Summary
The parasite Trichomonas vaginalis is the causative agent of trichomoniasis, a prevalent sexually transmitted infection. Here, we report the cellular analyses of T. vaginalis tetraspanin 6 (TvTSP6). This family of membrane proteins has been implicated in cell adhesion, migration and proliferation in vertebrates. We observed that TvTSP6 expression is upregulated upon contact with vaginal ectocervical cells (VECs) and that parasite strains that are highly adherent to VECs express higher levels of TvTSP6 mRNA relative to poorly adherent strains. TvTSP6 is localized predominantly on the flagella of parasites cultured in the absence of host cells; however, adherence of the parasite to VECs initially results in a redistribution of the protein to intracellular vesicles and the plasma membrane of the main body of the cell. We found that a 16-amino-acid C-terminal intracellular tail of TvTSP6 is necessary and sufficient for flagellar localization and protein redistribution when the parasite is in contact with VECs. Additionally, deletion of the C-terminal tail reduced parasite migration through Matrigel, a mimic of the extracellular matrix. Together, our data support roles for TvTSP6 in parasite migration in the host and sensory reception during infection.
Introduction
Trichomonas vaginalis, an extracellular flagellated protozoan, is the cause of trichomoniasis, a sexually transmitted infection that affects over 248 million people annually worldwide (World Health Organization, 2005). Although asymptomatic infection by T. vaginalis is common, multiple symptoms and pathologies can arise in both men and women, including vaginitis, urethritis, prostatitis, low-birth-weight infants, preterm delivery, premature rupture of membranes and infertility (Swygard et al., 2004; Fichorova, 2009). Additionally, infection by this parasite is associated with the development of cervical and prostate cancer (Gander et al., 2009; Stark et al., 2009; Sutcliffe et al., 2009) and an increased susceptibility to human immunodeficiency virus (HIV) infection (McClelland et al., 2007; Van Der Pol et al., 2008). These severe complications and high incidence of infection underscore the need to identify new diagnostic methods and drug targets and to advance vaccine development. Understanding the mechanisms by which T. vaginalis colonizes the host is central to developing strategies to prevent infection. Despite the prevalence of trichomoniasis, the underlying biochemical processes that lead to pathogenesis are poorly defined (Fiori et al., 1999; Hirt et al., 2011; Ryan et al., 2011). As an extracellular organism, surface proteins are likely to play important roles in the initial adherence to mucosal tissue as well as the long-term survival of the pathogen on mucosal surfaces. We have previously identified ∼ 400 putative T. vaginalis surface proteins (de Miguel et al., 2010); however, with the exception of two, their possible roles in pathogenesis remain undefined.
One class of proteins identified were the tetraspanins (TSPs). This family of cell surface proteins has a common structure that includes four hydrophobic transmembrane domains, two extracellular domains characterized by a high concentration of hydrophilic amino acids, and short intracellular amino and carboxyl tails (Hemler, 2005). TSPs modulate a variety of fundamental biological processes such as adhesion, migration, proliferation and fusion by functioning as organizers of multimolecular membrane complexes, termed ‘tetraspanin-enriched microdomains’ (Hemler, 2003; 2005; Pols and Klumperman, 2009). The extensive spectrum of biological functions in which TSP involvement has been implicated is compatible with their wide distribution in multiple cell types and organisms, and indicates their functional importance. As a number of these processes are likely critical for T. vaginalis to colonize its host, we hypothesize that TSPs could act as modulators of host : pathogen interactions. In mammalian cells, a ‘transmembrane linker’ model for TSPs has been proposed (Hemler, 1998). In this model, TSP extracellular domains link to integrins, whereas cytoplasmic domains link to intracellular signalling enzymes such as phosphatidylinositol 4-kinase and PKC (Hemler, 1998; Yauch and Hemler, 2000; Zhang et al., 2001). Here, we have examined a T. vaginalis TSP, called TvTSP6, that was identified in the plasma membrane surface proteome (de Miguel et al., 2010). Our data indicate roles for this protein in parasite migration and sensory reception during infection and provide support for a transmembrane linker model in which the C-terminal tail of a TSP links with intracellular pathways to mediate these activities.
Results
TvTSP6 localizes to the flagellar membrane of parasites cultured in the absence of host cells
We have previously determined the surface proteome of T. vaginalis to identify proteins on the surface of the parasite that might be involved in pathogenesis (de Miguel et al., 2010). Among others, these analyses revealed the presence of three members of the TSP family. As mammalian TSPs participate in adhesion and migration, which are processes T. vaginalis uses to colonize its host, we have examined one of these proteins called TvTSP6 (TVAG_460770). The gene encoding TvTSP6 was cloned and expressed under the control of the α-SCS promoter as a C-terminally haemagglutinin (HA)-tagged protein in T. vaginalis strain B7RC2. We then determined the localization of the tagged protein in transfected cells using immunofluorescence assays. As predicted, TvTSP6 was found to be present on the plasma membrane (Fig. 1A). The protein was also found to localize to intracellular vesicles (Fig. 1B). In addition to these locations, the protein is present on the flagellar membrane (Fig. 1A and C). On virtually all cells for which the flagella was in the visible plane, the bulk of the TvTSP6 signal was found on the flagella membrane (see arrow, Fig. 1A), with lesser signal on the plasma membrane and/or intracellular vesicles. TvTSP6 was detectable only on the flagella in ∼ 20% of the cells (Fig. 1D). The TvTSP6 signal is typically stronger on the flagellar membrane than on the plasma membrane or vesicles. This is likely due both to a greater abundance of the protein on flagellar membranes and to a fusion of multiple signals as the four anterior flagella tend to cluster.
Fig. 1.
