDL-C
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | β | SE | β | SE | |
| Intercept | 171.6* | 2.4 | 119.8* | 2.1 | 4.12 * | 0.04 | 41.40* | 0.70 | 130.1* | 2.3 |
| Age | −0.082‡ | 0.027 | −0.232* | 0.025 | −0.00083§ | 0.00042 | 0.2085* | 0.0087 | −0.298* | 0.028 |
| Age2 | −0.039* | 0.002 | −0.032* | 0.002 | −0.00026* | 0.00003 | 0.00001 | 0.00051 | −0.039* | 0.002 |
| Baseline exam date | −0.907* | 0.038 | −1.026* | 0.034 | −0.00070 | 0.00060 | 0.1298* | 0.0137 | −1.036* | 0.038 |
| CRF | −2.024* | 0.106 | −1.961* | 0.097 | −0.07237* | 0.00181 | 1.4381* | 0.0340 | −3.521* | 0.105 |
| Age × CRF | 0.068* | 0.010 | 0.038* | 0.009 | 0.00067 * | 0.00016 | 0.0200* | 0.0031 | 0.047* | 0.010 |
| Waist circumference | 0.250* | 0.030 | 0.211* | 0.027 | 0.00914 * | 0.00065 | −0.1648* | 0.0114 | 0.428* | 0.034 |
| Percent body fat | 0.613* | 0.045 | 0.582* | 0.042 | 0.00623 * | 0.00083 | −0.1156* | 0.0153 | 0.729* | 0.046 |
