Summary
The extracellular protozoan parasite Trichomonas vaginalis causes the most prevalent non-viral sexually transmitted human infection, yet the pathogenesis of infection is poorly understood, and host cell receptors have not been described. The surface of T. vaginalis is covered with a glycoconjugate called lipophosphoglycan (LPG), which plays a role in the adherence and cytotoxicity of parasites to human cells. T. vaginalis LPG contains high amounts of galactose, making this polysaccharide a candidate for recognition by the galactose-binding galectin family of lectins. Here we show that galectin-1 (gal-1) is expressed by human cervical epithelial cells and binds T. vaginalis LPG. Gal-1 binds to parasites in a carbohydrate-dependent manner that is inhibited in the presence of T. vaginalis LPG. Addition of purified gal-1 to cervical epithelial cells also enhances parasite binding, while a decrease in gal-1 expression by small interfering RNA (siRNA) transfection decreases parasite binding. In contrast, the related galectin-7 (gal-7) does not bind T. vaginalis in a carbohydrate-dependent manner, and is unable to mediate attachment of parasites to host cells. Our data are consistent with the presence of multiple host cell receptors for T. vaginalis of which gal-1 is the first to be identified and highlight the importance of glycoconjugates in host–pathogen interactions.
Introduction
Trichomonas vaginalis, an extracellular aerotolerant protozoan, is the cause of trichomoniasis, the most prevalent non-viral sexually transmitted infection in the world (WHO, 2001). Over 170 million people are infected with T. vaginalis annually worldwide, and an annual incidence of 5 million infections in the USA has been reported (Cates, 1999; WHO, 2001). Although asymptomatic infection by T. vaginalis is common, multiple symptoms and pathologies can arise in both men and women, including vaginitis, urethritis, prostatitis, low-birth weight infants and preterm delivery, premature rupture of membranes and infertility (Minkoff et al., 1984; Gardner et al., 1986; Cotch et al., 1997; Reimer et al., 1999). Additionally, infection by this parasite is associated with the development of cervical cancer (Zhang and Begg, 1994; Viikki et al., 2000) and an increased susceptibility to human immunodeficiency virus (HIV) infection (Sorvillo and Kerndt, 1998; Sorvillo et al., 2001). Despite the serious consequences of infection, the pathogenic mechanisms of T. vaginalis are not well studied.
Because T. vaginalis is an obligate extracellular pathogen, adherence to epithelial cells is critical for parasite survival (Petrin et al., 1998). Adherence of the parasite to a variety of epithelia indicates that T. vaginalis has a promiscuous mechanism for attachment to host cells and/or the ability to use multiple adhesion factors. However, surface molecules on host cells that can be utilized by the parasite for attachment have not been identified. Moreover, the lack of a well-defined animal model of T. vaginalis infection has amplified the difficulty in the search for human host cell molecules involved in parasite attachment. While some studies have examined putative parasite surface proteins as possible candidates to mediate T. vaginalis adhesion to host cells, data are sparse and controversial (Addis et al., 2000; Hirt et al., 2007). Recent studies on parasite factors that mediate adhesion to host cells have focused on the abundant surface lipophosphoglycan (LPG) for which there is sound evidence for a role in attachment to host cells (Bastida-Corcuera et al., 2005; Fichorova et al., 2006).
The high density of LPG molecules on the parasite surface (∼3 × 106 molecules cell−1) (Singh, 1994) suggests that it is an important molecule for the parasite, as was previously described for the LPG on the surface of another protozoan parasite Leishmania major (Spath et al., 2003a,b). Our recent studies comparing wild-type T. vaginalis with mutants with altered surface LPG have demonstrated that LPG mutants are significantly less adherent and less cytotoxic to human ectocervical cells in vitro (Bastida-Corcuera et al., 2005). The finding that LPG is an attachment factor for the parasite led us to ask whether carbohydrate-binding proteins expressed by human cells are capable of binding to T. vaginalis LPG. The saccharide structure of T. vaginalis LPG has not been determined, but monosaccharide composition analyses have revealed that galactose and glucosamine are the most abundant monosaccharides (Singh, 1994; Bastida-Corcuera et al., 2005; Fichorova et al., 2006). Labelling of the parasite surface with plant lectins RCA120 and wheat germ agglutinin confirm that terminal galactose and N-acetyl glucosamine saccharides are accessible for lectin binding (Singh et al., 1994; Mirhaghani and Warton, 1998; Bastida-Corcuera et al., 2005). A family of lectins called galectins specifically binds galactose-containing carbohydrate structures, with many galectins having a preference for poly-N-acetyl lactosamine (Galβ1 →4GlcNAcβ1) (Rabinovich et al., 2002). Thus, we reasoned that galectins may be capable of binding to T. vaginalis LPG.
A number of diverse, mammalian galectins unified by structurally conserved carbohydrate recognition domains (CRDs) have been described (Camby et al., 2006). The prototypic galectins consist of one CRD and usually form homodimers, which allow them to cross-link two proteins by the recognition of similar carbohydrate structures. Galectin functions have been best described in inflammatory response homeostasis, where the bivalent or multivalent carbohydrate-binding properties of these proteins allow them to mediate cell–cell or cell–matrix interactions (Brewer et al., 2002; Rabinovich et al., 2002; Rabinovich and Gruppi, 2005; He and Baum, 2006). The cell–cell interactions that galectins facilitate can also be exploited by microbial pathogens (Rabinovich and Gruppi, 2005). Galectin-mediated attachment of intracellular pathogens to human cells as an initial step for establishing infection has been reported for L. major, Trypanosoma cruzi, HIV, Pseudomonas aeruginosa and Candida albicans (Gupta et al., 1997; Fradin et al., 2000; Pelletier et al., 2003; Kleshchenko et al., 2004; Ouellet et al., 2005). An insect-specific form of galectin, PpGalec, is also involved in the attachment of L. major to the midgut of its sandfly vector (Beverley and Dobson, 2004; Kamhawi et al., 2004).
In contrast to intracellular pathogens that primarily need to adhere to the host cell transiently prior to invasion, adhesion of the extracellular pathogen T. vaginalis is required for the parasite to both establish and maintain infection, and thus host cell receptors play a critical role throughout infection. In this study, we tested whether galectins act as receptors to facilitate interaction between T. vaginalis and cervical epithelial cells. We demonstrate that galectin-1 (gal-1) binds to T. vaginalis LPG and crosslinks parasites to host cells, identifying the first host cell receptor for this prevalent human pathogen.