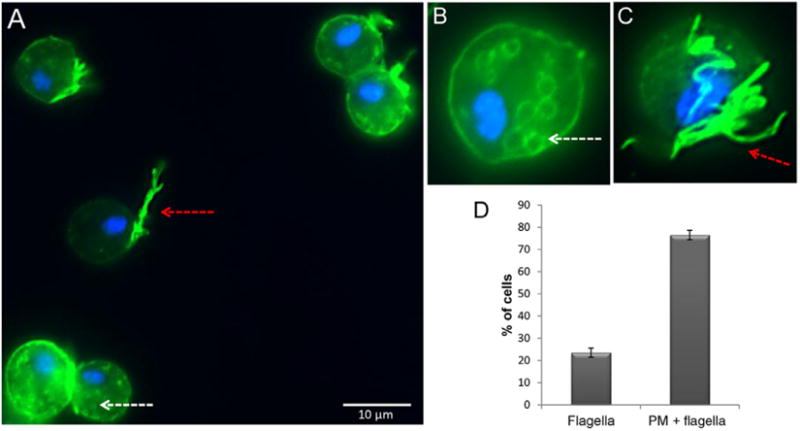
Subcellular localization of TvTSP6 in T. vaginalis transfectants.
A–C. Cells exogenously expressing TvTSP6 with a C-terminal haemagglutinin (HA) tag stained for immunofluorescence microscopy using a mouse anti-HA antibody. The nucleus (blue) was also stained with 4′,6′-diamidino-2-phenylindole (DAPI). Red arrows indicate the flagella and white arrows indicate intracellular vesicles. Scale bar, 10 μm.
D. Percentage of cells with TSP6 detected on the flagella only (flagella) or flagella and plasma membrane (flagella + PM) ± the standard deviation of the mean is indicated. One hundred parasites were counted in triplicate in four independent experiments.
TvTSP6 mRNA is more abundant in parasites that are highly adherent and cytolytic to vaginal ectocervical cells (VECs)
We compared TvTSP6 mRNA levels between six strains with different capacities to adhere to VECs. Three strains, T1, G3 and SD10 which are poorly adherent and are five-to 15-fold less adherent than strains B7268, B7RC2 and SD7 (de Miguel et al., 2010), were compared. As shown in Fig. 2A, qPCR analyses revealed that the expression level of TvTSP6 correlates with the adherence and cytotoxicity of the strains. TvTSP6 is five-, seven- and ten-fold more highly expressed in adherent strains B7268, B7RC2 and SD7, respectively, relative to the less adherent G3 strain (Fig. 2A). The greater abundance in mRNA expression observed in highly adherent versus less adherent parasites is consistent with an involvement of TvTSP6 in host : parasite interactions.
Fig. 2.
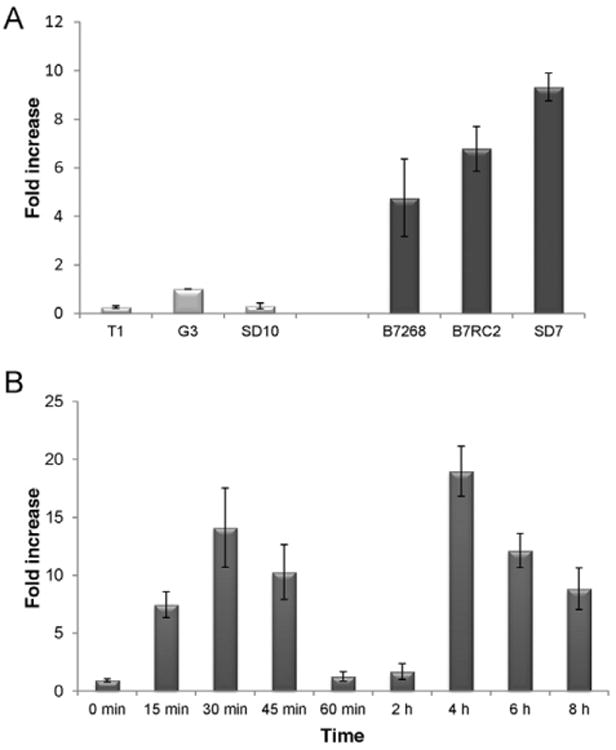
Expression analysis of TvTSP6.
A. mRNA expression levels of TvTSP6 in previously described (de Miguel et al., 2010) strains with different adherence capacities were analysed by qPCR. Data are expressed as fold increase compared with the poorly adherent G3 strain ± the standard deviation of the mean. Every sample and points of the standard curve were carried out in duplicates in three independent experiments.
B. Endogenous TvTSP6 mRNA expression upon exposure to host cells. B7RC2 parasites were exposed to VECs and the kinetics of TSP6 expression was analysed by qPCR. Data are expressed as fold increase compared with time 0 min ± the standard deviation of the mean. TvTSP6 mRNA levels are upregulated in contact with VECs, increasing ∼14-fold at 30 min. Levels subsequently decrease dramatically at 60 min and 2 h and then increased again ∼19-fold after 4 h of exposure to VECs. Every sample and point on the standard curve were carried out in duplicates in three independent experiments.
TvTSP6 is redistributed upon binding of the parasite to host cells
To evaluate a possible involvement of TvTSP6 in host–parasite interactions, parasites transfected with the TvTSP6–HA constructs described above were exposed to VECs for 0.5 to 6 h, immunostained with an anti-HA antibody and examined using fluorescence microscopy (Fig. 3). As shown in Fig. 1, in the absence of host cells, TvTSP6 is found preferentially on the flagellar membrane and present at lower levels on the plasma membrane and intracellular vesicles of parasites. Upon exposure to VECs we found that the TvTSP6 localization changes. Within 30 min of contact with host cells, the protein is found on the plasma membrane and flagella membrane (> 95%), whereas after 3 h ∼ 15% of the parasites have TvTSP6 protein only on the flagella and after 6 h > 50% of the cells have flagellar-only TvTSP6 (Fig. 3).
Fig. 3.