| Fasting glucose | 0.086* | 0.016 | 0.024 | 0.013 | 0.00289 * | 0.00025 | 0.0042 | 0.0044 | 0.083* | 0.015 |
| Diastolic BP | 0.218* | 0.020 | 0.134* | 0.018 | 0.00248 * | 0.00035 | 0.0334* | 0.0060 | 0.184* | 0.019 |
| Systolic BP | 0.061* | 0.015 | 0.005 | 0.014 | 0.00114 * | 0.00026 | 0.0322* | 0.0047 | 0.030§ | 0.015 |
| Current smoker | 0.932 | 0.555 | 0.594 | 0.509 | 0.03612† | 0.00996 | −0.5015‡ | 0.1747 | 1.319§ | 0.552 |
| Heavy drinker | 4.160* | 0.696 | 0.292 | 0.626 | 0.00596 | 0.01015 | 3.1648* | 0.2277 | 0.650 | 0.685 |
| Physical inactivity | 1.999* | 0.378 | 1.314† | 0.347 | 0.00687 | 0.00707 | 0.5160* | 0.1078 | 1.509* | 0.370 |
Waist circumference and percent body fat were normalized by CRF.
p < 0.0001;
p < 0.001;
p < 0.01;
p < 0.05.
BP = blood pressure. Other abbreviations as in Tables 1 and 4.
Graphical trajectories for lipids and lipoproteins with aging
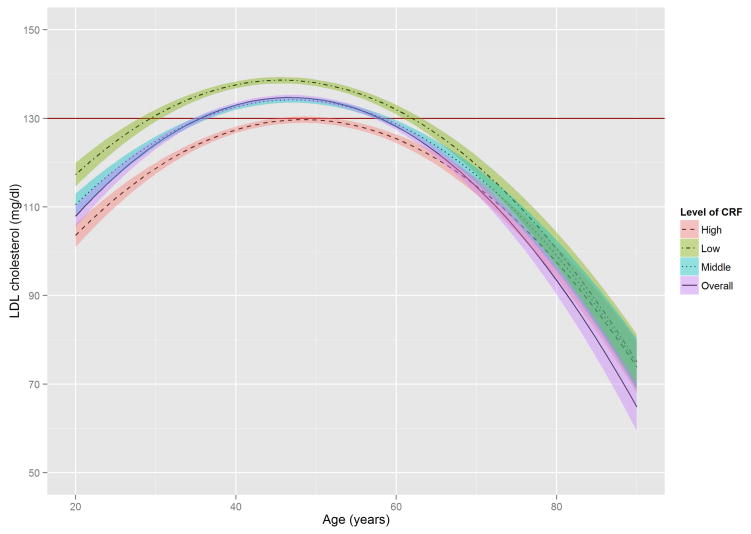
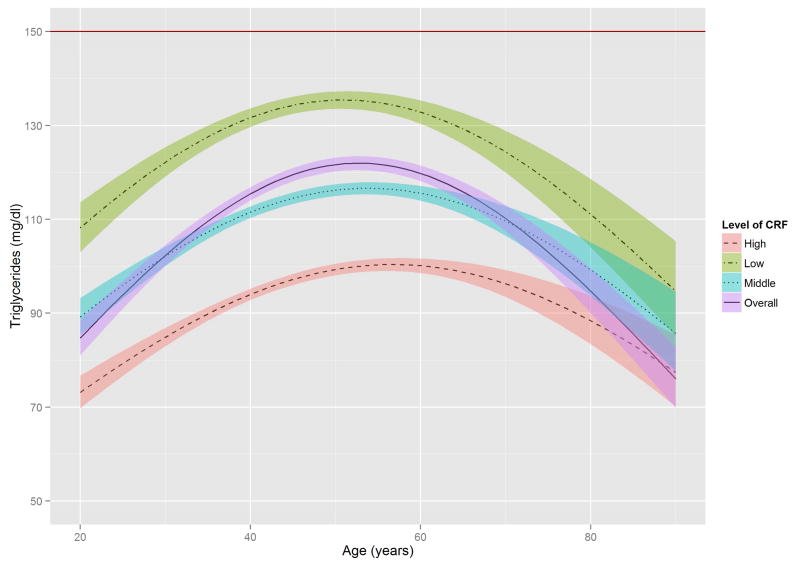
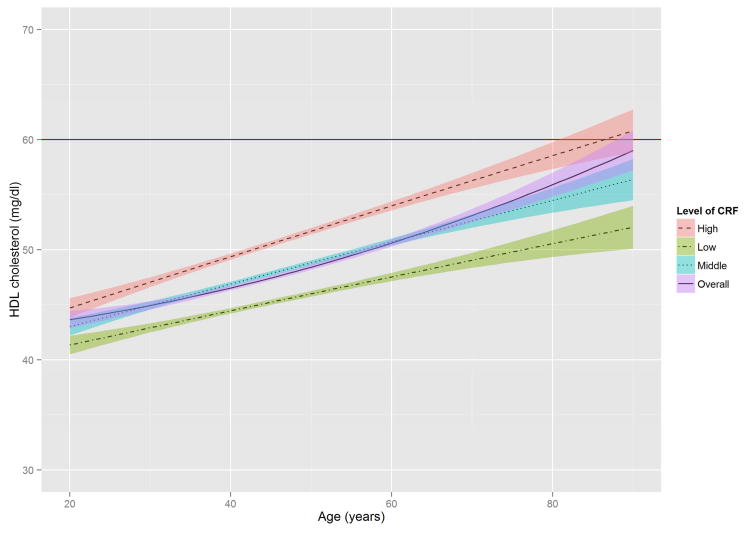