Results
Gal-1, but not galectin-7, may mediate T. vaginalis adherence to cervical epithelial cells
Trichomonas vaginalis LPG contains galactose and N-acetyl glucosamine in high proportions (Bastida-Corcuera et al., 2005), indicating that N-acetyl lactosamine structures that can be recognized by galectins may exist in the molecule. The ability of oligomeric galectins to bind to both parasites and host cells could allow host cell galectins to serve as attachment factors for T. vaginalis. To test whether galectins play a role in adherence of this extracellular parasite, we have focused our studies on prototypic galectins that are usually expressed by human epithelial cells, gal-1 and galectin-7 (gal-7). While gal-1 is widely expressed in many types of epithelial cells, gal-7 expression is restricted to stratified squamous epithelia (Rabinovich et al., 2002; Camby et al., 2006; Saussez and Kiss, 2006). We first tested two recently established human cervical epithelial cell lines (Fichorova and Anderson, 1999) for the expression of these galectins. Both ectocervical (Ect1 E6/E7) and endocervical (End1 E6/E7) cell lines express gal-1 mRNA as determined by reverse transcription polymerase chain reaction (RT-PCR) (data not shown). However, only the ectocervical cells express gal-7 mRNA (data not shown). Expression of the corresponding proteins was confirmed by Western blot analysis of cell lysates (Fig. 1A). HeLa cells that express gal-1, but not gal-7 (Park et al., 2001), were included as a control for galectin expression (Fig. 1A). In agreement with the RT-PCR data, whereas both cervical cell lines produce gal-1, gal-7 is made only by the ectocervical cells (Fig. 1A). The production of gal-7 by the ectocervical cells is consistent with the finding that of the two cell lines, only the ectocervical cell line has characteristics of stratified squamous epithelia (Fichorova and Anderson, 1999). As a negative control, expression of gal-2, a prototypic galectin generally not expressed in squamous epithelia, was not detected in either of the cervical epithelial cell lines by RT-PCR (data not shown).
Fig. 1.
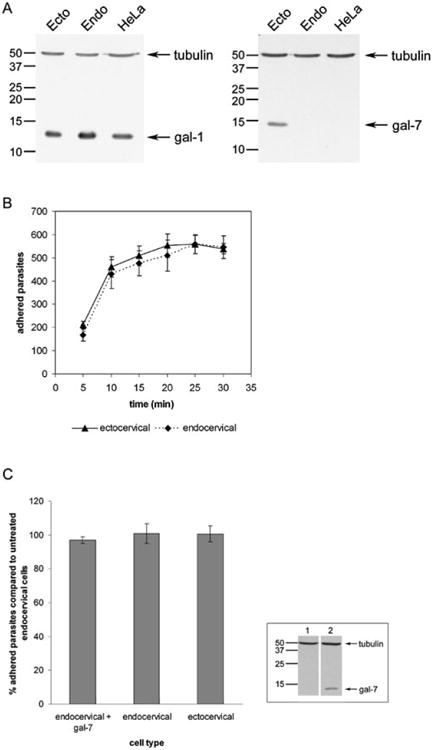
Gal-1, but not gal-7, may mediate T. vaginalis adherence to cervical epithelial cells.
A. Western blot analysis of cell lysates with indicated galectin antibodies and control tubulin antibody. Molecular weights are indicated in kDa. Lanes: (1) Ecto = ectocervical cells; (2) Endo = endocervical cells; (3) HeLa cells (control).
B. Time-course of T. vaginalis binding to ectocervical (gal-7-positive) or endocervical (gal-7-negative) cells. Data are expressed as total counted adhered parasites ± the standard deviation of the mean. A representative experiment of three independent experiments is shown.
C. Parasite binding to endocervical cells after pre-incubation with recombinant gal-7 was compared with binding to untreated endo- and ectocervical cells. Data are expressed as per cent parasites adhered compared with untreated endocervical cells ± the standard deviation of the mean. A representative experiment of three independent experiments is shown. Inset: Western blot analysis of gal-7-negative endocervical cell lysates pre-incubated with either no protein (1) or gal-7 (2). Blots were probed with both gal-7 and tubulin antibodies as indicated. Molecular weights are indicated in kDa.
The differential expression of gal-7 by the cervical epithelial cells allowed us to test whether gal-7 is involved in cross-linking parasites to host cells. We first tested the ability of parasites to bind the two cell lines in a time-dependent assay (Fig. 1B). Parasites were found to bind equally well to both cervical epithelial cell lines at all time points tested (Fig. 1B). These data suggest that the difference in gal-7 expression does not contribute to the binding of T. vaginalis to cervical epithelial cells, although the data do not exclude the possibility that another unknown protein can compensate for the lack of gal-7 in the endocervical cells. To address this issue and to further test whether gal-7 mediates parasite attachment to host cells, we added recombinant gal-7 to the endocervical cells to compensate for the absence of gal-7. Gal-7 binding to cells was confirmed by Western blot of whole-cell lysates (Fig. 1C, inset). Following incubation with gal-7, cells were washed to remove excess protein, and parasite adhesion to cells after 30 min was assessed (Fig. 1C). Parasites bound equally to cells that were pre-incubated with gal-7 compared with untreated endocervical cells (Fig. 1C), and bound equally well to untreated endo- and ectocervical cells in parallel binding assays as seen previously (Fig. 1B and C). Together, these data indicate that the difference in gal-7 expression by the cervical epithelial cells does not lead to a difference in T. vaginalis adherence, and do not support a role for gal-7 binding of the parasite to host cells.
Gal-1 binds to T. vaginalis in a carbohydrate-dependent manner
To further test whether galectins expressed by cervical epithelial cells potentially aid in parasite attachment to host cells, purified recombinant gal-1 and gal-7 were tested for the ability to bind and agglutinate T. vaginalis. Live parasites (PA strain) were incubated with either gal-1 or gal-7 in the presence of the non-competing sugar sucrose or the competing sugar lactose to assess whether binding was carbohydrate-mediated. Following incubation, binding and agglutination of parasites by the galectins was assessed by phase-contrast microscopy (Fig. 2A). Parasites were agglutinated by gal-1 in the presence of sucrose, but not in the presence of the competing sugar lactose (Fig. 2A), indicating a specific carbohydrate-dependent interaction between gal-1 and T. vaginalis. In contrast, gal-7 did not agglutinate parasites in the presence of either sucrose or lactose (Fig. 2A). These data demonstrate the ability of gal-1, but not gal-7, to cross-link T. vaginalis via a carbohydrate-mediated interaction.
Fig. 2.