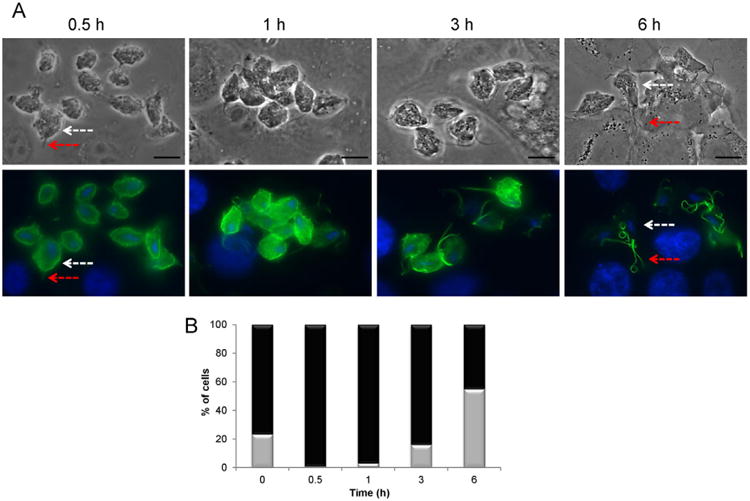
Subcellular localization of T. vaginalis TvTSP6 of parasites bound to VECs.
A. Parasites expressing the TvTSP6–HA-tagged construct were bound to VECs for 0.5, 1, 3 and 6 h and TvTSP6 was localized using an anti-HA antibody. The localization changed from primarily flagella in the absence of host cells (Fig. 1) to primarily plasma membrane and intracellular vesicles, returning to its original flagellar localization at 6 h post host cell exposure. Red arrows indicate the flagella and white arrows indicate intracellular vesicles and the plasma membrane of the main body of the cell. Under these conditions, the host cells are still intact at 6 h. Scale bar, 10 μm.
B. Percentage of cells with TSP6 present predominantly on the flagella (grey columns) or flagella and plasma membrane (black columns) during exposure to VECs. One hundred parasites were counted in triplicate in four independent experiments.
We have also exposed untransformed parasites to VECs and have followed the expression of endogenous TvTSP6 by qPCR (Fig. 2B). In agreement with immunofluorescence data shown in Fig. 3, TvTSP6 was found to be upregulated upon contact with VECs, increasing ∼ 14-fold after 30 min. Expression subsequently decreased between 30 min and 2 h and then increased again ∼ 19-fold after 4 h of exposure to VECs (Fig. 2B). The observed bipartite upregulation of TvTSP6 over time suggests multiple functions for this protein. Since in vitro attachment of the parasites is mostly completed within ∼20 min of exposure to the VECs (Okumura et al., 2008), the second wave of upregulation suggests TvTSP6 plays a role in events occurring downstream of adherence.
The C-terminal tail of TvTSP6 is essential for flagellar targeting and protein redistribution upon parasite binding to VECs
As depicted in Fig. 4A, TSPs span the membrane four times, and have two external domains and short N-terminal and C-terminal tails. The C-terminal cytoplasmic tail has been shown to play critical roles in determining the functional consequences of ligand binding for mammalian TSPs (Zhang et al., 2002; Latysheva et al., 2006). Therefore, we investigated whether the C-terminal tail of TvTSP6 contributes to the targeting of the protein to the flagellar membrane. An expression construct lacking the 16-amino-acid C-terminal tail (TvTSP6ΔCt) was trans-fected into parasites (Fig. 4A). Unlike full-length TvTSP6, TVTSP6ΔCt does not localize to the flagella (Fig. 4B). Hundreds of parasites with visual flagella were examined and no flagellar TvTSP6 was detected (data not shown). Instead, TVTSP6ΔCt localized exclusively to the plasma membrane and intracellular vesicles and its localization did not change when transfected parasites bound to VECs (Fig. 4C) in stark contrast to the redistribution observed for full-length TvTSP6 (Fig. 3A). These data demonstrate that the cytoplasmic tail is necessary for both flagellar targeting and cellular redistribution of the protein upon the binding of the parasite to host cells.
Fig. 4.
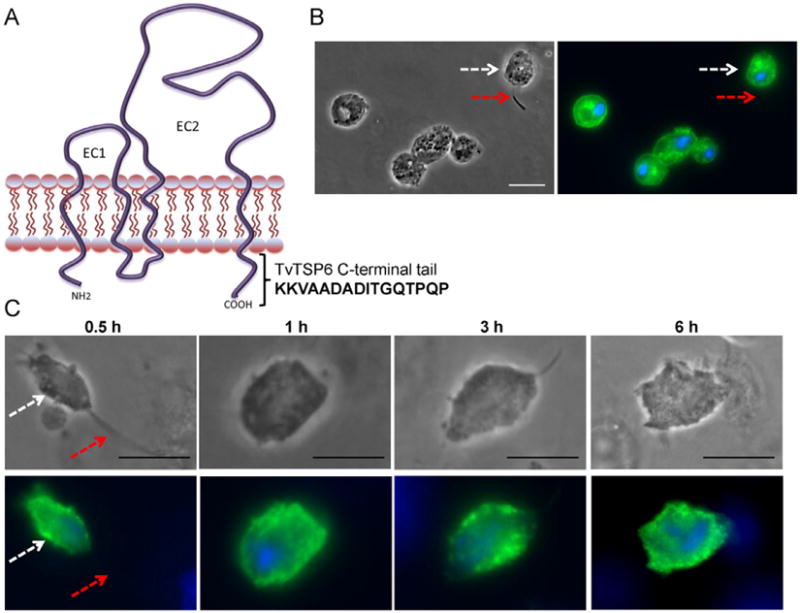
Subcellular localization of TvTSP6ΔCt in absence and presence of host cells using immunofluorescence microscopy.
