Abstract
While efforts have been made over the years, the exact cause of keratoconus (KC) remains unknown. The aim of this study was to identify alterations in endogenous metabolites in the tears of KC patients compared with age-matched healthy subjects. Three groups were tested: 1) Age-matched controls with no eye disease (N=15), 2) KC – patients wearing Rigid Gas permeable lenses (N=16), and 3) KC – No Correction (N=14). All samples were processed for metabolomics analysis using LC-MS/MS. We identified a total of 296 different metabolites of which >40 were significantly regulated between groups. Glycolysis and gluconeogenesis had significant changes, such as 3-phosphoglycerate and 1,3 diphopshateglycerate. As a result the citric acid cycle (TCA) was also affected with notable changes in Isocitrate, aconitate, malate, and acetylphosphate, up regulated in Group 2 and/or 3. Urea cycle was also affected, especially in Group 3 where ornithine and aspartate were up-regulated by at least 3 fold. The oxidation state was also severely affected. Groups 2 and 3 were under severe oxidative stress causing multiple metabolites to be regulated when compared to Group 1. Group 2 and 3, both showed significant down regulation in GSH-to-GSSG ratio when compared to Group 1. Another indicator of oxidative stress, the ratio of lactate – pyruvate was also affected with Groups 2 and 3 showing at least a 2-fold up regulation. Overall, our data indicate that levels of metabolites related to urea cycle, TCA cycle and oxidative stress are highly altered in KC patients.
Keywords: Keratoconus, metabolomics, oxidative stress, rigid gas permeable lenses, pentacam
1. Introduction
Keratoconus (KC) is a non-inflammatory corneal thinning disease(Krachmer et al., 1984). In KC, the main characteristic is the deviation from the normal corneal contour and physical structure that can have devastating effects on the patient's best-corrected visual acuity (Gordon et al., 2006; Moreira et al., 2007; Wagner et al., 2007; Wahrendorf, 2006). Changes in corneal biomechanical properties leading to corneal thinning can make the individual more susceptible to traumatic ocular injury (Al-Hussain et al., 2004). KC s main characteristic is the cone-shaped corneal protrusion and is seen in all ethnical populations, but more commonly in the Middle East. Both sexes can be affected, but males more often than females. The mean age of onset is at 17 years (Bechrakis et al., 1994). The incidence rate is still debatable, though large studies estimate 50 to 230 cases per 100,000 people in the general population (Rabinowitz, 1998). Depending on the severity, visual quality can be so adversely affected as to require surgical intervention.
The origin of the disease remains unknown and research has been extended over the last decade in order to ultimately unravel the etiology KC and may be able to correct the disease at its origin in the near future. Many genetics studies are currently on going or have been completed, but no common gene defect has been (Nielsen et al., 2013). Interestingly, less than 10% of cases are believed to be of familial origin (Aldave et al., 2006). Other onset mechanisms include mechanical eye rubbing and contact lens wearing both contributing to activation of wound-healing mechanisms and signaling pathways (Yeniad et al., 2009). Biochemical studies have also been performed indicating that the role of proteolytic enzyme digestion and the involvement of interleukin-1 (IL-1) are possible causative factors in some cases of KC(Rabinowitz, 1998).
It is known that the stability and quality of the tear film plays an important role in the optical quality of the eye (Montes-Mico et al., 2010). In KC there are a number of studies investigating the proteome of the tear fluids from patients diagnosed with KC compared to individuals without KC (Cheng et al., 2001; Nielsen et al., 2005; Nielsen et al., 2006; Srivastava et al., 2006). The most recent and extensive proteome study revealed(Chaerkady et al., 2013) new as well as previously reported proteins that are regulated in KC patients. The authors identified proteins from normal donor and KC corneas a total of 932 and 1157 proteins in the corneal epithelium and the stroma, respectively. Previous study on the KC epithelium detected 200–500 from which 19 were differentially expressed proteins(Srivastava et al., 2006). Another recent study identified 104 epithelial and 44 stromal proteins(Joseph et al., 2011). Between these studies there are a few proteins that are consistently been reported and regulated in KC patients. Collagen XII, transketolase, and TGFBI have all been reported to be decreased (Chaerkady et al., 2013; Cheng et al., 2001; Joseph et al., 2011) in KC stromal proteome.
The aim of this study was to identify alterations in tear metabolism of KC patients compared with age-matched healthy subjects. As Rigid Gas Permeable lenses(RGP) may pose significant mechanical stress to the cornea, tears from the KC group of patients were analyzed separately dependent on whether the patients wore RGP lenses. Corneal RGPs has been shown to induce topographical changes on the cornea in normal control individuals (Braun and Anderson Penno, 2003),(Wang et al., 2002)] as well as in subjects with KC (Szczotka et al., 1996). In normal subjects, alterations in corneal curvatures were observed and were directly related to the number of years of lens wear (Braun and Anderson Penno, 2003; Wang et al., 2002). In KC subjects, alterations in corneal curvature (Hwang et al., 2010; Zadnik et al., 2005; Zadnik and Mutti, 1987), shape (Gundel et al., 1996; Zadnik et al., 2005; Zadnik and Mutti, 1987), thickness (Jinabhai et al., 2012), and anterior surface (Jinabhai et al., 2012; Zadnik and Mutti, 1987) have been reported again all linked to the long term wear of RGPs. A recent study (Romero-Jimenez et al., 2014) has shown short term corneal changes of RGPs fitted in KC subjects. Authors found that the anterior cornea flattens and increases in thickness within a 14 day period of RGP wear.
This is the first study, to the author’s knowledge, to identify key metabolites in human tears for the study of KC disease in order to provide clues for the treatment of the defect. Furthermore, recent studies linking metabolomics to genomic(Adamski and Suhre, 2013; Gieger et al., 2008) profiling suggests that there may be even greater opportunities for us to approach the disease and grasp a greater understanding of the underlying mechanisms involved.
2. Methods
2.1. Subject recruitment
Thirty patients referred to the Department of Ophthalmology, Aarhus University Hospital for KC was asked to provide a tear sample for testing. All patients underwent a standard clinical examination including refraction, measurement of best corrected visual acuity, slit-lamp examination, and Pentacam HR Scheimpflug tomography. Fifteen patients referred for refractive surgery for myopia underwent similar examination and served as control group. Tears were collected in capillary glass tubes from the mid-temporal side of the tear meniscus and were collected only in the morning hours to ensure consistency. The volume obtain was on average 5–7ul per patient. Care was taken not to stimulate tear secretion during collection.