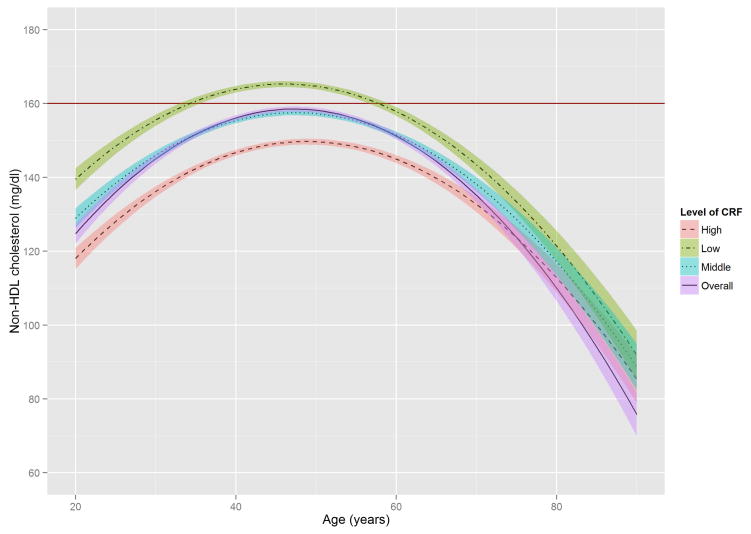
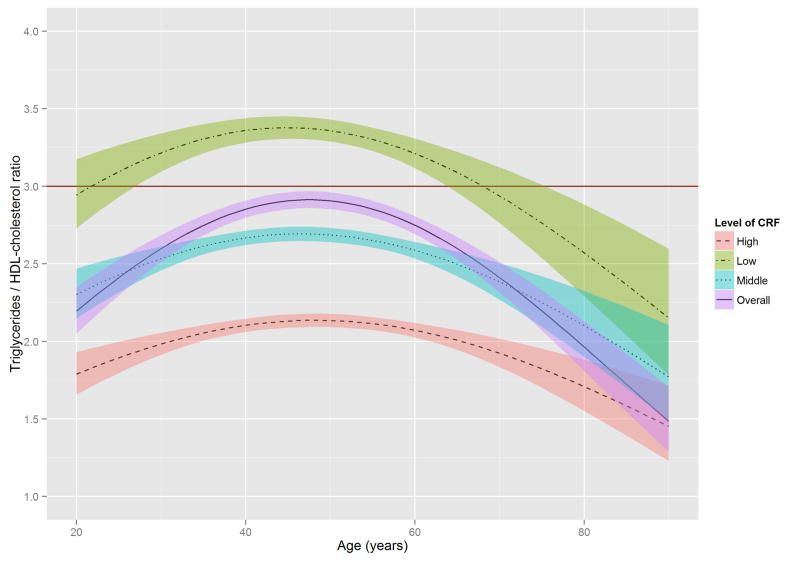
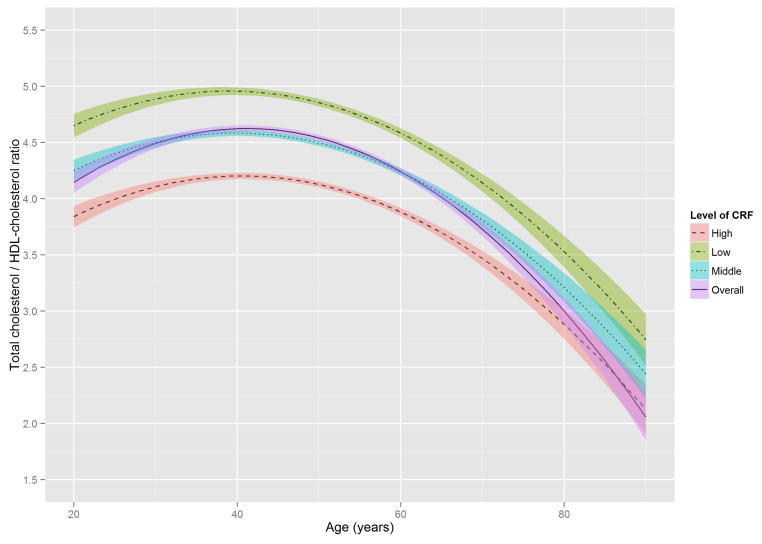
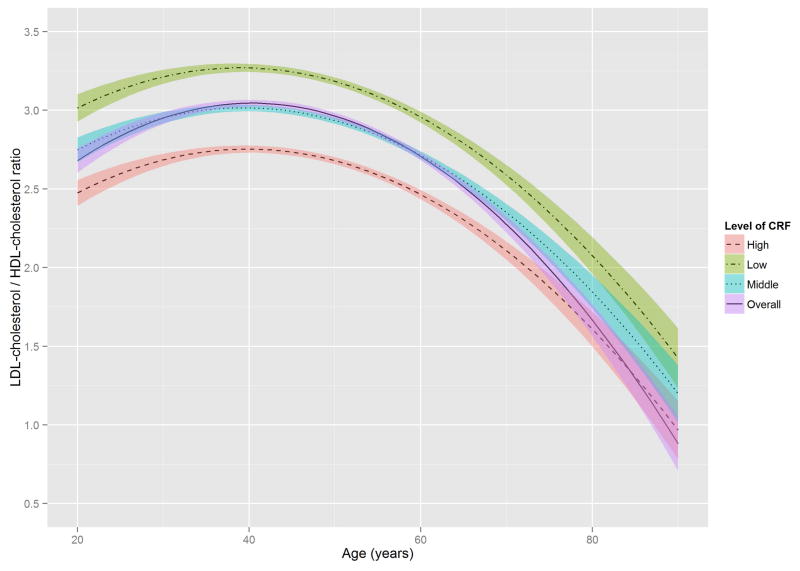
Figure 2 shows the estimated trajectories of lipids and lipoproteins with aging, and corresponding 95% confidence bands on the basis of baseline CRF level. When the WC residual was added to model 2, the estimates of age-squared changed by over 10% in TG, HDL-C, and non HDL-C, and the significant association with age-squared in HDL-C disappeared (Online Table S1). TC and TG tended to increase with age until the early 50s, while LDL-C and non-HDL-C tended to increase with age until around the late 40s, after which decreased trends were observed. In contrast, HDL-C tended to increase slightly over all age periods. All 3 lipid and lipoprotein ratio variables, including TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C tended to increase with age until the early to mid-40s, with decreased trends observed after the 40s.