A. The predicted structure of TvTSP6 in the plasma membrane is shown. EC1 and EC2 = extracellular domains; cytoplasmic NH2 and C-terminal tails are depicted, and the 16 amino acids deleted from the C-terminal tail of TvTSP6ΔCt are shown.
B. Subcellular localization of TvTSP6ΔCt in the absence of host cells is detected using an anti-HA antibody. Flagella (red arrows) and the main body of the cell (white arrows) were indicated. Note the lack of signal in the flagella.
C. Subcellular localization of TSP6ΔCt in parasites bound to VECs. No change in localization is observed. One hundred parasites were counted in triplicate in four independent experiments. Scale bar, 10 μm.
The C-terminal tail of TvTSP6 is sufficient for flagella targeting and protein redistribution upon the binding of parasites to VECs
The T. vaginalis genome encodes nine TSP genes (http://www.trichdb.org) and all are predicted to have a short C-terminal cytoplasmic tail following the fourth transmembrane domain (N. de Miguel and P.J. Johnson, unpubl. data). To further analyse the role of the C-terminal tail of TvTSP6 we prepared two HA-tagged, chimeric constructs replacing the C-terminal cytoplasmic tail of two different TSPs (TSP1 and TSP3) with the 16-amino-acid C-terminal tail of TvTSP6. Non-chimeric constructs expressing TSP1–HA and TSP3–HA were also prepared and transfected into parasites. As shown in Fig. 5, TSP1–HA and TSP3–HA are detected primarily on the plasma membrane and intracellular vesicles, with a very faint signal found on the flagellar membrane. In contrast, localization of the chimeric proteins (TSP1CT6 and TSP3CT6) is similar to that of full-length TvTSP6, being detected predominantly on the flagella, with less signal on the plasma membrane and vesicles (Fig. 5).
Fig. 5.
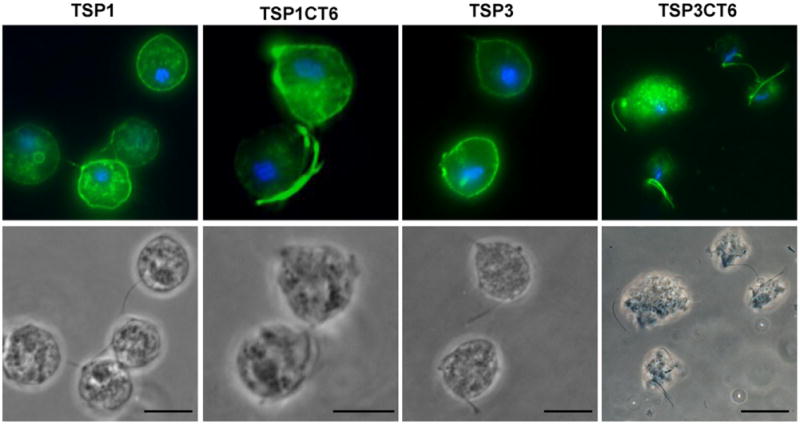
Subcellular localization of TSP1, TSP1CT6, TSP3 and TSP3CT6 in absence of host cells. Cells expressing C-terminal HA-tagged versions of TSP3 (TVAG_280860), TSP3CT6 (chimera with the C-terminal tail of TSP3 replaced by the C-terminal tail of TvTSP6), TSP1 (TVAG_019180) and TSP1CT6 (chimera with the C-terminal tail of TSP1 replaced by the C-terminal tail of TvTSP6) were stained for immunofluorescence microscopy using an anti-HA antibody. The nucleus (blue) was also stained with DAPI. Scale bar, 10 μm. Note the intensity of flagella signal on the chimeric proteins TSP1CT6 and TSP3CT6 relative to wild-type TSP1 and TSP3 proteins.
To address whether the C-terminal tail of TvTSP6 is responsible for protein redistribution upon contact with host cells, TSP1CT6- and TSP3CT6-transfected parasites were allowed to bind to VECs. A change in localization of TSP1CT6 and TSP3CT6 that mirrors that observed for TvTSP6 was seen when transfected parasites bound VECs (Figs 6 and S1). These results indicate that the C-terminal tail of TvTSP6 is sufficient for protein redistribution upon VECs exposure.
Fig. 6.
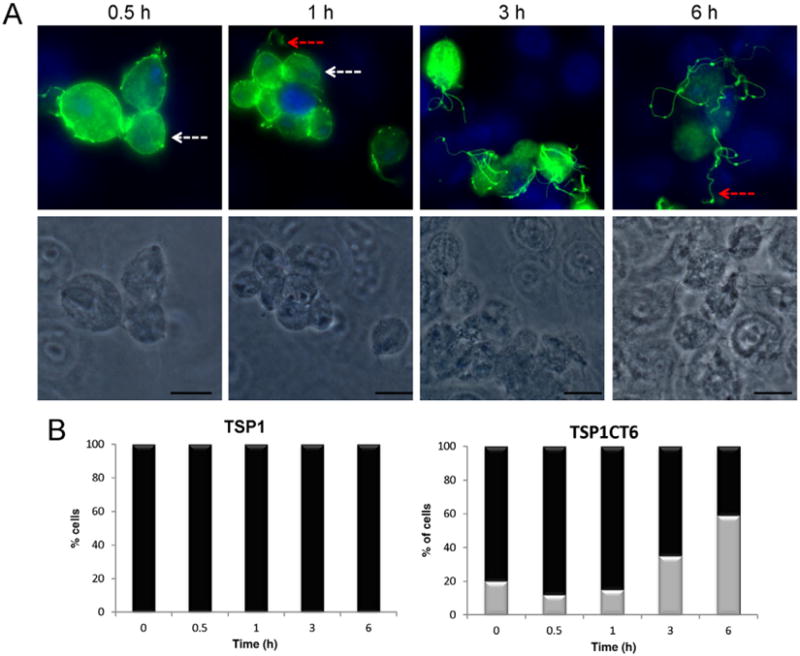
Subcellular localization of the TvTSP1CT6 protein in parasites bound to VECs.
