Abstract
The embryonic spinal cord is known to be rich in retinoic acid, and several indirect lines of evidence point to a dorsoventral concentration difference of this compound. Previous measurements of dorsoventral retinoic acid levels, however, showed only minor differences. By a combination of microdissection and bioassay techniques, we compared retinoic acid levels with retinaldehyde dehydrogenase levels along spinal cords from early embryonic to postnatal mice. Both parameters vary in parallel, indicating that the principal reason for regional retinoic acid differences in the developing spinal cord is different levels of retinoic acid-generating enzyme. Consistent with previous reports, we observed overall quite high synthesis, decreasing with age, and no dorsoventral difference throughout much of the spinal cord length. In two locations, however, ventral synthesis exceeds dorsal synthesis by several orders of magnitude. These hot spots colocalize with the origins of the limb innervations. They are highest during early stages of limb innervation and disappear slowly postnatally. The synthesis hot spots are likely to create local retinoic acid diffusion halos, which may influence the survival of neurons in the limb regions of the spinal cord and which probably promote innervation of the developing limbs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler K., Edlund T., Jessell T. M., Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993 May 21;73(4):687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Chen Y., Huang L., Russo A. F., Solursh M. Retinoic acid is enriched in Hensen's node and is developmentally regulated in the early chicken embryo. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10056–10059. doi: 10.1073/pnas.89.21.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert M. C., Linney E., LaMantia A. S. Local sources of retinoic acid coincide with retinoid-mediated transgene activity during embryonic development. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6572–6576. doi: 10.1073/pnas.90.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Haskell B. E., Stach R. W., Werrbach-Perez K., Perez-Polo J. R. Effect of retinoic acid on nerve growth factor receptors. Cell Tissue Res. 1987 Jan;247(1):67–73. doi: 10.1007/BF00216548. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Thaller C., Eichele G. Evidence that Hensen's node is a site of retinoic acid synthesis. Nature. 1992 Sep 17;359(6392):237–241. doi: 10.1038/359237a0. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C., Landmesser L. Motoneurone projection patterns in the chick hind limb following early partial reversals of the spinal cord. J Physiol. 1980 May;302:581–602. doi: 10.1113/jphysiol.1980.sp013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M., Holder N. Retinoic acid and development of the central nervous system. Bioessays. 1992 Jul;14(7):431–438. doi: 10.1002/bies.950140702. [DOI] [PubMed] [Google Scholar]
- Mavilio F. Regulation of vertebrate homeobox-containing genes by morphogens. Eur J Biochem. 1993 Mar 1;212(2):273–288. doi: 10.1111/j.1432-1033.1993.tb17660.x. [DOI] [PubMed] [Google Scholar]
- McCaffery P., Lee M. O., Wagner M. A., Sladek N. E., Dräger U. C. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development. 1992 Jun;115(2):371–382. doi: 10.1242/dev.115.2.371. [DOI] [PubMed] [Google Scholar]