Figure 2. The Estimated Trajectories of Lipids and Lipoproteins With Aging by Levels of CRF.
The purple solid line represents a crude overall trajectory; the other lines represent each category of CRF (orange dotted-dashed line for high CRF, blue dotted line for middle CRF, and green dashed line for low CRF), assuming constant WC residual (−0.179, mean of the residual). The shaded areas represent 95% confidence intervals for each CRF category. The red horizontal solid line in each panel indicates the established abnormal cutoff for lipid and lipoproteins: (A) LDL-C ≥130 mg/dl; (B) TG ≥150 mg/dl; (C) ≥60 mg/dl for HDL-C; (D) ≥160 mg/dl for non-HDL-C; (E) TG/HDL-C ≥3.0. No abnormal cutoff was established for (F) total cholesterol/HDL-cholesterol ratio and (G) LDL-cholesterol/HDL-cholesterol ratio. CRF was categorized into low (lower than 33.3th percentile), middle (those within 33.3th to 66.7th percentile) and high CRF categories (higher than 66.7th percentile), according to the distribution of age-standardized CRF at baseline.
CRF = cardiorespiratory fitness; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG = triglycerides; WC = waist circumference.
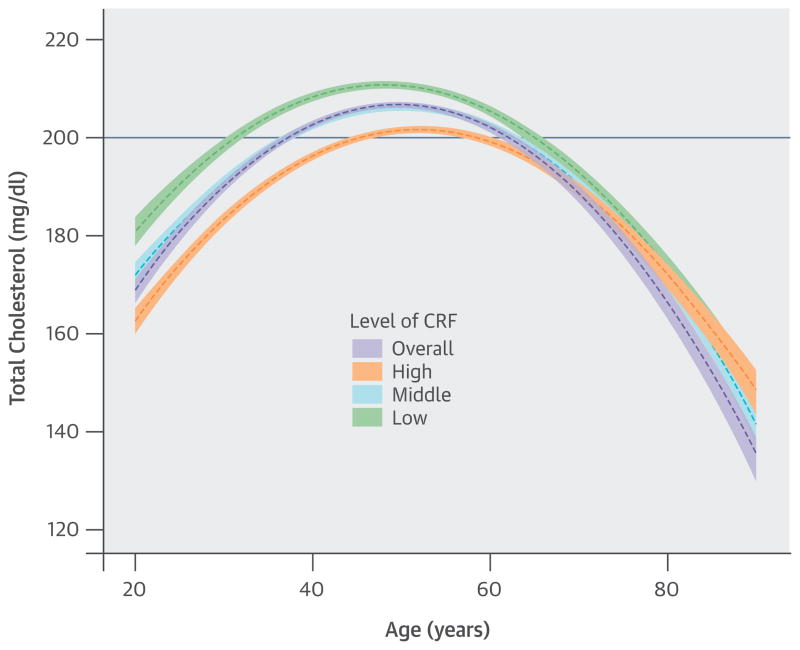
In these trajectories, men with low CRF showed a higher risk of developing abnormal lipid and lipoprotein profiles around the mid-20s through the late 30s. Notably, abnormal values for TC and LDL-C in men with low CRF were observed around 15 years earlier than in those with high CRF. For non-HDL-C and TG/HDL-C ratio, no period for abnormal values was observed in those with high CRF. The trajectories of the TC/HDL-C and LDL-C/HDL-C ratios were similar to those of the TG/HDL-C ratio.