A. Parasites transfected with TvTSP1CT6 containing a C-terminal HA tag were bound to VECs for 0.5, 1, 3 and 6 h and the protein was localized using an anti-HA antibody. Note the change in localization from plasma membrane and intracellular vesicles to flagellar localization at 3 and 6 h post host cell exposure. Flagella (red arrows) and the main body of the cell (white arrows) were indicated. Scale bar, 10 μm.
B. Percentage of cells transfected with TvTSP1 and TvTSP1CT6 with the respective protein detected on the flagella only (grey columns) or flagella and plasma membrane (black columns) during exposure to VECs. One hundred parasites were counted in triplicate in four independent experiments.
As shown in Fig. 4A, there are two threonines (T) in the 16-amino-acid TvTSP6 tail. To assess whether phosphorylation of these threonines is required for the targeting and distribution of the protein, the two residues were mutated to alanine. The localization of the mutant protein was indistinguishable from that of the full-length wild-type protein (data not shown). Hence, the two threonine residues present in the C-terminal tail are not necessary for flagellar targeting or protein redistribution.
TvTSP6 plays a role in parasite migration
To adhere to epithelial cells of the host's urogenital tract, T. vaginalis must migrate through the extracellular matrix (ECM) that surrounds these cells. As mammalian TSPs are known to be involved in cellular migration (Hemler, 1998; Yauch and Hemler, 2000; Zhang et al., 2001), we tested whether TvTSP6 facilitates parasite migration through the ECM. A commercial invasion system in which parasites are placed in a cell culture insert and assessed for their ability to pass through 8 μm diameter pores impregnated with ECM proteins (Matrigel™) was used. Parasites were transfected with either an episomal vector expressing TvTSP6 or TVTSP6ΔCt expressing the truncated C-terminal version of TvTSP6 (see Fig. 4A). At increasing times post inoculation, the number of parasites that migrated through the Matrigel and crossed into the bottom chamber was counted. After 3 h, 84% of the TvTSP6-transfected parasites had migrated into the lower chamber compared to 70% of TvTSP6ΔCt transfectants (Fig. 7). Six hours after inoculation the differences were even more pronounced, with 100% of the TvTSP6 transfectants having migrated through the Matrigel, compared to 70% for the TVTSP6ΔCt transfectants. Migration of parasites mock-transfected with an empty vector was similar to that observed for TvTSP6 transfectants (data not shown). These results indicate a role for TvTSP6 in migration wherein the C-terminal tail modulates migration as its deletion substantially reduces the percentage of migrating parasites.
Fig. 7.
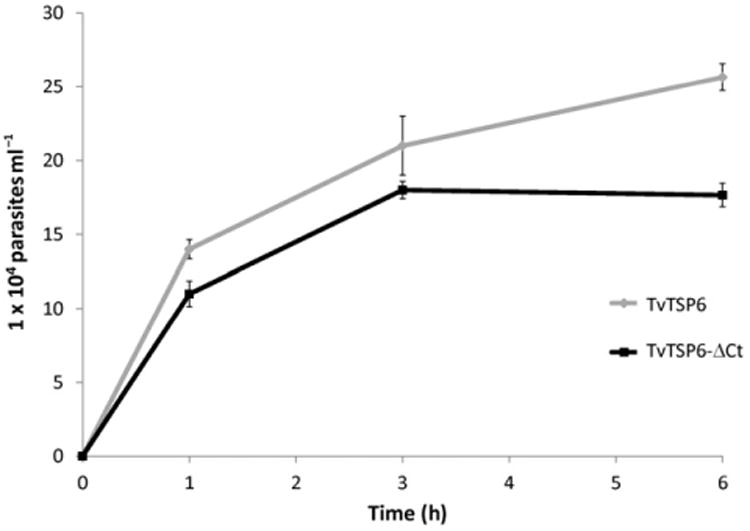
Cell migration through Matrigel. Migration of parasites transfected with EpNEO, an empty episomal expression vector (black line), or TvTSP6ΔCt, a truncated C-terminal version of TvTSP6 (grey line), was determined over a 6 h period. Every sample in each point of the curve was carried out in triplicates. Error bars indicate standard deviations. A representative experiment of six independent experiments is shown. Note that after 6 h, 100% of the TvTSP6-transfected parasites had migrated into the lower chamber compared to 70% of TVTSP6ΔCt transfectants.
Discussion
In this study we have identified a flagellar protein, TvTSP6, of the parasite T. vaginalis that is a member of the TSP family of integral membrane proteins. TSPs have primarily been studied in mammals and worms where they have been shown to reside in the plasma membrane and the membranes of intracellular vesicles (Hemler, 2003; 2005; Hong et al., 2005; Tran et al., 2006; Huang et al., 2007; Pearson et al., 2012). Although TvTSP6 is predominantly in the flagellar membrane when the parasite is not in contact with host cells, we found that it redistributes to the plasma membrane of the cell body and intracellular vesicles upon initial binding to host cells (VECs). Interestingly, TvTSP6 then reappears primarily on the flagella within 3 h of exposure to VECs. As the flagella is known to function in sensory reception in addition to motility (Scholey, 2003), redistribution of TvTSP upon host cell exposure suggests a role for this protein in sensing external host-derived stimuli.
Interestingly, the only sequenced parasitic protist genomes that contain TSP genes are those of T. vaginalis and Entamoeba histolytica (N. de Miguel, our unpublished BLAST analyses) and the gene does not appear to have been conserved in Trypanosoma, Leishmania, Plasmodium or Toxoplasma. This suggests that TSPs may be specifically involved in the pathogenesis of a subgroup of extracellular mucosal parasites. Consistent with this we found that expression levels of TvTSP6 correlate with the ability of different T. vaginalis strains to adhere to host VECs. Additionally, expression is upregulated upon adherence, consistent with a role for TvTSP6 in parasite: host cell interactions.