2.2. Pentacam
All individuals participating in the study were examined using the Pentacam HR (Oculus, Optikgeräte GmbH). A variety of values were collected. As shown in Table 1 the mean age for healthy individuals was 38 (range from 31 to 47 years). For KC individuals that received RGP treatment the average age was 29.3 (range 21 to 58 years). For KC individuals with no correction the average age was 29.7 (range 20 to 51). There were no statistically significant differences in age between the study groups. The mean corneal thickness for the control group was 551.9, for the RGP treated group was 451 and for the no correction group was recorded as 455.6 (Table 1). Maximum keratometric (Kmax) average value for the controls was 43.9, the RGP group was 53.9 and the No correction group average value was 52.7 (Table 1). Patients with RGP lenses were instructed to leave lenses out for at least one week prior to tear collection.
Table 1. Pentacam data obtained from subjects participated in this study.
Summary of the mean ages, corneal thickness (Ct min) and maximum keratometric (Kmax) values for Group 1: Controls, Group 2: KC-RGP, and Group 3: KCno correction. Mean age for Group 1 was 37.2, Group 2 was 29.5, and for Group 3 the average age was 29.6. No statistically significant differences were found. The Ct min for Group 1 was 551, for Group 2 was 451 and Group 3 was recorded as 455. Both Groups 2 and 3 were statistically different compared to Group 1 (p<0.05). Kmax average value for the controls was 43.7, the KC-RGP group was 53.9 and the KC-No correction group average value was 52.7
| Age (years) | Ct min (um) | Kmax (D) | |
|---|---|---|---|
| Control | 38 ± 7.02 | 551.9 ± 7.96 | 43.9 ± 0.35 |
| KC-RGP | 29.3 ± 9.18 | 451 ± 6.23 | 53.9 ± 1.31 |
| KC- No Correction | 29.7 ± 9.27 | 455.6 ± 12.43 | 52.7 ± 1.46 |
2.3. Tear metabolite extraction
All samples were collected and processed as previously reported(Yuan et al., 2012). Briefly, samples were centrifuged (14,000 g, 10 min, 4°C) in ice-cold 80% MeOH. Supernatants were incubated on dry ice. Plasma metabolites were extracted from the tear samples twice in 80% ice-cold MeOH. Metabolite extracts were vortexed and centrifuged (14,000 g, 10 min, 4° C). Supernatants were evaporated and stored at −80° C until further analysis.
2.4. Targeted Mass Spectrometry
Targeted mass spectrometry was used for sample processing, as previously described(Karamichos et al., 2014; Webhofer et al., 2013; Yuan et al., 2012). Briefly, samples were re-suspended using 20 µL HPLC grade water and 5–7 µl was injected into a hybrid 5500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC HPLC system (Shimadzu, Columbia, MD)(Webhofer et al., 2013). A total of 256 endogenous water soluble metabolites were analyzed using selected reaction monitoring (SRM). Some metabolites were targeted in both positive and negative ion mode, for a total of 289 SRM transitions, using positive/negative ion polarity switching. Approximately 10–14 data points were acquired per detected metabolite. Samples were delivered to the mass spectrometer via hydrophilic interaction chromatography (HILIC) where gradients were run as previously described(Karamichos et al., 2014; Webhofer et al., 2013; Yuan et al., 2012). Peak areas from the total ion current for each metabolite SRM transition were integrated using MultiQuant v2.0 software (AB/SCIEX).
2.5. Pathway Enrichment Analysis
Pathway enrichment (representation) analysis was performed using an online freeware program; Metaboanalyst (www.Metaboanalyst.ca) as previously described(Karamichos et al., 2014; Webhofer et al., 2013; Yuan et al., 2012). Briefly, we performed the analysis using only the metabolites that were up or down regulated by 2:1 ratio as indicated by our statistical analysis. We chose 2:1 ratio cutoff, as previously described(Karamichos et al., 2014) in order to ensure that we included only the vastly abundant metabolites. Only metabolites that were present on all biological samples were considered for further analysis. Data is plotted as fold enrichment in order to highlight those abundant metabolites that are being affected (Booth et al., 2013).
2.6. Statistics
Data was analyzed for significant variations (p<0.05) using one way ANOVA and Tukey's multiple comparisons test
2.7. Ethics
Tear collection was considered part of the highly specialized clinical and para-clinical evaluation of patients referred to the department. The study met the tenets of the Declaration of Helsinki. Tear samples were anonymized before analysis. Informed, consent was obtained from all participants. Permission from the Regional Ethics Committee for the Central Region of Denmark for tear collection has been obtained.
3. Results
3.1. Pathway Enrichment Analysis
We have identified 296 endogenous water soluble metabolites of which more than 40 were significantly regulated between groups. Significance was established based on a 2:1 ratio cutoff. We investigated all three groups: Group 1: Age-matched controls with no eye disease, Group 2: KC – RGP lenses, and Group 3: KC – No Correction. More specifically, in Group 1, we found 9 metabolites significantly up-regulated when compared to Group 2 and 3. In contrast, 50 and 48 metabolites were significantly upregulated in Group 2 and 3 respectively.
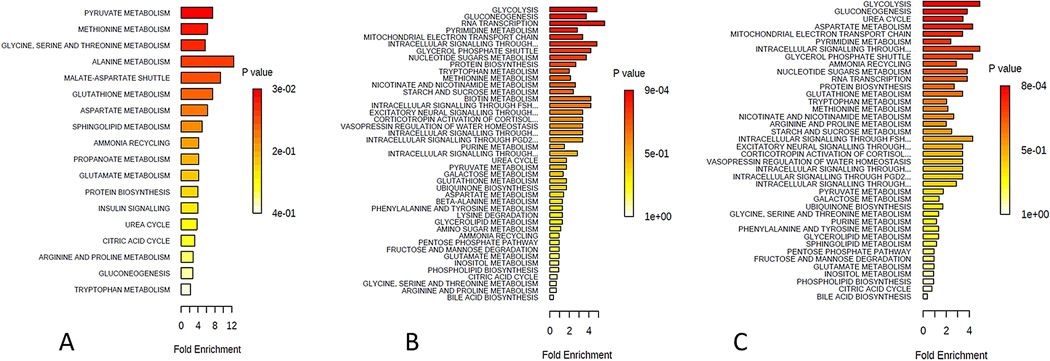
In Figure 1 the predicted metabolic pathways that are affected based on the raw data is shown in all three Groups. Only those metabolites that were up regulated by at least 2:1 ration were included in the analysis. Figure 1A shows the predicted metabolic pathways affected in Group 1. According to P values (shown in red) the most affected pathways are those of pyruvate, methionine, and glycine-serine and threonine metabolism. Alanine and malate-aspartate shuttle metabolism were predicted to be significantly affected. All of these are linked to the TCA cycle regulation(Lane, 2009; Lowenstein, 1969; Monty et al., 2003), while pyruvate and malate-aspartate shuttle are also involved in glycolysis(Monty et al., 2003).