Sensitivity analyses
When data were analyzed from subjects with no abnormal lipid profiles, diabetes, or hypertension at baseline, and no known diabetes or hypertension at follow-ups, the overall results are not very different from those of original dataset. After adjusting for all covariates, TC, LDL-C, TG, and non-HDL-C showed consistently inverse associations with age-squared (all p < 0.0001), indicating inverted U-shaped quadratic trends with aging. There were significant inverse associations of CRF with TC, LDL-C, TG, and non HDL-C and a positive association with HDL-C (all p < 0.0001) (Online Table S2). Similar results were found in the subgroup analysis for subjects with low-fat or low-cholesterol dietary habits (Online Table S3).
Discussion
Our findings from a prospective study of 11,418 men support that CRF is associated inversely with TC, LDL-C, TG, and non-HDL-C, and is associated positively with HDL-C. Men with higher CRF had more favorable lipid profiles than those with lower CRF from their early 20s until their early 60s or mid-70s. In addition, the trajectories of TC, LDL-C, TG, and non-HDL-C had inverted U-shaped quadratic relationships with aging. There was a significant interaction between age and CRF in each trajectory, with CRF influencing the aging trajectories of lipids and lipoproteins mainly in young to middle-aged men, but with little effect on the elderly. Furthermore, men with lower CRF levels reached abnormal TC (≥200 mg/dl), LDL-C (≥130 mg/dl), non-HDL-C (≥160 mg/dl), and TG/HDL-C ratio (≥3) before 40 years of age, notably earlier than in those with higher CRF levels.
Overall trends of lipids and lipoproteins with aging over the adult lifespan were generally consistent with the results of previous studies (9,10,12,13). It is likely that multiple lifestyle factors may be involved in these age-related lipid changes, including changes in BF distribution (13). Overall BF tends to increase, especially in the abdominal region, which causes insulin resistance, the potential mechanism underlying dyslipidemia. Under conditions of abdominal obesity, free fatty acids (FFAs) are released abundantly from enlarged adipose tissue. The increased flux of FFAs from the blood to the liver promotes hepatic TG synthesis, overproduction of TG containing very low-density lipoprotein (VLDL), and increased apolipoprotein B in the liver (32). This altered lipid metabolism contributes to the insulin resistance that leads to elevated FFA levels (33).
Several epidemiologic studies have shown that TC, LDL-C, and TG decrease after middle age (11,13–15). Decreased cholesterol concentrations with advancing age could be explained by the relationship of the inflammatory response to common chronic diseases (16), reduction of cholesterol absorption due to metabolic and hormonal changes with aging (34), and poor health status (13). However, these effects may be minimized in the present study because subjects with a history of myocardial infarction, stroke, and cancer were excluded. In addition, the results of sensitivity analysis after excluding subjects with diabetes or hypertension were consistent with our main finding. It is also possible that those who live longer have a more favorable lipid profile in terms of selective survival.
Previous studies demonstrated that HDL-C decreased (13–15) or did not change with advancing age (12,16). However, HDL-C tended to increase with age when mean HDL-C was compared among representative U.S. men with almost 10-year differences between the survey periods, supposing that those in the same age group were the same cohort (for instance, mean HDL-C of men aged 40 to 49 in the survey of 1988–1994 vs. that of men aged 50 to 59 in the survey of 1999–2002) (9,11). The present study also showed the trend of increasing HDL-C with age. In addition, despite a significant interaction of age·CRF, the graphical illustration of HDL-C trajectories with age are not so prominent, perhaps due to the generally small variation of HDL-C with age (9,11). Thus, a future study using a population with a wider range of HDL-C is warranted to more clearly discern the protective effect of CRF on the age-related HDL-C trajectory.
The distribution of TG in the age-TG trajectory was relatively low compared with that of other lipids and lipoprotein in the trajectories of TC, LDL-C, and non-HDL-C (Figure 2), using suggested cutoffs for elevated CHD risk (3,31). In addition, TG concentrations at baseline were around 10 to 20 mg/dl lower than those from national data over a similar period (11), possibly due to the characteristics of this study’s healthy population. Although the trajectories for HDL-C were below the 60 mg/dl cutoff for high HDL-C over most of the lifespan (Figure 2), they were not significantly different from the HDL-C distribution in the national survey (11). However, the age-related trajectory for the composite variable of TG/HDL-C ratio, which is a surrogate marker for insulin resistance and a CHD risk factor (30), showed a pattern similar to those of TC, LDL-C, and non-HDL-C, in which men with lower CRF reached the abnormal cutoff values considerably earlier. The TC/HDL-C and LDL-C/HDL-C ratios, both of which are generally considered better predictors of CHD risk than a single profile of lipids and lipoproteins (35), showed a similar age-related trajectory pattern to the TG/HDL-C ratio when cutoffs for abnormal levels were set at ≥4.5 for the TC/HDL-C ratio and ≥3.0 for the LDL-C/HDL-C ratio (36,37).