Tetraspanins are characterized by the presence of N-terminal and C-terminal intracellular tails that have been implicated in ‘outside-in’ signalling in mammalian cells (Zhang et al., 2002; Latysheva et al., 2006). Here we have demonstrated that the 16 amino acids composing the C-terminal tail of TvTSP6 are necessary and sufficient for TSP localization to the flagellar membrane. Using domain-swapping experiments the C-terminal tail of TvTSP6 was shown to be capable of redirecting and preferentially targeting T. vaginalis TSP1 and TSP3 to the flagellar membrane. We propose that this 16-amino-acid tail is recognized by a protein(s) involved in flagellar targeting, thus mediating the association of TvTSP6 with the flagellar membrane protein trafficking machinery (Tobin and Beales, 2009). Little is known about the mechanisms used for targeting membrane proteins to the flagella; however, for the parasite Trypanosoma brucei, lipid rafts and palmitoylation appear to play crucial roles (Emmer et al., 2009; 2010). In this regard, it is notable that mammalian TSPs are known to reside in microdomains within the plasma membrane and to be palmitoylated (Hemler, 2005). We have also determined that TvTSP6 is palmitoylated (N. de Miguel et al., unpubl. data).
The pathobiology of T. vaginalis is multifaceted and involves the adhesion to and alteration of various mucosal landmarks: mucus, epithelial cell barrier, ECM, innate and adaptive immune cells, and bacterial microflora. These interactions are thought to be critical in initiating and maintaining infections (Fiori et al., 1999; Lehker and Alderete, 2000; Noel et al., 2010; Hirt et al., 2011; Ryan et al., 2011). Using an in vitro system that mimics the ECM, we demonstrated that TvTSP6 influences the migration of the parasite. When the C-terminal tail of this protein was deleted, the cell migration through Matrigel was reduced. We propose that the TvTSP6 C-terminal tail mutant (TVTSP6ΔCt) has a dominant negative effect on endogenous TvTSP6, similar to that observed upon the deletion of the C-terminal tail of the TSP protein CD151 (Zhang et al., 2002). This might be explained by the mutant TVTSP6ΔCt shifting the stoichiometric balance of endogenous wild-type TvTSP6 via the formation of oligomeric complexes between the two proteins. This in turn could disrupt critical TvTSP6 tail-dependent interactions involved in cellular migration, such as the interaction of the tail with proteases that degrade ECM. However, this is only one of several possible explanations; defining the mechanism underlying the observed reduction in migration awaits future studies. Although mammalian TSPs have been shown to be involved in cell migration (Liu et al., 2007; Powner et al., 2011) it also remains unclear which cellular processes are affected in this system.
The migration of T. vaginalis through Matrigel requires at least two activities: cell motility and degradation of ECM proteins. Mammalian TSPs are known to regulate cell motility (Maecker et al., 1997; Yanez-Mo et al., 1998; 2009) providing precedent for a possible role for TvTSP6 in promoting motility, a property of the parasite thought to be important for adhering to host cells. Alternatively, TvTSP6 may play a role in ECM degradation. Consistent with this, in mammalian cells, specific TSPs regulate proteolytic activity of metalloproteases (Sugiura and Ber-ditchevski, 1999; Lafleur et al., 2009) and/or are involved in post-adhesion signalling (Hemler, 2003; 2005).
A transmembrane linker role has also been proposed for TSPs (Hemler, 1998) wherein extracellular domains of the protein link to external proteins while cytoplasmic domains link to intracellular enzymes to mediate, for example, ‘outside-in’ signalling (Hemler, 1998; Yauch and Hemler, 2000; Zhang et al., 2001). Similarly, intracellular TSP tail domains are thought to associate with signalling molecules such as PtdIns 4-K (Berditchevski et al., 1997; Yauch and Hemler, 2000) and PKC (Zhang et al., 2001). Our observations that the C-terminal cytoplasmic tail of TvTSP6 is involved in the redistribution of the protein upon exposure to host cells and parasite migration add further support for TSPs acting as transmembrane linkers. In conclusion, our results support a function for TvTSP6 in sensory reception and migration of T. vaginalis. Additional studies will be required to better define how these functions are mediated by TvTSP6, the first flagellar protein identified in this extracellular parasite.
Experimental procedures
Parasites, cell culture and media
The T. vaginalis strain B7RC2 (PA strain, ATCC 50167) was cultured in Diamond's Trypticase-yeast extract-maltose (TYM) medium supplemented with 10% horse serum, penicillin, streptomycin (Invitrogen) and iron as previously described (Clark and Diamond, 2002). Parasites were grown at 37°C and passaged daily. The human cervical ectocervical cell line Ect1 E6/E7 (ectocervical, ATCC CRL-2614), referred to as VECs, were grown as previously described (Fichorova et al., 1997) in keratinocyte-SFM complemented with provided recombinant protein supplements, penicillin, streptomycin and cultured at 37°C/5% CO2.
Quantitative PCR (qPCR)
RNA was extracted from ∼ 4 × 106 T. vaginalis from strains with different adherence capacities (de Miguel et al., 2010) using TRIzol (Invitrogen) following the manufacturer's instructions. For exposure to host cells, ∼ 107 B7RC2 parasites were incubated with vaginal epithelial cells (VECs) for various times, unattached parasites were removed and RNA was subsequently prepared from attached parasites and VECs scraped from the plate. Total RNA was treated with amplification grade DNase I (Invitrogen) and reverse transcribed using SuperScript III reverse transcriptase and oligo(dT) primers (Invitrogen). Real-time PCRs were performed using Brilliant SYBR Green qPCR Master Mix (Stratagene), a 150–450 nM concentration of each primer, and 200–500 ng of cDNA in a 20 μl reaction volume using an Eppen-dorf Mastercycler and realplex v.1.5 (Eppendorf). Parallel reactions performed without reverse transcriptase were included as negative controls. During the exponential phase of the qPCR, threshold cycle (CT) and base lines were set according to Eppendorf Mastercycler protocols. Data from different samples were interpolated from standard curves ran for each primer set and then normalized against the tubulin housekeeping gene. Every experimental and standard curve sample was tested in duplicate in three independent experiments. qPCR primer pair sequences were as follows: TUB-F, GTCTCG GCACACTCCTTCTC and TUB-R, AGACGTGGGAATGGAAC AAG; TvTSP6-qPCR-F, GCCAACTGTAAGCGAAAC; TvTSP6-qPCR-R, CGAAGACAATGATAACAATAGC.