- McCaffrery P., Posch K. C., Napoli J. L., Gudas L., Dräger U. C. Changing patterns of the retinoic acid system in the developing retina. Dev Biol. 1993 Aug;158(2):390–399. doi: 10.1006/dbio.1993.1197. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Perez-Castro A. V., Toth-Rogler L. E., Wei L. N., Nguyen-Huu M. C. Spatial and temporal pattern of expression of the cellular retinoic acid-binding protein and the cellular retinol-binding protein during mouse embryogenesis. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8813–8817. doi: 10.1073/pnas.86.22.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek M., Jessell T. M., Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Quinn S. D., De Boni U. Enhanced neuronal regeneration by retinoic acid of murine dorsal root ganglia and of fetal murine and human spinal cord in vitro. In Vitro Cell Dev Biol. 1991 Jan;27(1):55–62. doi: 10.1007/BF02630895. [DOI] [PubMed] [Google Scholar]
- Reynolds K., Mezey E., Zimmer A. Activity of the beta-retinoic acid receptor promoter in transgenic mice. Mech Dev. 1991 Dec;36(1-2):15–29. doi: 10.1016/0925-4773(91)90068-h. [DOI] [PubMed] [Google Scholar]
- Rossant J., Zirngibl R., Cado D., Shago M., Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991 Aug;5(8):1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Ruberte E., Dolle P., Chambon P., Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. II. Their differential pattern of transcription during early morphogenesis in mouse embryos. Development. 1991 Jan;111(1):45–60. doi: 10.1242/dev.111.1.45. [DOI] [PubMed] [Google Scholar]
- Ruberte E., Friederich V., Chambon P., Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. III. Their differential transcript distribution during mouse nervous system development. Development. 1993 May;118(1):267–282. doi: 10.1242/dev.118.1.267. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Sundin O., Eichele G. An early marker of axial pattern in the chick embryo and its respecification by retinoic acid. Development. 1992 Apr;114(4):841–852. doi: 10.1242/dev.114.4.841. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Thaller C., Hofmann C., Eichele G. 9-cis-retinoic acid, a potent inducer of digit pattern duplications in the chick wing bud. Development. 1993 Jul;118(3):957–965. doi: 10.1242/dev.118.3.957. [DOI] [PubMed] [Google Scholar]
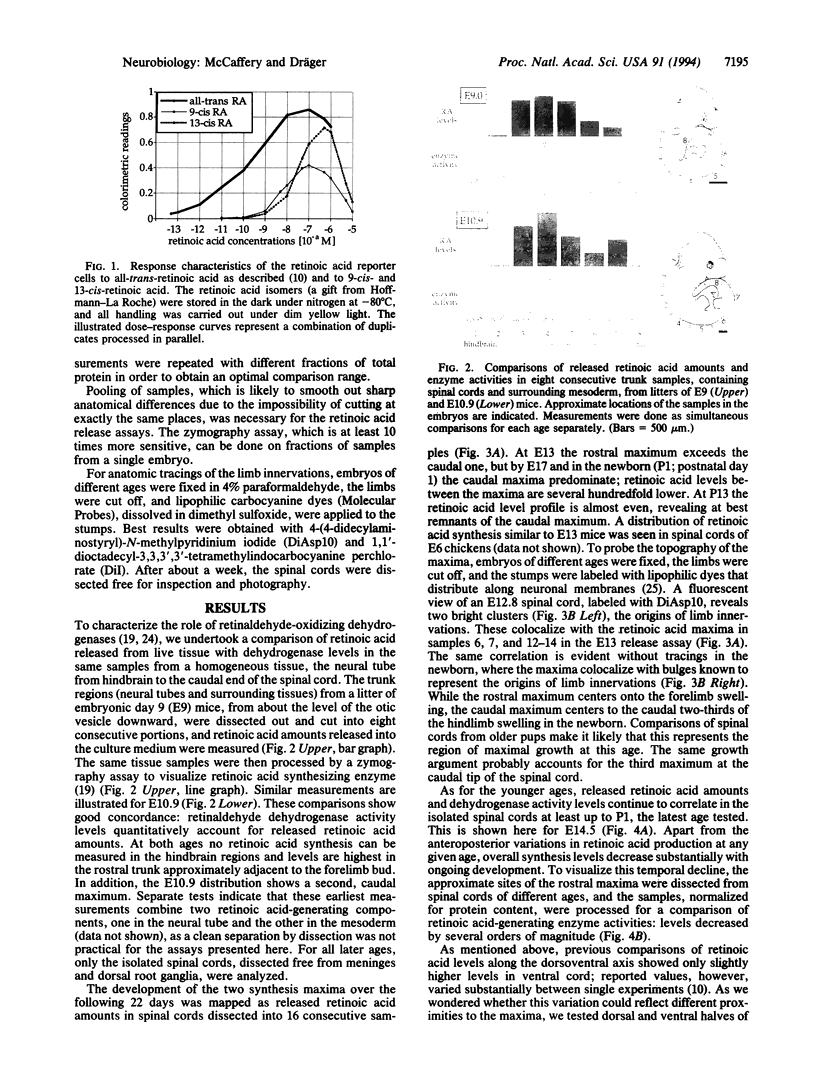
- Wagner M., Han B., Jessell T. M. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992 Sep;116(1):55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Wuarin L., Sidell N., de Vellis J. Retinoids increase perinatal spinal cord neuronal survival and astroglial differentiation. Int J Dev Neurosci. 1990;8(3):317–326. doi: 10.1016/0736-5748(90)90038-4. [DOI] [PubMed] [Google Scholar]
- Yamada T., Pfaff S. L., Edlund T., Jessell T. M. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993 May 21;73(4):673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- Zgombić-Knight M., Satre M. A., Duester G. Differential activity of the promoter for the human alcohol dehydrogenase (retinol dehydrogenase) gene ADH3 in neural tube of transgenic mouse embryos. J Biol Chem. 1994 Mar 4;269(9):6790–6795. [PubMed] [Google Scholar]