Prior studies indicate that CRF is independently and inversely associated with dyslipidemia (18,19), whereas CRF improves lipid and lipoprotein profiles through mechanisms that increase lipoprotein lipase activity in skeletal muscle, resulting in an increased TG clearance rate, improved HDL-C, and enhanced transport of lipids and lipoproteins from the peripheral circulation and tissues to the liver (19,38). The effect of physical activity and exercise on improving lipid and lipoprotein profiles may differ for different age groups (39). A significant interaction between age and CRF was found in the present study, in which CRF influenced the aging trajectories of lipids and lipoproteins mainly among young to middle-aged men, but had little effect on the elderly. Specifically, CRF was consistently a protective factor for abnormal lipid and lipoprotein profiles, and this prominent effect most frequently appeared between the early 20s and early 60s for TC, LDL-C, and non-HDL-C, and between the early 20s and early 70s for TG and HDL-C. Our findings showed that abnormal LDL-C began to occur at around 30 years of age in men with low CRF levels and around the mid-40s in men with high CRF levels. Abnormal TC levels also began to occur in the early 30s in men with low CRF levels, but were delayed until the mid-40s in men with high CRF levels. Furthermore, men with low CRF levels reached abnormal TG/HDL-C ratios and non-HDL-C levels around the early 20s and mid-30s, respectively, but men with high CRF levels kept their values for TG/HDL-C ratio and non-HDL-C below the abnormal cutoffs over the entire lifespan. These findings indicate that men with higher CRF are more likely to reach abnormal values for lipids and lipoproteins about at least a decade later than men in the low CRF category. This suggests that improving CRF levels may delay the period of elevated serum lipids and lipoproteins. These data are especially clinically relevant for LDL-C because the recent American College of Cardiology/American Heart Association guideline shows that aerobic physical activity reduces LDL-C by 3.0 to 6.0 mg/dl on average (17). In addition, although the treatment targets for LDL-C and non-HDL-C are abandoned by some, but not all (40), LDL-C and non-HDL-C still play important roles in defining the risk group for prevention of atherosclerotic CVD (41).
Strengths and limitations
Strengths of our study include its prospective study design, permitting longitudinal measures of lipids, lipoproteins, and time-varying covariates for assessing trajectories with aging. Using a relatively large sample size over the entire lifespan, stable estimates were obtained in the main analysis and in sensitivity analyses. However, our study has limitations. First, loss to follow-up may affect the validity of the study results, although this is not a substantial issue in the ACLS (42). The present study includes relatively healthy men who primarily were white and from middle-to-upper socioeconomic status, which might reduce the generalizability of the results, but also potentially minimizes residual confounding by socioeconomic status. Secondly, there was no information on lipid-lowering medication. Thus, we excluded those who reported a history of high cholesterol or high TG at baseline and at each follow-up visit to minimize the likely impact of lipid-lowering medication on lipids and lipoprotein trajectories. Also, a sensitivity analysis was performed to determine whether the presence of metabolic abnormalities would potentially affect the consistency of the results at baseline and follow-up. Thirdly, due to small sample size, 95% confidence intervals in each trajectory tended to be wider in older individuals, which may render the estimates of trajectories less precise. Finally, longitudinal changes in lipids and lipoproteins may be affected by dietary habit changes (16) that were not included in the models. However, analysis of the subgroup with records of low-fat or low-cholesterol diets did not make much difference (Online Table S3).
Conclusions
TC, LDL-C, TG, and non-HDL-C gradually increase until the mid-40s to early 50s, with subsequent declines thereafter; in contrast, HDL-C increases steadily with aging in healthy men. Independent of time, varying lifestyle, and metabolic characteristics, a higher CRF has a significant contribution to maintaining favorable lipid and lipoprotein profiles, especially in young to middle-aged men. Therefore, promoting CRF may contribute to possible delay of dyslipidemia and its related atherosclerosis and CVD.