Plasmid construction and exogenous protein expression in T. vaginalis
The TvTSP6, TVTSP6ΔCt, TSP1, TS1DCt, TSP3 and TSP3DCt constructs were generated using the following primer pairs:
TvTSP6-ATG-NdeI, AAACATATGATGGCGCTTAACGGAAAGT;
TvTSP6-R-KpnI, TTTGGTACCTGGTTGTGGTGTTTGGCCTG;
TvTSP6-ΔCt-KpnI, TTTGGTACCGTACATGCAGGAAATAACAAT;
TSP1-ATG-NdeI, CATATGATGACCTGCTGTTCATGCA;
Tsp1-R-Kpn1, GGTACCAACGTATGTGATGCCTTCCTT;
TSP1DCT-R, TTTGGTACCTGGATCTTCGTAGCAGAAAGCGT;
TSP3-ATG-NdeI, AAACATATGATGACTTGCTGTTCATGCA;
TSP3-R-KpnI, AAAGGTACCAGAATATGAAGAACTAGAAT;
TSP3-ΔCt-R, AAAGGTACCATGTGGTTTGTAGCAGCATCC.
NdeI and KpnI restriction sites were engineered into the 5′- and 3′-primers respectively. PCR fragments were generated using standard procedures and the resulting fragments were then cloned into the Master-Neo-(HA)2 plasmid (Dyall et al., 2000) to generate constructs to transfect into T. vaginalis. To generate the TSP1CT6 and TSP3CT6 constructs, two oligos of the C-terminal tail of TvTSP6: (TvTSP6C-F) GTACCAAGGTTGCT GCCGATGCTGATATCACAGGCCAAACACCACAACCAG and (TvTSP6CT-R) GTACCTGGTTGTGGTGTTTGGCCTGTGATAT CAGCATCGGCAGCAACCTTG, were annealed and cloned in frame into the TSP1ΔCT and TSP3ΔCt construct respectively. Electroporation of T. vaginalis strain B7RC2 was carried out as described previously (Delgadillo et al., 1997) with 50 μg of circular plasmid DNA. Transfectants were selected with 100 μg ml−1 G418 (Sigma).
Immunolocalization experiments
Parasites in the absence of host cells were incubated at 37°C on glass coverslips as previously described (de Miguel et al., 2010). To assess TSP6 localization in parasites attached to VECs, parasite were incubated at 37°C with VECs for 0.5, 1, 3 and 6 h, followed by 3× PBS washes to remove unbound parasites. All further incubations were carried out at room temperature. Cells were washed in PBS with 5% sucrose (PBS-S) and fixed in 4% formaldehyde for 20 min. After three washes, cells were permeabilized with 0.2% Triton X-100 in PBS for 15 min, blocked with 3% BSA in PBS (PBS-BSA) for 30 min, incubated with a 1:1000 dilution of anti-HA primary antibody (Covance, Emeryville, CA, USA) diluted in PBS–BSA, washed and then incubated with a 1:5000 dilution of Alexa Fluor-conjugated secondary antibody (Molecular Probes). The cover-slips were mounted onto microscope slips using ProLong Gold antifade reagent with 4′,6′-diamidino-2-phenylindole (Invitrogen). Stained parasites were examined using an Axioscope 2 epifluorescence microscope (Zeiss), and images were recorded with an AxioCam camera and processed with the AxioVision 3.2 program (Zeiss).
Migration assays
Migration assays were performed on Matrigel invasion chambers (BD Biocoat, Beckton Dickinson Labware). Approximately 2.5 × 105 serum-starved transfected parasites were added to the upper chamber. In the bottom well, complete TYM media plus 20% v/v of VECs-conditioned media were added as a chemoat-tractant. The cells were incubated at 37°C over a time course to allow migration. The numbers of parasites that crossed the Matrigel and entered the bottom chamber were counted automatically using a Z1 coulter particles counter (Beckman). The number of cells in each of three wells per time point was counted and the standard error was calculated and plotted. As a control, the migration capacity of parasites transfected with an empty vector (EpNEO) was compared.
Supplementary Material
Fig. S1. Subcellular localization of the TvTSP3CT6 protein in parasites bound to VECs. Parasites transfected with TvTSP3CT6 containing a C-terminal HA tag were bound to VECs for 0.5, 1, 3 and 6 h and the protein was localized using an anti-HA antibody. Note the change in localization from plasma membrane and intracellular vesicles to primarily a flagellar localization at 3 and 6 h post host cell exposure. The red arrow indicates the flagella and the white arrow indicates intracellular vesicles and the plasma membrane of the main body of the cell. Scale bar, 10 μm.
Acknowledgments
We thank Dr Clea Mantini for her contribution to the project, Drs Brian Janssen, Gil Lustig, Olivia Twu, Anh Vu and Yael Wexler-Cohen for critical comments on the manuscript and our colleagues in the lab for helpful discussions. This work was supported by the National Institute of Health R01 Grant AI069058 and ANPCyT (Agencia Nacional de Promocion Cientifica y Tecnologica) Grant PICT-2011-0279. A. R. was supported by a UCLA Eugene V. Cota-Robles Fellowship and the Howard Hughes Medical Institute's Gilliam Fellowship for Advanced Study.