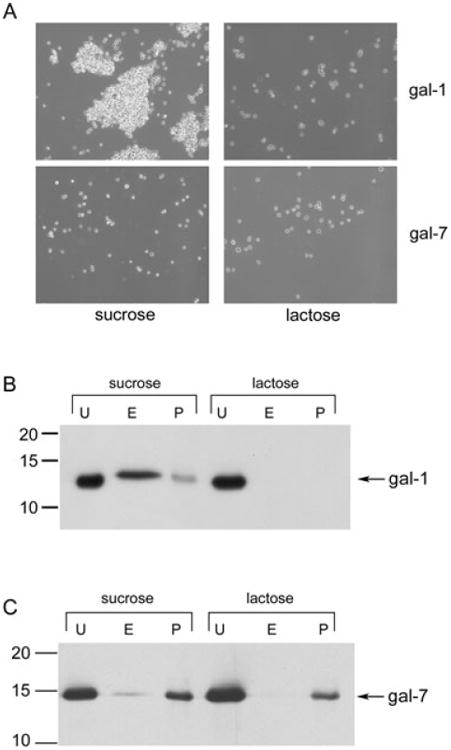
Gal-1 binding to T. vaginalis is carbohydrate-dependent.
A. A total of 106 PA strain parasites were incubated with 0.5 μg (0.35 μM) of recombinant gal-1 or gal-7 in the presence of 200 mM sucrose or lactose. Parasite agglutination after incubation with galectin was observed by phase-contrast microscopy.
B and C. A total of 106 T1 strain parasites were incubated with 0.5 μg (0.35 μM) of gal-1 (B) or gal-7 (C) in the presence of 200 mM sucrose or lactose as indicated. Unbound galectin (U) was collected by centrifugation. Parasite-bound galectin was then eluted (E) with lactose, eluted galectin was separated by centrifugation and 15% of the total collected eluted galectin was loaded. Galectin that remained associated with parasites (P) was also assessed. Fractions were analysed by Western blot. Molecular weights are indicated in kDa.
To confirm gal-1 binding to T. vaginalis and to examine whether binding is strain-specific, galectin binding assays (Pelletier et al., 2003) were then performed using our laboratory strain T1, and binding was accessed by SDS-PAGE and Western blotting. Purified recombinant gal-1 was incubated with live T1 parasites in the presence of the non-competing sugar sucrose or the competing sugar lactose. After incubation on ice, unbound gal-1 in the supernatant was separated by centrifugation and collected. Parasites were washed and then incubated with the competing sugar lactose to elute parasite-bound gal-1. The eluted gal-1 in the supernatant was separated by centrifugation and collected. Gal-1 remaining associated with the parasite was also assessed. Analyses of unbound, eluted and parasite-associated fractions demonstrated that T. vaginalis bound to gal-1 in the presence of the non-competing sugar sucrose, as gal-1 was detected in both the eluted and parasite-associated fractions (Fig. 2B). Assessment of gal-1 binding to parasites in the presence of sucrose by densitometric analysis of Western blots revealed that 70–80% of parasite-bound gal-1 was eluted with lactose. In contrast, T. vaginalis did not bind to gal-1 in the presence of the competing sugar lactose (Fig. 2B), reinforcing that gal-1 binding to T. vaginalis is carbohydrate-mediated and providing data that this interaction is not strain-specific.
In contrast to the carbohydrate-mediated interaction of gal-1 with parasites, T. vaginalis bound gal-7 in the presence of both sucrose and lactose (Fig. 2C), and binding was not competed or eluted with lactose (Fig. 2C). These data demonstrate that although gal-7 binds T. vaginalis, this interaction is not carbohydrate-mediated. These data are in agreement with the ability of gal-1, but not gal-7, to agglutinate parasites (Fig. 2A), as only lectin-mediated binding of dimerized gal-7 would be expected to agglutinate parasites. Furthermore, the combined data confirms that because gal-7 does not bind to parasites in a carbohydrate-dependent manner, it would not be expected to cross-link T. vaginalis to cervical epithelial cells, as shown in Fig. 1C. These data led us to focus on whether gal-1 can mediate an interaction between parasites and host cells.
Gal-1 binds to T. vaginalis LPG
As shown above, gal-1 binds T. vaginalis in a carbohydrate-dependent manner (Fig. 2). LPG is the major surface polysaccharide on the surface of T. vaginalis, and contains relatively high amounts of galactose and N-acetyl glucosamine predicted to be recognized by gal-1. In previous studies, we established chemical mutants of T. vaginalis with altered LPG that have dramatically decreased cell binding capabilities (Bastida-Corcuera et al., 2005). These mutant parasites have over 50% less galactose and glucosamine in their LPG molecules compared with wild-type LPG (Bastida-Corcuera et al., 2005). To determine whether gal-1 specifically binds T. vaginalis LPG, we tested gal-1 binding to the LPG mutant parasites (2E2 and 4-12 strains) in the presence of sucrose using the galectin binding assay (Fig. 3A). Gal-1 binding to the parental wild-type (PA strain) parasites was included as a positive control (Fig. 3A). Both LPG mutant parasites exhibited no detectable binding to gal-1 compared with the wild-type PA strain (Fig. 3A). None of the parasite strains bound gal-1 in the presence of lactose, as seen previously (data not shown). These data correlate well with the finding that the mutant parasite LPGs have less galactose and glucosamine, and further supports that gal-1 binding to parasites is dependent on carbohydrates present in parasite surface LPG.
Fig. 3.
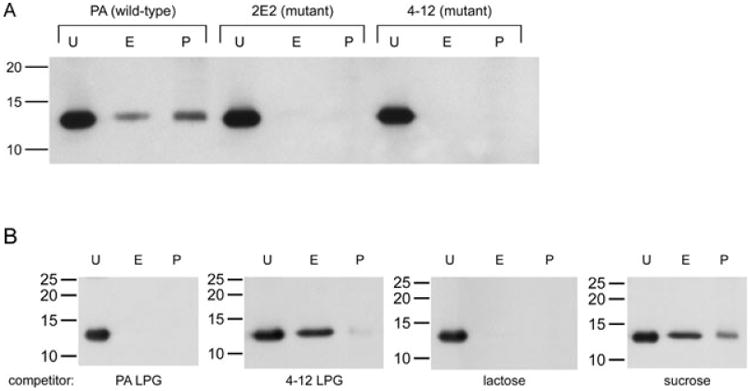
Gal-1 binds to T. vaginalis LPG.
A. A total of 106 wild-type (PA strain) and LPG mutant (2E2 and 4-12) parasites were incubated with 0.5 μg (0.35 μM) of gal-1 in the presence of 200 mM sucrose. Unbound (U), eluted (E) and parasite-retained (P) galectin was collected and loaded as described in Fig. 2B. Fractions were analysed by Western blot. Molecular weights are indicated in kDa.