Figure 1.
Summary of pathway enrichment analysis for Group 1: Controls, Group 2: KCRGP, and Group 3: KC-no correction. Above is a display of the diversity of signaling pathways that are enriched on the basis of all the metabolites passing filtering criteria. The most significant p-values are in red while the least significant are in yellow and white. A) Predicted metabolic pathways affected in Group 1 B) predicted pathway regulation for Group 2, and C) Predicted metabolic pathways affected in Group 3.
Figure 1B shows predicted pathway regulation for Group 2. The three most significant pathways affected were glycolysis, gluconeogenesis (GNG), and RNA transcription. Similarly to Group 1, these are all involved in the TCA cycle indicating metabolic regulation between normal individuals and the ones diagnosed with KC. Furthermore, mitochondrial electron transport chain and was also affected. Very similar to Group 2, Group 3 predicted pathways (Figure 1C) included glycolysis, GNG, and urea cycle. Aspartate metabolism and mitochondrial electron transport chain were fourth and fifth, respectively, most significantly affected pathways.
Surprisingly the top two pathways between Group 2 and 3 were identical: glycolysis and GNG. Based on these results we further analyzed the individual metabolites involved in TCA cycle, glycolysis/GNG, and urea cycle.
3.2. Metabolites Regulation
Tricarboxylic acid cycle (TCA)
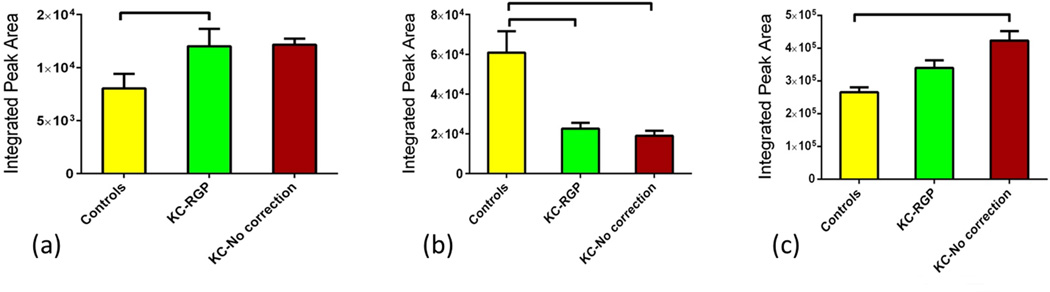
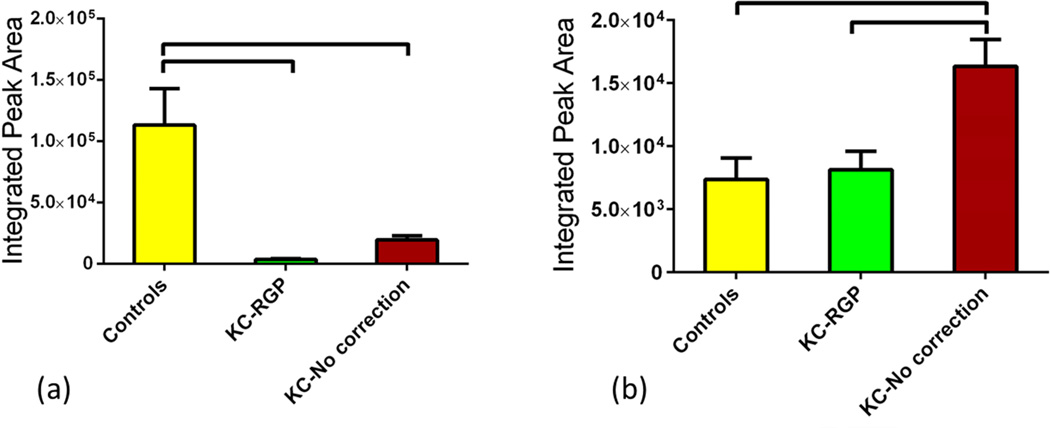
The citric acid cycle is a key component of the metabolic pathway by which all aerobic organisms generate energy. Its regulation is vital to cell survival and tissues. We investigated the metabolism of human tears collected from the three groups. We found several individual metabolites significantly regulated between those groups that are known for their part in TCA cycle. Three primary metabolites in TCA cycle were significantly regulated. Isocitrate was significantly up-regulated in Group 2 (Figure 2a; p<0.05) when compared to Group 1. Opposite pattern was seen with aconitate where Group 1 showed the highest expression when compared to the other two groups (Figure 2b; p<0.001). No significant difference was seen between Group 2 and 3. Malate was significantly up regulated in Group 3 but no significant difference was shown between the Groups 1 and 2 (p<0.01; Figure 2c). Furthermore, we found several intermediates to be regulated between groups. Acetylphosphate was one of them and was up-regulated both in Groups 2 and 3 by 3 and 4 fold respectively (p<0.05; Figure 3a). It is important that TCA cycle is carefully regulated by the cell. Any dysfunction in TCA cycle large amounts of metabolic energy would be wasted in the over production of ATP. In our study, ATP was significantly up-regulated in Group 3 indicating an anomaly on the TCA cycle metabolism (Figure 3b; p<0.0001). In support of the latter, another intermediate metabolite that was found to be significantly regulated (Figure 3c; p<0.0001) was Malonyl-CoA. As seen in Figure 3c the metabolite was barely detected in Groups 2 and 3 when compared to Group 1. Malonyl-CoA is formed by acetyl-CoA and is a precursor for fatty acids. ATP is also involved in glycolysis and GNG discussed below.