Supplementary Material
Supplemental Table S1. Population average coefficients and standard errors (SE) for age, CRF, and waist circumference on the lipids and lipoproteins from Generalized Estimating Equation methods
Supplemental Table S2. Population average coefficients and standard errors (SE) for all predictors on the lipids and lipoproteins from Generalized Estimating Equation methods in the subjects without metabolic abnormalities at baseline* and follow-up
Supplemental Table S3. Population average coefficients and standard errors (SE) for all predictors on the lipids and lipoproteins from Generalized Estimating Equation methods in the subjects who had the records about low-fat or low-cholesterol dietary habit
CENTRAL ILLUSTRATION. Trajectories of Total Cholesterol With Aging.
The purple dashed line represents a crude overall trajectory; the other dashed lines represent each category of CRF (orange for high CRF, light blue for middle CRF, and green for low CRF), assuming constant WC residual (−0.179, mean of the residual). Shaded areas represent 95% confidence intervals for each CRF category. The solid blue horizontal line indicates the established abnormal cutoff of ≥200 mg/dl for TC. CRF = cardiorespiratory fitness; TC = total cholesterol; WC = waist circumference.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Cardiorespiratory fitness is more strongly associated with the aging trajectories of lipids and lipoproteins in young and middle-aged men than in older men, and promoting cardiorespiratory fitness can delay the development of dyslipidemia.
TRANSLATIONAL OUTLOOK: Additional research is needed to determine whether cardiorespiratory fitness could change the age-related trajectories of lipids and lipoproteins in patients taking lipid-lowering medications.
Acknowledgments
Grant Support: This work was supported by National Institutes of Health grants AG06945, HL62508, and DK088195. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank the Cooper Clinic physicians and technicians for collecting the baseline data and Cooper Institute staff for data entry and management.
ABBREVIATIONS LIST
- BMI
body mass index
- CHD
coronary heart disease
- CRF
cardiorespiratory fitness
- CVD
cardiovascular disease
- GEE
generalized estimating equations
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TC
total cholesterol
- TG
triglycerides
- WC
waist circumference
Footnotes
Disclosures: Dr. Blair is a member of the advisory board for Technogym, Santech, and Clarity and has received unrestricted research grants from The Coca-Cola Company, Technogym, and BodyMedia. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger PS, Smith DA, Mehta AE, et al. AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012;18 (Suppl):1–78. doi: 10.4158/ep.18.s1.1. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. JAMA. 1987;257:2176–80. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 5.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Law MR, Wald NJ, Rudnicka A. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Y, Blumenthal RS, Flaws JA, et al. Non–high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–9. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 8.Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. Am J Cardiol. 2000;86:943–9. doi: 10.1016/s0002-9149(00)01127-9. [DOI] [PubMed] [Google Scholar]
- 9.Carroll MD, Kit BK, Lacher DA, et al. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;308:1545–54. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 10.Schubert CM, Rogers NL, Remsberg KE, et al. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes (Lond) 2006;30:251–60. doi: 10.1038/sj.ijo.0803129. [DOI] [PubMed] [Google Scholar]
- 11.Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–81. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 12.Siervogel RM, Wisemandle W, Maynard LM, et al. Serial changes in body composition throughout adulthood and their relationships to changes in lipid and lipoprotein levels. The Fels Longitudinal Study. Arterioscler Thromb Vasc Biol. 1998;18:1759–64. doi: 10.1161/01.atv.18.11.1759. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PWF, Anderson KM, Harris T, et al. Determinants of Change in Total Cholesterol and HDL-C With Age: The Framingham Study. J Gerontol. 1994;49:M252–7. doi: 10.1093/geronj/49.6.m252. [DOI] [PubMed] [Google Scholar]