B. A total of 106 PA strain wild-type parasites were incubated with 0.5 μg (0.35 μM) of gal-1 in the presence of 200 mM wild-type (PA) LPG, mutant (4-12) LPG, lactose (negative control) or sucrose (positive control). Unbound (U), eluted (E) and parasite-retained (P) fractions were analysed by Western blot as described in Fig. 2B. Molecular weights are indicated in kDa.
To further test whether gal-1 binds specifically to T. vaginalis LPG, isolated LPG from wild-type and mutant parasites was used as a competitive inhibitor in the gal-1 binding assay. Wild-type strain (PA) parasites were incubated with gal-1 in the presence of ∼200 mM PA LPG or mutant (2E2 and 4-12 strains) LPG, replacing sucrose or lactose as a competitor. Parasites binding to gal-1 in the presence of 200 mM of these sugars were also performed in parallel assays as controls. Unbound, eluted and parasite-associated fractions were analysed by Western blot (Fig. 3B). LPG isolated from the wild-type strain (PA) competed for gal-1 binding to parasites, comparable to the results obtained when gal-1 binding was competed with lactose (Fig. 3B). In contrast, when either mutant (2E2 and 4-12) LPG was used as a competitor, gal-1 was still able to bind to parasites, comparable to the level of binding using sucrose as a competitor (Fig. 3B and data not shown). These results are consistent with the inability of gal-1 to bind to the mutant parasites (Fig. 3A). Together, these data demonstrate that gal-1 binds specifically to the LPG structure present on the surface of the PA parasite, and although the LPGs of the mutant parasites still contain some galactose and glucosamine (Bastida-Corcuera et al., 2005), the specific carbohydrate structure in PA LPG recognized by gal-1 is lost in the mutant parasites.
Addition of gal-1 enhances parasite binding to ectocervical cells
The data shown thus far provide strong evidence for interactions between T. vaginalis LPG and gal-1. To begin testing whether gal-1 facilitates interaction between parasites and host epithelial cells, we added various concentrations of purified recombinant gal-1 to ectocervical cell monolayers to saturate the cell surface with gal-1. Binding of gal-1 to cells was confirmed by Western blot of whole-cell lysates, and densitometric analysis indicated that an average of 66% more gal-1 was present on cells incubated with recombinant protein (data not shown). Following incubation with gal-1, cells were washed to remove excess protein to prevent parasite agglutination. Final washes of ectocervical cells were tested for the presence of gal-1 and were found to be negative (data not shown). Parasite binding to cells pre-incubated with gal-1 was increased 15–20% compared with untreated cells, regardless of concentration (Fig. 4A). This relatively uniform increase in parasite binding confirms that the cell surface was saturated upon addition of the lowest concentration of gal-1. As a negative control, we added recombinant gal-7, instead of gal-1, to the cells. As was seen with the endocervical cells (Fig. 1C), pre-incubating these ectocervical cells with gal-7 did not enhance parasite adhesion compared with untreated cells (Fig. 4A). These data underscore the specificity of binding enhancement by gal-1.
Fig. 4.
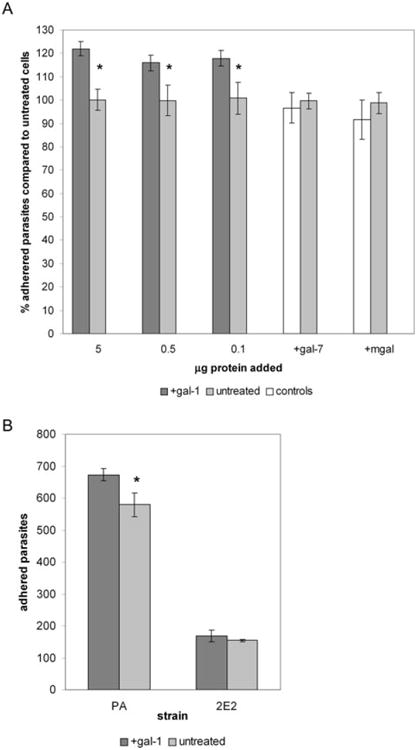
Addition of gal-1 enhances the adherence of T. vaginalis to ectocervical cells.
A. Various concentrations of recombinant gal-1, 0.5 μg of recombinant gal-7 or 5 μg of mgal in 0.5 ml of PBS were pre-incubated with ectocervical cells. Parasite binding to galectin-treated cells was compared with binding to untreated cells. Data are expressed as per cent parasites adhered compared with untreated cells. Asterisks indicate data are statistically significant, P < 0.05.
B. Binding of wild-type (PA) or LPG mutant (2E2) parasites to ectocervical cells pre-incubated with 0.5 μg of gal-1 compared with binding to untreated cells. Data are expressed as the number of adhered parasites within five 20× magnification fields per coverslip. Asterisk indicates data are statistically significant, P < 0.01. For both panels, a representative experiment of at least three independent experiments is shown.
Galectins of the prototype family such as gal-1 non-covalently homodimerize to form a molecule with two identical lectin-binding domains that are capable of cross-linking cells (Camby et al., 2006). To test whether the dimeric form of gal-1 is required for the enhancement of T. vaginalis binding to host cells, we pre-incubated ectocervical cells with a monomeric form of gal-1 (mgal) generated by site-directed mutagenesis. This mutant gal-1 retains carbohydrate binding activity, but is unable to dimerize (Cho and Cummings, 1996). Pre-incubating the ectocervical cells with the highest concentration of mgal did not increase parasite binding to cells compared with untreated cells (Fig. 4A). These data show that the dimeric form of gal-1 is required to mediate an interaction between parasites and host cells, indicating that ectocervical cell surface glycoconjugates and T. vaginalis LPG are cross-linked by gal-1. Furthermore, these data demonstrate that the multivalency of gal-1 is a significant contributor to T. vaginalis adhesion to cells by providing a high avidity of binding.
To confirm that LPG is the major parasite surface glycoconjugate involved in gal-1-mediated attachment of parasites to host cells, we next tested binding of our LPG mutant (2E2 strain) parasites to ectocervical cells pre-incubated with gal-1 (Fig. 4B). Because the LPG mutant parasites have such a dramatic cell-binding defect (85% decreased binding compared with PA strain) (Bastida-Corcuera et al., 2005), the number of 2E2 parasites added to ectocervical cells was doubled to increase the number of bound mutant parasites, so that differences in parasite binding could be clearly distinguished. However, adding twice the amount of LPG mutant parasites still resulted in a 75% reduction in binding to ectocervical cells compared with the wild-type PA parasites (Fig. 4B). Pre-incubation of the ectocervical cells with gal-1 did not enhance binding of 2E2 parasites to cells compared with untreated cells (Fig. 4B). Pre-incubation of the ectocervical cells with gal-1 in parallel binding assays again increased the binding of wild-type PA parasites ∼15% compared with untreated controls (Fig. 4B). These results further validate that LPG is the specific surface glycoconjugate that binds to gal-1, and suggest that the reduction in binding of the LPG mutant parasites to host cells (Bastida-Corcuera et al., 2005) is in part due to the inability of these parasites to bind gal-1.