Figure 2.
Three groups were tested and analyzed: Group 1: Controls, Group 2: KCRGP, and Group 3: KC-no correction. Three primary citric acid cycle metabolites were significantly regulated. (a) Isocitrate was significantly up-regulated in Group 2 (p<0.05). (b) Aconitate showed the highest expression in Group 1 when compared to the other two groups (p<0.001). No significant difference was seen between Group 2 and 3. (c) Malate was significantly up regulated in Group 3 compared to Group 1 and 2 (p<0.01) but no significant difference was shown between the two.
Figure 3.
Three groups were tested and analyzed: Group 1: Controls, Group 2: KCRGP, and Group 3: KC-no correction. Three intermediate citric acid cycle metabolites were significantly regulated. (a) Acetylphosphate was up-regulated in both Groups 2 and 3 (p<0.05). (b) ATP was significantly up-regulated in Group 3 (p<0.0001) when compared to Group 1 and 2. Malonyl-CoA was barely detected in Groups 2 and 3 when compared to Group 1(p<0.0001).
Glycolysis and Gluconeogenesis
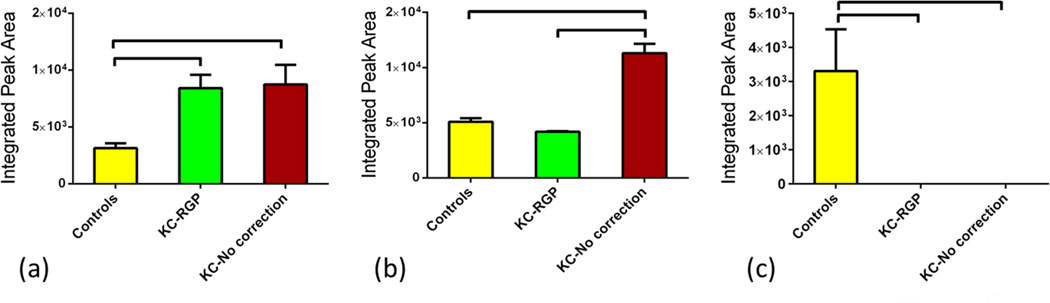
Glycolysis and GNG are vital metabolic pathways for the survival of the cells. Glycolysis converts glucose into pyruvate and enters the TCA cycle whereas GNG generates glucose. In our study we found several metabolic intermediates that are linked to these pathways; 1,3 diphopshateglycerate was significantly up regulated (Figure 4a) in Groups 2 and 3 (p<0.0001) when compared to Group 1 which was barely detectable. Group 3 was also significantly lower than Group 2 (p<0.001). This intermediate metabolite is critical in the formation of ATP(Alberts, 2001; Germann and Stanfield, 2002) and it correlates with the ATP up regulation shown above (Figure 3b). Another intermediate metabolite that we found to significantly be upregulated in Group 3 was 3-phosphoglycerate (Figure 4b). It was found significantly upregulated in Group 3 when compared to both Groups 1 and 2 (p<0.05 and p<0.01 respectively).
Figure 4.
Three groups were tested and analyzed: Group 1: Controls, Group 2: KCRGP, and Group 3: KC-no correction. Two intermediate Glycolysis/gluconeogenesis metabolites were significantly regulated. (a) 1,3 diphopshateglycerate was significantly up-regulated in Groups 2 and 3 (p<0.0001). Group 3 was also significantly lower than Group 2 (p<0.001). (b) 3-phosphoglycerate was significantly up regulated in Group 3 when compared to Group 1 (p<0.05) and Group 2 (p<0.01).
Urea cycle metabolism
Three of the primary metabolites in the Urea cycle were significantly regulated between the three groups. Ornithine showed the highest level expression in Group 1 and was significantly higher when compared to Groups 2 and 3 (Figure 5a; p<0.01). Interestingly ornithine expression levels were at their lowest on Group 2. Aspartate was only up-regulated Group 3 when compared to Group 1 and Group 2 (Figure 5b; p<0.05).
Figure 5.
Three groups were tested and analyzed: Group 1: Controls, Group 2: KCRGP, and Group 3: KC-no correction. Two primary Urea cycle metabolites were significantly regulated. (a) Ornithine showed the highest level expression in Group 1 when compared to Groups 2 and 3 (p<0.01). (b) Aspartate was significantly upregulated in Group 3 (p<0.05).
Oxidation state (redox) metabolism
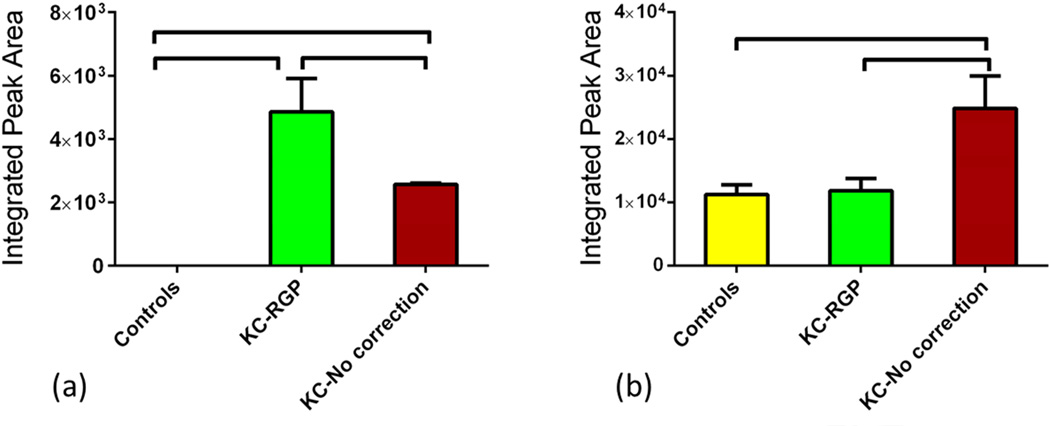
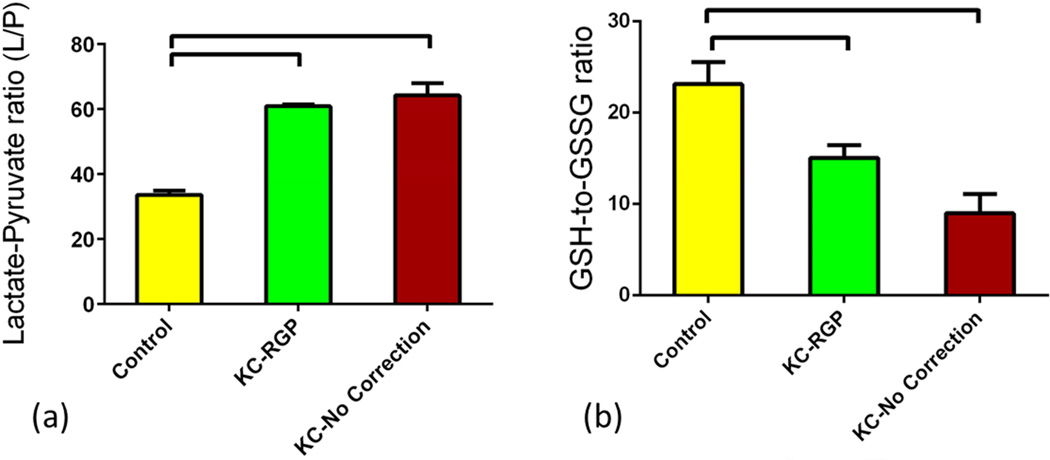
Previous reports suggested that oxidative stress is involved in KC(Behndig et al., 2001; Buddi et al., 2002; Gondhowiardjo and van Haeringen, 1993; Gondhowiardjo et al., 1993; Kenney et al., 2000). Accumulation of reactive oxygen species (ROS) can damage cells by reacting with proteins, DNA, and membrane phospholipids. In this study we investigated metabolites that are known to be key players in oxidative stress. Lactate/pyruvate (L/P) relative ratio is an indicator of oxidative stress. Increase of this ratio suggests higher oxidative stress levels. In our study, the ratio was significantly regulated between the three groups. Figure 6a shows ratio regulation on all three groups. L/P ratio was significantly up-regulated (p<0.0001), at least 2 fold, in both Group 2 and 3 when compared to Group 1. Another indicator of oxidative stress levels the Glutathione metabolite. There are two forms of Glutathione: Reduced (GSH) and oxidized (GSSG) states. A decreased GSH-to-GSSG ratio is considered indicative of oxidative stress(Chai et al., 1994; Zitka et al., 2012). In our tear study, the ratio was significantly lower in Groups 2 and 3 (~2 fold) when compared to Group 1 (Figure 6b; p<0.05 and p<0.001 respectively), confirming the oxidative stress levels in KC patients even in terms of tear quality.