Footnotes
Supporting information: Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Berditchevski F, Tolias KF, Wong K, Carpenter CL, Hemler ME. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:2595–2598. doi: 10.1074/jbc.272.5.2595. [DOI] [PubMed] [Google Scholar]
- Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgadillo MG, Liston DR, Niazi K, Johnson PJ. Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc Natl Acad Sci USA. 1997;94:4716–4720. doi: 10.1073/pnas.94.9.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SD, Koehler CM, Delgadillo-Correa MG, Bradley PJ, Plumper E, Leuenberger D, et al. Presence of a member of the mitochondrial carrier family in hydrogenosomes: conservation of membrane-targeting pathways between hydrogenosomes and mitochondria. Mol Cell Biol. 2000;20:2488–2497. doi: 10.1128/mcb.20.7.2488-2497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci. 2009;122:867–874. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol. 2009;83:185–189. doi: 10.1016/j.jri.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- Fiori PL, Rappelli P, Addis MF. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes Infect. 1999;1:149–156. doi: 10.1016/s1286-4579(99)80006-9. [DOI] [PubMed] [Google Scholar]
- Gander S, Scholten V, Osswald I, Sutton M, van-Wylick R. Cervical dysplasia and associated risk factors in a juvenile detainee population. J Pediatr Adolesc Gynecol. 2009;22:351–355. doi: 10.1016/j.jpag.2009.01.070. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- Hirt RP, de Miguel N, Nakjang S, Dessi D, Liu YC, Diaz N, et al. Trichomonas vaginalis pathobiology new insights from the genome sequence. Adv Parasitol. 2011;77:87–140. doi: 10.1016/B978-0-12-391429-3.00006-X. [DOI] [PubMed] [Google Scholar]
- Hong IK, Kim YM, Jeoung DI, Kim KC, Lee H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med. 2005;37:230–239. doi: 10.1038/emm.2005.31. [DOI] [PubMed] [Google Scholar]
- Huang H, Sossey-Alaoui K, Beachy SH, Geradts J. The tetraspanin superfamily member NET-6 is a new tumor suppressor gene. J Cancer Res Clin Oncol. 2007;133:761–769. doi: 10.1007/s00432-007-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur MA, Xu D, Hemler ME. Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell. 2009;20:2030–2040. doi: 10.1091/mbc.E08-11-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latysheva N, Muratov G, Rajesh S, Padgett M, Hotchin NA, Overduin M, Berditchevski F. Syntenin-1 is a new component of tetraspanin-enriched microdomains: mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol Cell Biol. 2006;26:7707–7718. doi: 10.1128/MCB.00849-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker MW, Alderete JF. Biology of trichomonosis. Curr Opin Infect Dis. 2000;13:37–45. doi: 10.1097/00001432-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Liu L, He B, Liu WM, Zhou D, Cox JV, Zhang XA. Tetraspanin CD151 promotes cell migration by regulating integrin trafficking. J Biol Chem. 2007;282:31631–31642. doi: 10.1074/jbc.M701165200. [DOI] [PubMed] [Google Scholar]
- McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- de Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohls-chlegel JA, Johnson PJ. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics. 2010;9:1554–1566. doi: 10.1074/mcp.M000022-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel CJ, Diaz N, Sicheritz-Ponten T, Safarikova L, Tachezy J, Tang P, et al. Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC Genomics. 2010;11:99. doi: 10.1186/1471-2164-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 2008;10:2078–2090. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MS, Pickering DA, McSorley HJ, Bethony JM, Tribolet L, Dougall AM, et al. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis. 2012;6:e1564. doi: 10.1371/journal.pntd.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Powner D, Kopp PM, Monkley SJ, Critchley DR, Berditchevski F. Tetraspanin CD9 in cell migration. Biochem Soc Trans. 2011;39:563–567. doi: 10.1042/BST0390563. [DOI] [PubMed] [Google Scholar]
- Ryan CM, de Miguel N, Johnson PJ. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem. 2011;51:161–175. doi: 10.1042/bse0510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- Stark JR, Judson G, Alderete JF, Mundodi V, Kuck-noor AS, Giovannucci EL, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. J Natl Cancer Inst. 2009;101:1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Berditchevski F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) J Cell Biol. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe S, Alderete JF, Till C, Goodman PJ, Hsing AW, Zenilman JM, et al. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int J Cancer. 2009;124:2082–2087. doi: 10.1002/ijc.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swygard H, Sena AC, Hobbs MM, Cohen MS. Trichomoniasis: clinical manifestations, diagnosis and management. Sex Transm Infect. 2004;80:91–95. doi: 10.1136/sti.2003.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Library Cataloguing-in-Publication Data. 2005. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates; pp. 1–36. [Google Scholar]
- Yanez-Mo M, Alfranca A, Cabanas C, Marazuela M, Tejedor R, Ursa MA, et al. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha3 beta1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Hemler ME. Specific interactions among transmembrane 4 superfamily (TM4SF) proteins and phosphoinositide 4-kinase. Biochem J. 2000;351(Pt 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem. 2001;276:25005–25013. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- Zhang XA, Kazarov AR, Yang X, Bontrager AL, Stipp CS, Hemler ME. Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol Biol Cell. 2002;13:1–11. doi: 10.1091/mbc.01-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Subcellular localization of the TvTSP3CT6 protein in parasites bound to VECs. Parasites transfected with TvTSP3CT6 containing a C-terminal HA tag were bound to VECs for 0.5, 1, 3 and 6 h and the protein was localized using an anti-HA antibody. Note the change in localization from plasma membrane and intracellular vesicles to primarily a flagellar localization at 3 and 6 h post host cell exposure. The red arrow indicates the flagella and the white arrow indicates intracellular vesicles and the plasma membrane of the main body of the cell. Scale bar, 10 μm.