Silencing of gal-1 in ectocervical cells leads to a reduction of parasite binding
Epithelial cell lines from the urogenital tract that lack gal-1 expression have not been reported (Walzel et al., 1995; Maquoi et al., 1997; Vicovac et al., 1998; Ellerhorst et al., 1999; Lahm et al., 2001; van den Brule et al., 2003; von Wolff et al., 2005). Given the lack of such cell lines, we chose to further analyse the role and contribution of gal-1 in T. vaginalis adhesion to host cells using an RNAi approach. To this end, we transfected gal-1-specific small interfering RNAs (siRNAs) into our ectocervical cell line to knock down endogenous gal-1 expression. Transfected cells were tested for gal-1 expression and parasite binding 3 days post transfection. Gal-1 mRNA was consistently knocked down in gal-1 siRNA-transfected cells (gal-1 knock-down cells) to 3–5% compared with untreated cells, as assessed by quantitative PCR (qPCR) (Table 1). The knock-down of gal-1 mRNA resulted in protein levels that were about 18% of the level of gal-1 in untreated cells, as determined by Western blot of cell lysates (Table 1). As the transcript level of gal-1 in knockdown cells was low (3–5% remaining mRNA, Table 1), these data indicate that the remaining gal-1 protein is due to the relative stability of the protein, and that the levels cannot be further reduced by this technique. As a specificity control, the mRNA and protein levels of the non-targeted gal-7 in the gal-1 knock-down cells were also assessed. Gal-7 transcript and protein levels were slightly increased in the gal-1 knock-down cells (Table 1). This increase in gal-7 expression may indicate that the gal-1 knock-down cells are compensating for the lack of gal-1 by upregulating gal-7 expression. However, given that the addition of gal-7 does not affect T. vaginalis binding to cells (Figs 1C and 4A), we reasoned that the small increase in gal-7 expression in the gal-1 knock-down cells would not contribute to parasite binding. As a negative control for both the specific effect of knocking down gal-1 and for non-specific effects resulting from activation of the RNAi pathway, we also transfected ectocervical cells with gal-7 siRNAs. Gal-7 transcript levels were knocked down to about 17% of untreated cells, which resulted in protein levels of about 26% of untreated cells (Table 1). Gal-1 transcript and protein levels were not significantly affected in the gal-7 knock-down cells (Table 1). Control cells treated with transfection reagent only (no siRNA, mock-transfected) showed no statistically significant changes in gal-1 mRNA, resulting in no changes in gal-1 protein (Table 1), and similarly showed no significant changes in gal-7 mRNA or protein levels (Table 1). Together these data show that gal-1 and gal-7 are specifically and effectively knocked down by the transfected siRNA oligos.
Table 1.
Gal-1 and gal-7 expression in siRNA-transfected cells.
| Sample | % gal-1 mRNA | % gal-1 protein | % gal-7 mRNA | % gal-7 protein |
|---|---|---|---|---|
| Untreated | 100.5 ± 0.9 | 100.1 ± 0.5 | 100.9 ± 1.8 | 100.4 ± 0.8 |
| Mock-transfected | 79.2 ± 28.7 | 106.5 ± 16.8 | 109.8 ± 42.2 | 105.8 ± 19.8 |
| Gal-1 siRNA-transfected | 4.0 ± 0.7 | 17.7 ± 6.6 | 162.5 ± 69.2 | 128.8 ± 21.8 |
| Gal-7 siRNA-transfected | 135.4 ± 54.5 | 96.4 ± 28.3 | 17.1 ± 6.2 | 26.2 ± 2.3 |
Combined data of at least three independent experiments are expressed as average percentage compared with untreated cells ± standard deviation of the mean. All data were normalized, then compared with untreated cells.
We next tested parasite adhesion to the transfected cells. Parasites bound equally well to untreated and mock-transfected cells (data not shown). As mock-transfected cells do not significantly differ from untreated cells in gal-1 and gal-7 expression (Table 1) or parasite adherence, these cells were compared with cells transfected with either gal-1 or gal-7 siRNAs. In time-course binding assays, parasites bound equally well to both negative control gal-7 knock-down cells and mock-tranfected control cells (Fig. 5A). These results are consistent with the data shown in Figs 1 and 2 that gal-7 is not involved in parasite attachment to host cells and show that activation of the RNAi pathway per se does not affect parasite binding. Although parasites bound equally well to both gal-7 knock-down and mock-transfected cells within a given experiment, variation was observed between experiments. For this reason, data are presented from a single, representative experiment performed in triplicate. However, consistent results were obtained for at least three independent experiments.
Fig. 5.
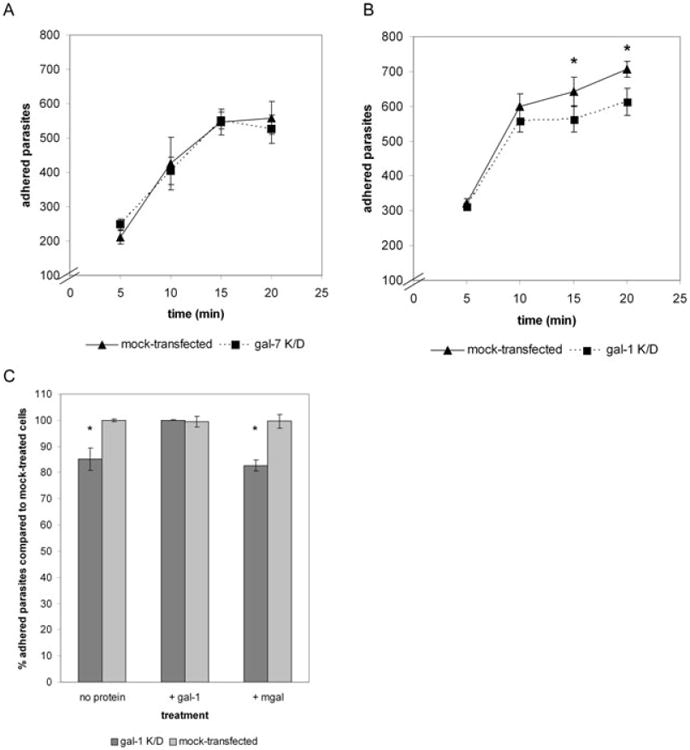
Silencing of gal-1 in ectocervical cells leads to a reduction in T. vaginalis adhesion.