Figure 6.
Three groups were tested and analyzed: Group 1: Controls, Group 2: KCRGP, and Group 3: KC-no correction. Two indicators of oxidative stress metabolism were significantly regulated. (a) Lactate/pyruvate (L/P) was significantly up-regulated (p<0.0001) in both Group 2 and 3 when compared to Group 1. Reduced (GSH)/oxidized (GSSG) glutathione ratio (GSH-to-GSSG) was significantly down regulated in Groups 2 (p<0.05) and 3 (p<0.001) when compared to Group 1.
4. Discussion
KC seem to be a multifactorial disease(Chaerkady et al., 2013; Kenney et al., 2000) and the initiation process is still a mystery. The clinical hallmark of KC is corneal thinning and bulging and results in compromised visual acuity(Rabinowitz, 1998). KC is associated with a variety of factors and events such as ECM destruction, increased activity of proteolytic enzymes, decrease of collagen types that normally exist in healthy cornea, and altered lamellae patterns (Rabinowitz, 1998; Romero-Jimenez et al., 2010). Understanding how all these mechanisms relate is not easy and we are far from it. It is critical, however crucial to understand and being able to modulate and control these events, if we are going to prevent KC pathogenesis.
Over the last decade, increasing number of studies has highlighted the importance of oxidative stress in KC pathogenesis (Greiner et al., 1985; Karamichos et al., 2014; Kenney et al., 2000; Risa et al., 2004). Several studies have reported changes in antioxidant status of KC corneas(Greiner et al., 1985; Kenney et al., 2000; Risa et al., 2004). Some non-enzymatic antioxidants such as glutathione, cysteine, uric acid, and tyrosine, play a key role in the regulation of cellular redox status and protection of the cells(Birben et al., 2012). Arnal and co-authors (Arnal et al., 2011) reported a decreased glutathione content and overall antioxidant capacity in KC corneas. Saijyothi and co-authors (Saijyothi et al., 2012)reported decreased levels of glutathione in KC tear film linking oxidative stress in KC corneas to tear film. We recently reported oxidative stress in HKCs while cultured in a conventional 2D system or our 3D model (Karamichos et al., 2014). Interestingly our findings here correlate with our findings using KC-derived cells. KC-derived cells showed high levels of oxidative stress both in conventional 2D cultures and 3D constructs. The aim of the current study was to investigate tear metabolism in KC diagnosed subjects that have been treated differently. We found significant modulation of metabolites that are oxidative stress indicators such as glutathione.
Metabolomics has been used successfully in ocular diseases (Fu et al., 2011; Greiner et al., 1985; Klyce, 1981; Nguyen and Bonanno, 2012; Risa et al., 2004) and it is rapidly becoming important in several disease diagnosis. Using this technique we investigated the metabolic differences between various groups. We analyzed tears from healthy subjects with no ocular history and KC patients who were RGP lens wearers and uncorrected KC patients. Our data indicates almost identical defects between patients with no correction and those fitted with RGP lenses. RGP lenses can cause mechanical and physiological stress on the cornea that could lead to changes in tear metabolites (Esgin and Erda, 2002; Holden et al., 1985; Liu and Pflugfelder, 2000; Miller, 1968). In the study, metabolite changes were however not affected by RGP lens wear to any major extent. Understanding the metabolic mechanisms behind KC disease is important if we are going to treat or at least arrest the disease. Our data here indicates that there are multiple metabolic differences between healthy and KC diagnosed subjects, independent of RGP wear.