- 14.Garry PJ, Hunt WC, Koehler KM, et al. Longitudinal study of dietary intakes and plasma lipids in healthy elderly men and women. Am J Clin Nutr. 1992;55:682–8. doi: 10.1093/ajcn/55.3.682. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Weijenberg MP, Feskens EJ, Kromhout D. Age-related changes in total and high-density-lipoprotein cholesterol in elderly Dutch men. Am J Public Health. 1996;86:798–803. doi: 10.2105/ajph.86.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Medicine Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carnethon MR, Gidding SS, Nehgme R, et al. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 20.Kokkinos PF, Holland JC, Narayan P, et al. Miles run per week and high-density lipoprotein cholesterol levels in healthy, middle-aged men: A dose-response relationship. Arch Int Med. 1995;155:415–20. [PubMed] [Google Scholar]
- 21.Kelley GA, Kelley KA, Vu Tran Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Obes (Lond) 2005;29:881–93. doi: 10.1038/sj.ijo.0802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 23.Jackson AS, Sui X, Hébert JR, et al. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–7. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Sui X, Lavie CJ, et al. Effects of cardiorespiratory fitness on blood pressure trajectory with aging in a cohort of healthy men. J Am Coll Cardiol. 2014;64:1245–53. doi: 10.1016/j.jacc.2014.06.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sui X, Jackson AS, Church TS, et al. Effects of cardiorespiratory fitness on aging: glucose trajectory in a cohort of healthy men. Ann Epidemiol. 2012;22:617–22. doi: 10.1016/j.annepidem.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson AW, Lee DC, Sui X, et al. Muscular strength is inversely related to prevalence and incidence of obesity in adult men. Obesity (Silver Spring) 2010;18:1988–95. doi: 10.1038/oby.2009.422. [DOI] [PubMed] [Google Scholar]
- 28.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 29.Jones RH, Ackerson LM. Serial correlation in unequally spaced longitudinal data. Biometrika. 1990;77:721–31. [Google Scholar]
- 30.Li C, Ford ES, Meng YX, Mokdad AH, et al. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity. Cardiovasc Diabetol. 2008;7:4. doi: 10.1186/1475-2840-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 2006;14 (Suppl 1):41S–49S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- 33.Lebovitz HE. Insulin resistance–a common link between type 2 diabetes and cardiovascular disease. Diabetes, Obes Metab. 2006;8:237–49. doi: 10.1111/j.1463-1326.2005.00521.x. [DOI] [PubMed] [Google Scholar]
- 34.Gylling H, Strandberg T, Tilvis R, et al. Regulation of serum cholesterol level in middle-aged and elderly men. Relation of cholesterol absorption and synthesis to lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 1994;14:694–700. doi: 10.1161/01.atv.14.5.694. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan S, Glick H, Criqui M, et al. Cholesterol measures to identify and treat individuals at risk for coronary heart disease. Am J Prev Med. 2003;25:50–7. doi: 10.1016/s0749-3797(03)00092-8. [DOI] [PubMed] [Google Scholar]
- 36.Gimeno-Orna JA, Faure-Nogueras E, Sancho-Serrano MA. Usefulness of total cholesterol/HDL-cholesterol ratio in the management of diabetic dyslipidaemia. Diabetic Medicine. 2005;22:26–31. doi: 10.1111/j.1464-5491.2004.01341.x. [DOI] [PubMed] [Google Scholar]
- 37.Nicklas BJ, Penninx BW, Ryan AS, et al. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–20. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 38.Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sciences Rev. 1994;22:477–522. [PubMed] [Google Scholar]
- 39.Mittendorfer B, Klein S. Effect of aging on glucose and lipid metabolism during endurance exercise. Int J Sport Nutr Exerc Metab. 2001;11 (Suppl):S86–91. doi: 10.1123/ijsnem.11.s1.s86. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Yehuda O, DeMaria AN. LDL-cholesterol targets after the ACC/AHA 2013 guidelines: evidence that lower is better? J Am Coll Cardiol. 2014;64:495–7. doi: 10.1016/j.jacc.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Macera CA, Jackson KL, Davis DR, et al. Patterns of non-response to a mail survey. J Clin Epidemiol. 1990;43:1427–30. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Population average coefficients and standard errors (SE) for age, CRF, and waist circumference on the lipids and lipoproteins from Generalized Estimating Equation methods
Supplemental Table S2. Population average coefficients and standard errors (SE) for all predictors on the lipids and lipoproteins from Generalized Estimating Equation methods in the subjects without metabolic abnormalities at baseline* and follow-up
Supplemental Table S3. Population average coefficients and standard errors (SE) for all predictors on the lipids and lipoproteins from Generalized Estimating Equation methods in the subjects who had the records about low-fat or low-cholesterol dietary habit