A. Time-course of T. vaginalis binding to negative control gal-7 siRNA-transfected (gal-7 K/D) cells compared with mock-transfected cells. Data are expressed as the number of adhered parasites within five 20× magnification fields per coverslip.
B. Time-course of T. vaginalis binding to gal-1 siRNA-transfected (gal-1 K/D) cells compared with mock-transfected cells. Data are expressed as the number of adhered parasites within five 20× magnification fields per coverslip. Asterisks indicate data are statistically significant, P < 0.05.
C. Gal-1 siRNA-transfected (gal-1 K/D) cells were pre-incubated with no protein, 0.5 μg of gal-1 or 0.5 μg of mgal. Parasite binding to galectin-treated gal-1 K/D cells was then compared with binding to untreated mock-transfected cells. Data are expressed as per cent parasites adhered compared with mock-transfected cells. Asterisks indicate differences are statistically significant, P < 0.01. For all panels, a representative experiment of at least three independent experiments is shown.
In contrast to what we observed with the gal-7 knockdown cells, parasite binding to the gal-1 knock-down cells was significantly decreased compared with the mock-transfected cells at the 15 and 20 min time points (Fig. 5B). Parasite binding to gal-1 knock-down cells was decreased about 9% at the 15 min time point and 16% at the 20 min time point compared with the mock-transfected cells (Fig. 5B). These data demonstrate that the reduction of gal-1 expression results in decreased parasite binding to cells with time. The data implicate gal-1 as an important molecule for parasite adhesion to host cells, and that the effect of gal-1 is on stable attachment of parasites to host cells. To directly test whether the decrease in parasite binding to gal-1 knock-down cells is specifically the result of the decrease in gal-1, we performed gal-1 rescue experiments. Because gal-1 associates with the cell membrane by binding surface glycoconjugates, we complemented the gal-1 deficiency in the gal-1 knockdown cells by incubating cells with recombinant gal-1. As negative controls, gal-1 knock-down cells were pre-incubated with either no protein or the monomeric mutant mgal. Cells were washed, and parasite binding to treated gal-1 knock-down cells was compared with untreated mock-transfected cells (Fig. 5C). Parasite binding to gal-1 knock-down cells in the absence of protein was reduced by about 17% compared with binding to mock-transfected control cells, consistent with our previous results (Fig. 5B and C). However, when recombinant gal-1 was added to the surface of gal-1 knock-down cells, the binding of parasites was restored to the levels of parasites bound to mock-transfected cells (Fig. 5C). Moreover, adding the negative control mgal did not restore binding to gal-1 knock-down cells (Fig. 5C), confirming that the dimeric form of gal-1 is required to mediate an interaction between parasites and host cells. These results further demonstrate that the decrease in binding to gal-1 siRNA-transfected cells is due solely to the decrease in gal-1 levels, and not secondary defects caused by the reduction in gal-1 levels or the activation of the RNAi pathway. Taken together, our data show that gal-1 expressed by cervical epithelial cells is involved in stably cross-linking T. vaginalis to host cells, revealing gal-1 as the first identified host cell receptor for this extracellular parasite.
Discussion
Trichomonas vaginalis is an extracellular human parasite that must bind to vaginal and cervical epithelial cells to establish and maintain infection (Schwebke and Burgess, 2004). Mammalian receptors responsible for parasite binding have not previously been identified. Here, we demonstrate that T. vaginalis binds to cervical epithelial cells via a carbohydrate-dependent interaction with the mammalian galactose-binding protein gal-1. We further show that this gal-1–parasite interaction is mediated by T. vaginalis LPG that densely coats the surface of the parasite. The most abundant monosaccharides in this LPG, galactose and N-acetyl glucosamine, appear to interact with one subunit of a homodimeric gal-1 while the other subunit binds to similar sugars on the surface of epithelial cells. This interaction serves to effectively crosslink the parasite to host cells.
In addition to the cervical epithelial cells used in this study, gal-1 is broadly expressed on various cell types that are potentially exposed to T. vaginalis during infection, including human placenta, prostate, endometrial and decidual tissue (Bozic et al., 2004; von Wolff et al., 2005; Camby et al., 2006). Thus gal-1 may be a general attachment factor for T. vaginalis, allowing adherence to the various epithelia the parasite is capable of colonizing. Further supporting this model is the ability of all tested adherent strains of T. vaginalis to bind gal-1 (Figs 2 and 3 and data not shown). Moreover, the presence of increased amounts of N-acetyl glucosamine on the surface of T. vaginalis has been correlated with increased pathogenicity (Kon et al., 1988). Conversely, we observed in this study that the loss of surface galactose and glucosamine in LPG mutants resulted in a gal-1 binding deficiency, and correlated with our previous observations that these mutants are severely compromised in their ability to adhere to host cells (Bastida-Corcuera et al., 2005). Taken together, these data strongly support a critical role for LPG–gal-1 binding in establishing this host–parasite interaction, although it does not exclude the possibility that other galectins may also contribute to this interaction.
Our data indicate that although gal-1 plays a significant role in host–parasite interaction, other, yet to be defined, host cell receptors are likely to also mediate parasite binding. The addition of ∼66% gal-1 to cells results in a 15–20% increase in parasite attachment (Fig. 4A), and conversely, the ∼80% reduction of endogenous gal-1 by siRNA transfection results in ∼15% decrease in parasite attachment (Fig. 5B and C). Likewise, our previous analyses (Bastida-Corcuera et al., 2005) indicate that parasite adhesion factors in addition to LPG are also likely to be involved in host–parasite interaction. Isolated LPG from T. vaginalis only reduces parasite binding to ectocervical cells by about 45%, indicating that LPG is not the only surface molecule involved in T. vaginalis attachment (Bastida-Corcuera et al., 2005). Given that binding to host epithelia is critical for the duration of this chronic infection, it is not surprising that multiple parasite adhesion factors have evolved. Indeed, the recently published sequence of the T. vaginalis genome has revealed a number of potential surface proteins that may also play a role in attachment to host cells (Carlton et al., 2007; Hirt et al., 2007). Thus, gal-1 may be the first of multiple host cell receptors identified for this extracellular parasite.
In addition to cell–cell interactions, galectins can mediate cell–matrix interactions. Gal-1 can specifically bind to the extracellular matrix (ECM) proteins laminin and placental fibronectin, promoting cell attachment to the ECM (Rabinovich et al., 2002; He and Baum, 2004; 2006). In this regard, it is important to note that T. vaginalis has also been reported to bind to matrix proteins including laminin and fibronectin (Silva Filho et al., 1998; Crouch and Alderete, 1999), and parasite surface carbohydrates have been implicated in the binding of laminin (Crouch and Alderete, 1999). Thus, T. vaginalis binding to gal-1 may also enhance parasite–matrix interactions, in addition to the enhancement of parasite– epithelial cell interaction described here.