Another indicator of oxidative stress was affected in KC diagnosed subjects, the TCA cycle. TCA cycle is a key component of many metabolic pathways. It is involved in synthesis of all three major groups: lipids, proteins, and carbohydrates (Barnes and Weitzman, 1986). The end product of this TCA is cellular energy. Inefficient cycling of TCA will lead to altered redox state. In cornea TCA cycle is the key source of energy required to maintain corneal transparency and cellular activity (Thies and Mandel, 1985). TCA cycle takes place in mitochondria and is relatively quiet under normal/healthy state, because of less abundant mitochondria in corneal epithelium (Friend, 1983; Maurice, 1965). Upon injury however it becomes activated and can lead to severe problems. In fact, in KC disease, mitochondrial DNA damage has been reported which agrees with our findings(Atilano et al., 2005). GNG and glycolysis are part of the same process and are vital in humans for maintaining blood glucose levels to normal. In cornea, GNG is important as glucose is used as fuel source and has been linked to wound healing(Nelson and Cox, 2000; Young, 1977). 3-phosphoglycerate regulated here is part of the Phosphoenolpyruvate or PEP metabolism that was also found to be significantly regulated in KC diagnosed subjects (data not shown). PEP holds the highest-energy phosphate bond found in any living organism and it participates in both glycolysis and GNG. Through metabolism to pyruvate, PEP, generates ATP and again this is indicative to the energy regulation differences in tears between normal and KC individuals, found here.
Overall our data suggests an altered quality of tears in KC patients that might provide clues to the progression of the disease. Supporting the latter, we found significant differences in Urea cycle key metabolites such as ornithine and citrulline. Organisms that cannot remove ammonia usually have to convert it quickly to another other substance, like urea or uric acid, in order to prevent toxicity. Insufficiency of the urea cycle is known for some genetic disorders (Brusilow and Horwich, 2001; Leonard and Morris, 2002). There are many studies that have suggested various genes associated with KC disease including SOD1, VSX1, COL6A1, COL8A1, and MMP9(Nowak and Gajecka, 2011). However none of the follow up studies has confirmed the role of these genes.
While the tear metabolome can be similar between ocular diseases such as dry eye and KC, the metabolites on KC patients have never been reported previously. In future studies, it would be interesting to include multiple ocular diseases and investigate tear metabolome differences and similarities.
Overall, we present a novel approach for KC investigations and it is certainly promising since the differences in metabolic activities could potentially suggest a metabolic treatment for KC disease.
5. Conclusions
Metabolomics measures the metabolite profile in body fluids or tissues and as such provides a profile of pathways and processes that are activated or altered in those samples. The results presented in this study strongly support further investigation of metabolomic analysis of ocular disease and more specifically KC. We found significant alterations in the oxidation state as well as the citric acid cycle and urea.
Highlights.
This is the first study to identify alterations in endogenous metabolites in KC tears.
TCA cycle and Urea cycle, both altered in KC individuals.
Severe oxidative stress levels were found in KC individuals.
Acknowledgments
The authors thank Min Yuan and Susanne Breitkopf for technical help with mass spectrometry experiments. The authors would also like to thank Dr. Robert E. Anderson for his input. This work was supported by National Institutes of Health/ National Eye Institute Grant 5R01EY023568 (D.K), 2P01CA120964 (J.M.A.) and Dana–Farber/Harvard Cancer Center Support Grant 5P30CA006516 (J.M.A.). Also, supported, in part, by an unrestricted grant from Research to Prevent Blindness, (New York, NY USA). The Pentacam HR at Aarhus University Hospital was kindly donated by Bagenkop-Nielsens Myopia Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D Karamichos, Email: dimitrios-karamichos@ouhsc.edu.
JD Zieske, Email: james_zieske@meei.harvard.edu.
H Sejersen, Email: henrseje@rm.dk.
A Sarker-Nag, Email: Akhee-SarkerNag@ouhsc.edu.
John M Asara, Email: jasara@bidmc.harvard.edu.
J Hjortdal, Email: jesper.hjortdal@dadlnet.dk.
REFERENCES
- Adamski J, Suhre K. Metabolomics platforms for genome wide association studies--linking the genome to the metabolome. Curr Opin Biotechnol. 2013;24:39–47. doi: 10.1016/j.copbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Al-Hussain H, Zeisberger SM, Huber PR, Giunta C, Steinmann B. Brittle cornea syndrome and its delineation from the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI): report on 23 patients and review of the literature. American journal of medical genetics. 2004;Part A 124A:28–34. doi: 10.1002/ajmg.a.20326. [DOI] [PubMed] [Google Scholar]
- Alberts B. Molecular Biology of the Cell. New York: Garland Science, New York: Garland Science; 2001. [Google Scholar]
- Aldave AJ, Yellore VS, Salem AK, Yoo GL, Rayner SA, Yang H, Tang GY, Piconell Y, Rabinowitz YS. No VSX1 gene mutations associated with keratoconus. Investigative ophthalmology & visual science. 2006;47:2820–2822. doi: 10.1167/iovs.05-1530. [DOI] [PubMed] [Google Scholar]
- Arnal E, Peris-Martinez C, Menezo JL, Johnsen-Soriano S, Romero FJ. Oxidative stress in keratoconus? Invest Ophth Vis Sci. 2011;52:8592–8597. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- Atilano SR, Coskun P, Chwa M, Jordan N, Reddy V, Le K, Wallace DC, Kenney MC. Accumulation of mitochondrial DNA damage in keratoconus corneas. Investigative ophthalmology & visual science. 2005;46:1256–1263. doi: 10.1167/iovs.04-1395. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Weitzman PD. Organization of citric acid cycle enzymes into a multienzyme cluster. FEBS letters. 1986;201:267–270. doi: 10.1016/0014-5793(86)80621-4. [DOI] [PubMed] [Google Scholar]
- Bechrakis N, Blom ML, Stark WJ, Green WR. Recurrent keratoconus. Cornea. 1994;13:73–77. doi: 10.1097/00003226-199401000-00012. [DOI] [PubMed] [Google Scholar]
- Behndig A, Karlsson K, Johansson BO, Brannstrom T, Marklund SL. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Investigative ophthalmology & visual science. 2001;42:2293–2296. [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. The World Allergy Organization journal. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth SC, Weljie AM, Turner RJ. Computational Tools for the Secondary Analysis of Metabolomics Experiments. Computational and structural biotechnology journal. 2013;4:e201301003. doi: 10.5936/csbj.201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DA, Anderson Penno EE. Effect of contact lens wear on central corneal thickness measurements. Journal of cataract and refractive surgery. 2003;29:1319–1322. doi: 10.1016/s0886-3350(03)00230-x. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Horwich AL. Urea cycle enzymes. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. McGraw-Hill; New York: 2001. p. 1909. [Google Scholar]
- Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50:341–351. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- Chaerkady R, Shao H, Scott SG, Pandey A, Jun AS, Chakravarti S. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. Journal of proteomics. 2013;87:122–131. doi: 10.1016/j.jprot.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai YC, Ashraf SS, Rokutan K, Johnston RB, Jr, Thomas JA. S-thiolation of individual human neutrophil proteins including actin by stimulation of the respiratory burst: evidence against a role for glutathione disulfide. Arch Biochem Biophys. 1994;310:273–281. doi: 10.1006/abbi.1994.1167. [DOI] [PubMed] [Google Scholar]
- Cheng EL, Maruyama I, SundarRaj N, Sugar J, Feder RS, Yue BY. Expression of type XII collagen and hemidesmosome-associated proteins in keratoconus corneas. Current eye research. 2001;22:333–340. doi: 10.1076/ceyr.22.5.333.5491. [DOI] [PubMed] [Google Scholar]
- Esgin H, Erda N. Corneal endothelial polymegethism and pleomorphism induced by daily-wear rigid gas-permeable contact lenses. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 2002;28:40–43. [PubMed] [Google Scholar]
- Friend J. Physiology of the cornea: metabolism and biochemistry. In: Smolin G, Thoft RA, editors. In the Cornea. Boston: Little, Brown and Co; 1983. [Google Scholar]
- Fu H, Khan A, Coe D, Zaher S, Chai JG, Kropf P, Muller I, Larkin DFP, George AJT. Arginine depletion as a mechanism for the immune privilege of corneal allografts. Eur J Immunol. 2011;41:2997–3005. doi: 10.1002/eji.201141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann WJ, Stanfield CL. Principles of Human Physiology. San Francisco: Benjamin Cummings, San Francisco: Benjamin Cummings; 2002. [Google Scholar]
- Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T, Mewes HW, Wichmann HE, Weinberger KM, Adamski J, Illig T, Suhre K. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. Plos Genet. 2008;4:e1000282. doi: 10.1371/journal.pgen.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondhowiardjo TD, van Haeringen NJ. Corneal aldehyde dehydrogenase, glutathione reductase, and glutathione S-transferase in pathologic corneas. Cornea. 1993;12:310–314. doi: 10.1097/00003226-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Gondhowiardjo TD, van Haeringen NJ, Völker-Dieben HJ, Beekhuis HW, Kok JH, van Rij G, Pels L, Kijlstra A. Analysis of corneal aldehyde dehydrogenase patterns in pathologic corneas. Cornea. 1993;12(2):146–154. doi: 10.1097/00003226-199303000-00010. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Steger-May K, Szczotka-Flynn L, Riley C, Joslin CE, Weissman BA, Fink BA, Edrington TB, Olafsson HE, Zadnik K, Clek Study G. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. American journal of ophthalmology. 2006;142:923–930. doi: 10.1016/j.ajo.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Greiner JV, Kopp SJ, Glonek T. Phosphorus nuclear magnetic resonance and ocular metabolism. Surv Ophthalmol. 1985;30:189–202. doi: 10.1016/0039-6257(85)90063-3. [DOI] [PubMed] [Google Scholar]
- Gundel RE, Libassi DP, Zadnik K, Barr JT, Davis L, McMahon TT, Edrington TB, Gordon MO. Feasibility of fitting contact lenses with apical clearance in keratoconus. Optometry and vision science : official publication of the American Academy of Optometry. 1996;73:729–732. doi: 10.1097/00006324-199612000-00002. [DOI] [PubMed] [Google Scholar]
- Holden BA, Sweeney DF, Vannas A, Nilsson KT, Efron N. Effects of long-term extended contact lens wear on the human cornea. Investigative ophthalmology & visual science. 1985;26:1489–1501. [PubMed] [Google Scholar]
- Hwang JS, Lee JH, Wee WR, Kim MK. Effects of multicurve RGP contact lens use on topographic changes in keratoconus. Korean journal of ophthalmology : KJO. 2010;24:201–206. doi: 10.3341/kjo.2010.24.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinabhai A, O'Donnell C, Radhakrishnan H. Changes in refraction, ocular aberrations, and corneal structure after suspending rigid gas-permeable contact lens wear in keratoconus. Cornea. 2012;31:500–508. doi: 10.1097/ICO.0b013e31820f777b. [DOI] [PubMed] [Google Scholar]
- Joseph R, Srivastava OP, Pfister RR. Differential epithelial and stromal protein profiles in keratoconus and normal human corneas. Experimental eye research. 2011;92:282–298. doi: 10.1016/j.exer.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Hutcheon AE, Rich CB, Trinkaus-Randall V, Asara JM, Zieske JD. In vitro model suggests oxidative stress involved in keratoconus disease. Scientific reports. 2014;4:4608. doi: 10.1038/srep04608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MC, Brown DJ, Rajeev B. Everett Kinsey lecture. The elusive causes of keratoconus: a working hypothesis. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 2000;26:10–13. [PubMed] [Google Scholar]
- Klyce SD. Stromal lactate accumulation can account for corneal oedema osmotically following epithelial hypoxia in the rabbit. J Physiol. 1981;321:49–64. doi: 10.1113/jphysiol.1981.sp013971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- Lane N. Life Ascending: The Ten Great Inventions of Evolution. W W Norton & Co Inc; 2009. [Google Scholar]
- Leonard JV, Morris AA. Urea cycle disorders. Seminars in neonatology : SN. 2002;7:27–35. doi: 10.1053/siny.2001.0085. [DOI] [PubMed] [Google Scholar]