Galectins not only facilitate interactions between cells and between cells and matrix, but also can profoundly affect cellular metabolism. Gal-1, for example, induces apoptosis of T cells, regulating the immune response (Perillo et al., 1995). Direct effects of galectin binding have also proven to play a role in other host–pathogen interactions, as gal-3 has been shown to be directly involved in inducing death in the pathogenic fungus C. albicans (Kohatsu et al., 2006). In this regard, galectins are protective molecules for the host. Whether the gal-1-mediated attachment of T. vaginalis is more beneficial to the parasite or the host remains to be determined. This study does however identify the first host cell receptor for T. vaginalis, providing insight into molecular mechanisms that may drive T. vaginalis pathogenesis.
Experimental procedures
Parasites, cell cultures and media
Trichomonas vaginalis strains B7RC2 (PA strain, ATCC 50 167), T1 (J.-H. Tai, Institute of Biomedical Sciences, Taipei, Taiwan), 2E2 and 4-12 (LPG mutant strains) (Bastida-Corcuera et al., 2005) were cultured in TYM medium supplemented with 10% horse serum, penicillin and streptomycin (Invitrogen), and iron (Clark and Diamond, 2002). Parasites were grown at 37°C and passaged daily. The human cervical epithelial cell lines Ect1 E6/E7 (ectocervical, ATCC CRL-2614) and End1 E6/E7 (endocervical, ATCC CRL-2615) were grown as previously described (Fichorova et al., 1997) in defined keratinocyte-SFM complemented with provided recombinant protein supplements and penicillin and streptomycin (Invitrogen) and cultured at 37°C/5% CO2.
Antibodies, recombinant proteins and LPG
Antibodies to the following recombinant human proteins were used in this study: gal-1 (Perillo et al., 1995), gal-7 (R&D Systems) and alpha-tubulin (Sigma). Recombinant human gal-1 and the monomeric gal-1 N-Gal-1 mutant (mgal) were purified as described previously (Pace et al., 2003; Fulcher et al., 2006). Recombinant human gal-7 was purchased from R&D Systems. LPG was isolated from T. vaginalis and quantified by densitometry as previously described (Bastida-Corcuera et al., 2005). No protein contamination in the LPG preparations was detected by silver staining of LPG samples run in SDS-PAGE gels.
Galectin binding assay
The galectin binding assay was performed as previously described (Pelletier and Sato, 2002), with the following modifications. Briefly, 2 × 106 live T. vaginalis parasites were washed and incubated with 0.5 mg (0.35 μM) of gal-1 or gal-7 in 200 mM sucrose, lactose or isolated LPG-containing binding buffer [20 mM potassium glutamate, 2 mM MgCl2, 20 mM Hepes, Complete Mini EDTA-free protease inhibitors cocktail containing serine and cysteine proteinase inhibitors (Roche Applied Science) and 2 mM DTT]. After incubation for 30 min on ice, parasites that bound gal-1 were visibly agglutinated. Parasites were pelleted at 5000 g for 5 min, unbound galectin in the supernatant was collected and 7.5% of this fraction was loaded onto SDS-PAGE gels. Parasites were washed several times, and then incubated for 30 min on ice with 200 mM lactose binding buffer to elute parasite-bound galectin. Parasites that were bound and agglutinated by gal-1 in the presence of sucrose were no longer agglutinated after incubation in lactose, indicating effective elution of gal-1. Parasites were pelleted, eluted galectin in the supernatant was collected and 15% of this fraction was loaded onto SDS-PAGE gels. Microscopic examination after galectin elution confirmed that parasites were still intact and viable. Parasites were washed several more times, lysed in SDS-PAGE sample buffer and 25% of this fraction was loaded onto SDS-PAGE gels. Collected fractions and parasites were run in 15% SDS-PAGE gels, transferred to PVDF (Bio-Rad) and probed with gal-1 or gal-7 antibodies (1:1000 for both antibodies).
Adherence to cervical epithelial cells
A modified version of the T. vaginalis cell binding assay was performed (Bastida-Corcuera et al., 2005). Briefly, cervical epithelial cells were seeded on 12 mm coverslips in 24-well plates at 3–4 × 105 cells per well in culture medium and grown to confluence at 37°C/5% CO2 for 2 days. Where indicated, triplicate wells of cells were washed and incubated with recombinant galectins in 0.5 ml of PBS for 30 min at 37°C/5% CO2. Parallel wells incubated with galectins were washed several times, trypsinized and lysed in SDS-PAGE sample buffer. Cell lysates were run on SDS-PAGE gels, blotted and probed with galectin antibodies to confirm that galectins were loaded onto cells. Cell monolayers were washed before the addition of parasites to remove excess galectin and prevent parasite agglutination. T. vaginalis parasites were labelled with 10 μM CellTracker Blue CMAC (Invitrogen), and approximately 105 labelled parasites in 0.5 ml of PBS were added to ∼3–4 × 105 cervical epithelial cells (1:3 parasite:cell ratio) in triplicate. Plates were incubated at 37°C/5% CO2 for 30 min or the indicated times. Coverslips were subsequently washed in PBS, fixed with 4% paraformaldehyde and mounted on slides. Five 20× magnification fields (approximately 800 epithelial cells per field) were analysed per coverslip with three coverslips per treatment. Fluorescent parasites adhered to host cells were counted using Scion Image for Windows, v. Beta 4.0.2 (Scion Corporation). Data are expressed as the number of adhered parasites in five fields per coverslip, or as a percentage of the control sample. All experiments were repeated at least three independent times.
siRNA transfection of ectocervical cells
Gal-1-specific siRNAs (Silencer pre-designed siRNAs #41 828 and 41 676, Ambion) or gal-7-specific siRNAs (Silencer pre-designed siRNAs #11 428 and 11 517, Ambion) were resuspended at 5 μM in RNase-free water and pooled. Ectocervical cells were reverse transfected with 150 μM pooled siRNAs per 105 cells using oligofectamine (Invitrogen) and plated in triplicate onto coverslips in 24-well plates in uncomplemented culture media. Untreated cells and mock-transfected (no siRNA, transfection reagent only) cells were plated in parallel as controls. One hundred microlitres of culture medium containing 3× concentration of recombinant supplements was added to cells 4 h post transfection. Cells were switched to fully complemented culture media the following day. Assessment of galectin knockdown and parasite binding assays was performed 3 days post transfection. Galectin RNA levels were assessed by qPCR (see below). To assess galectin protein levels, cell lysates were run in 15% SDS-PAGE gels, blotted and probed with galectin and tubulin antibodies. Signals were quantified by densitometry using the ImageJ program, v. 1.32j. All samples were normalized to tubulin and compared with untreated cells. Parasite binding assays to knock-down cells were performed in triplicate as described. All experiments were repeated at least three independent times.