- Liu Z, Pflugfelder SC. The effects of long-term contact lens wear on corneal thickness, curvature, and surface regularity. Ophthalmology. 2000;107:105–111. doi: 10.1016/s0161-6420(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Lowenstein JM. Methods in Enzymology, Volume 13: Citric Acid Cycle. Boston: Academic Press; 1969. [Google Scholar]
- Maurice DM. The regulation of corneal regulation. In: King JH, McTigue JW, editors. In the Cornea: World congress. London: Butterworths; 1965. [Google Scholar]
- Miller D. Contact lens-induced corneal curvature and thickness changes. Archives of ophthalmology. 1968;80:430–432. doi: 10.1001/archopht.1968.00980050432004. [DOI] [PubMed] [Google Scholar]
- Montes-Mico R, Cervino A, Ferrer-Blasco T, Garcia-Lazaro S, Madrid-Costa D. The tear film and the optical quality of the eye. The ocular surface. 2010;8:185–192. doi: 10.1016/s1542-0124(12)70233-1. [DOI] [PubMed] [Google Scholar]
- Monty K, Matthew PS, Matsudaira PT, Lodish HF, Darnell JE, Lawrence Z, Kaiser C, Arnold B. Molecular Cell Biology. Fifth Edition, 5th ed. San Francisco: W. H. Freemanp; 2003. p. 973. [Google Scholar]
- Moreira LB, Alchieri JC, Belfort R, Jr, Moreira H. [Psychological and social aspects of patients with keratoconus] Arquivos brasileiros de oftalmologia. 2007;70:317–322. doi: 10.1590/s0004-27492007000200023. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. USA: Worth Publishersp; 2000. p. 724. [Google Scholar]
- Nguyen TT, Bonanno JA. Lactate-H? transport is a significant component of the in vivo corneal endothelial pump. Invest Ophth Vis Sci. 2012;53:2020–2029. doi: 10.1167/iovs.12-9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K, Heegaard S, Vorum H, Birkenkamp-Demtroder K, Ehlers N, Orntoft TF. Altered expression of CLC, DSG3, EMP3, S100A2, and SLPI in corneal epithelium from keratoconus patients. Cornea. 2005;24:661–668. doi: 10.1097/01.ico.0000153556.59407.69. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Hjortdal J, Pihlmann M, Corydon TJ. Update on the keratoconus genetics. Acta Ophthalmol. 2013;91:106–113. doi: 10.1111/j.1755-3768.2012.02400.x. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Vorum H, Fagerholm P, Birkenkamp-Demtroder K, Honore B, Ehlers N, Orntoft TF. Proteome profiling of corneal epithelium and identification of marker proteins for keratoconus, a pilot study. Experimental eye research. 2006;82:201–209. doi: 10.1016/j.exer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Nowak DM, Gajecka M. The genetics of keratoconus. Middle East African journal of ophthalmology. 2011;18:2–6. doi: 10.4103/0974-9233.75876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Risa O, Saether O, Lofgren S, Soderberg PG, Krane J, Midelfart A. Metabolic changes in rat lens after in vivo exposure to ultraviolet irradiation: measurements by high resolution MAS 1H NMR spectroscopy. Invest Ophth Vis Sci. 2004;45:1916–1921. doi: 10.1167/iovs.03-1292. [DOI] [PubMed] [Google Scholar]
- Romero-Jimenez M, Santodomingo-Rubido J, Flores-Rodriguez P, Gonzalez-Meijome JM. Short-term corneal changes with gas-permeable contact lens wear in keratoconus subjects: A comparison of two fitting approaches. Journal of optometry. 2014 doi: 10.1016/j.optom.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2010;33:157–166. doi: 10.1016/j.clae.2010.04.006. quiz 205. [DOI] [PubMed] [Google Scholar]
- Saijyothi AV, Fowjana J, Madhumathi S, Rajeshwari M, Thennarasu M, Prema P, Angayarkanni N. Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Experimental eye research. 2012;103:41–46. doi: 10.1016/j.exer.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Srivastava OP, Chandrasekaran D, Pfister RR. Molecular changes in selected epithelial proteins in human keratoconus corneas compared to normal corneas. Molecular vision. 2006;12:1615–1625. [PubMed] [Google Scholar]
- Szczotka LB, Rabinowitz YS, Yang H. Influence of contact lens wear on the corneal topography of keratoconus. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 1996;22:270–273. [PubMed] [Google Scholar]
- Thies RS, Mandel LJ. Role of glucose in corneal metabolism. The American journal of physiology. 1985;249:C409–C416. doi: 10.1152/ajpcell.1985.249.5.C409. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Mashima Y, Murata H, Sato N, Ogata T. Corneal epithelium in keratoconus. Cornea. 1995;14:77–83. [PubMed] [Google Scholar]
- Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2007;30:223–232. doi: 10.1016/j.clae.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrendorf I. [How to live with keratoconus] Klin Monbl Augenheilkd. 2006;223:877–888. doi: 10.1055/s-2006-927144. [DOI] [PubMed] [Google Scholar]
- Wang X, McCulley JP, Bowman RW, Cavanagh HD. Time to resolution of contact lens-induced corneal warpage prior to refractive surgery. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 2002;28:169–171. doi: 10.1097/01.ICL.0000018042.02034.AB. [DOI] [PubMed] [Google Scholar]
- Webhofer C, Gormanns P, Reckow S, Lebar M, Maccarrone G, Ludwig T, Putz B, Asara JM, Holsboer F, Sillaber I, Zieglgansberger W, Turck CW. Proteomic and metabolomic profiling reveals time-dependent changes in hippocampal metabolism upon paroxetine treatment and biomarker candidates. J Psychiatr Res. 2013;47:289–298. doi: 10.1016/j.jpsychires.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Yeniad B, Alparslan N, Akarcay K. Eye rubbing as an apparent cause of recurrent keratoconus. Cornea. 2009;28:477–479. doi: 10.1097/ICO.0b013e31818d37fa. [DOI] [PubMed] [Google Scholar]
- Young JW. Gluconeogenesis in cattle: significance and methodology. Journal of dairy science. 1977;60:1–15. doi: 10.3168/jds.S0022-0302(77)83821-6. [DOI] [PubMed] [Google Scholar]
- Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadnik K, Barr JT, Steger-May K, Edrington TB, McMahon TT, Gordon MO Collaborative Longitudinal Evaluation of Keratoconus Study G. Comparison of flat and steep rigid contact lens fitting methods in keratoconus. Optometry and vision science : official publication of the American Academy of Optometry. 2005;82:1014–1021. doi: 10.1097/01.opx.0000192349.11525.de. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Mutti DO. Contact lens fitting relation and visual acuity in keratoconus. American journal of optometry and physiological optics. 1987;64:698–702. doi: 10.1097/00006324-198709000-00009. [DOI] [PubMed] [Google Scholar]
- Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]