Quantitative PCR (qPCR)
Total RNA was extracted from approximately 6 × 105 siRNA-transfected cells using Trizol (Invitrogen). RNA was treated with DNase, ethanol precipitated and reverse transcribed using SuperScript RT III and oligo dT primers (Invitrogen). Real-time PCR reactions were performed using 2 μl of cDNA, Brilliant SYBR Green QPCR Master Mix (Stratagene), and primers for gal-1 [5′-AACCTGGAGAGTGCCTTCGA-3′;5′-GTAGTTGATGGCCTC CAGGT-3′ (Lahm et al., 2001)], gal-7 [5′-ATGTCCAACGTCC CCCACAAG-3′; 5′-TGACGCGATGATGAGCACCTC-3′ (Lahm et al., 2001)] or GAPDH (5′-CATCTCTGCCCCCTCTGCTGA-3′; 5′-GGATGACCTTGCCCACAGCCT-3′) using an Eppendorf Mastercycler and realplex v. 1.5 (Eppendorf). Real-time PCR conditions were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 62°C for 30 s and 72°C for 1 min. All reactions were performed in duplicate per experiment. Parallel reactions performed without reverse transciptase were used as a negative control. Data were analysed using the Pfaffl method (Pfaffl, 2001). All samples were normalized to GAPDH and compared with untreated cells. Melting curve analyses were performed to ensure specific products, and all products were sequence verified.
Statistical analyses
All adherence data were analysed by the Student's t-test for independent samples.
Acknowledgments
We thank Dr Luciana Kohatsu for the suggestion of looking at gal-1 as a potential receptor for T. vaginalis, Mabel Pang for purifying recombinant gal-1 and assistance in purifying recombinant mgal, Dr Raina Fichorova at Brigham and Women's Hospital, Boston, MA, for the human cervical epithelial cells, and Dr Kent Hill at UCLA for use of the fluorescent microscope. We also thank lab members for helpful discussions.
References
- Addis MF, Rappelli P, Fiori PL. Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins. Infect Immun. 2000;68:4358–4360. doi: 10.1128/iai.68.7.4358-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. Trichomonas vaginalis lipophospho-glycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot Cell. 2005;4:1951–1958. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley SM, Dobson DE. Flypaper for parasites. Cell. 2004;119:311–312. doi: 10.1016/j.cell.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Bozic M, Petronijevic M, Milenkovic S, Atanackovic J, Lazic J, Vicovac L. Galectin-1 and galectin-3 in the trophoblast of the gestational trophoblastic disease. Placenta. 2004;25:797–802. doi: 10.1016/j.placenta.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- van den Brule F, Califice S, Garnier F, Fernandez PL, Berchuck A, Castronovo V. Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab Invest. 2003;83:377–386. doi: 10.1097/01.lab.0000059949.01480.40. [DOI] [PubMed] [Google Scholar]
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates W., Jr Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999;26:S2–S7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Characterization of monomeric forms of galectin-1 generated by site-directed mutagenesis. Biochemistry. 1996;35:13081–13088. doi: 10.1021/bi961181d. [DOI] [PubMed] [Google Scholar]
- Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotch MF, Pastorek JG, 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group. Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Alderete JF. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology. 1999;145(Part 10):2835–2843. doi: 10.1099/00221287-145-10-2835. [DOI] [PubMed] [Google Scholar]
- Ellerhorst J, Troncoso P, Xu XC, Lee J, Lotan R. Galectin-1 and galectin-3 expression in human prostate tissue and prostate cancer. Urol Res. 1999;27:362–367. doi: 10.1007/s002400050164. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun. 2006;74:5773–5779. doi: 10.1128/IAI.00631-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin C, Poulain D, Jouault T. Beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun. 2000;68:4391–4398. doi: 10.1128/iai.68.8.4391-4398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher JA, Hashimi ST, Levroney EL, Pang M, Gurney KB, Baum LG, Lee B. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J Immunol. 2006;177:216–226. doi: 10.4049/jimmunol.177.1.216. [DOI] [PubMed] [Google Scholar]
- Gardner WA, Jr, Culberson DE, Bennett BD. Trichomonas vaginalis in the prostate gland. Arch Pathol Lab Med. 1986;110:430–432. [PubMed] [Google Scholar]
- Gupta SK, Masinick S, Garrett M, Hazlett LD. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect Immun. 1997;65:2747–2753. doi: 10.1128/iai.65.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem. 2004;279:4705–4712. doi: 10.1074/jbc.M311183200. [DOI] [PubMed] [Google Scholar]
- He J, Baum LG. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 2006;417:247–256. doi: 10.1016/S0076-6879(06)17017-2. [DOI] [PubMed] [Google Scholar]
- Hirt RP, Noel CJ, Sicheritz-Ponten T, Tachezy J, Fiori PL. Trichomonas vaginalis surface proteins: a view from the genome. Trends Parasitol. 2007;23:540–547. doi: 10.1016/j.pt.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, et al. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Kleshchenko YY, Moody TN, Furtak VA, Ochieng J, Lima MF, Villalta F. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect Immun. 2004;72:6717–6721. doi: 10.1128/IAI.72.11.6717-6721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- Kon VB, Papadimitriou JM, Robertson TA, Warton A. Quantitation of concanavalin A and wheat germ agglutinin binding by two strains of Trichomonas vaginalis of differing pathogenicity using gold particle-conjugated lectins. Parasitol Res. 1988;75:7–13. doi: 10.1007/BF00931183. [DOI] [PubMed] [Google Scholar]
- Lahm H, Andre S, Hoeflich A, Fischer JR, Sordat B, Kaltner H, et al. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol. 2001;127:375–386. doi: 10.1007/s004320000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquoi E, van den Brule FA, Castronovo V, Foidart JM. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta. 1997;18:433–439. doi: 10.1016/s0143-4004(97)80044-6. [DOI] [PubMed] [Google Scholar]
- Minkoff H, Grunebaum AN, Schwarz RH, Feldman J, Cummings M, Crombleholme W, et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984;150:965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- Mirhaghani A, Warton A. Involvement of Trichomonas vaginalis surface-associated glycoconjugates in the parasite/target cell interaction. A quantitative electron microscopy study. Parasitol Res. 1998;84:374–381. doi: 10.1007/s004360050413. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, et al. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. 2005;174:4120–4126. doi: 10.4049/jimmunol.174.7.4120. [DOI] [PubMed] [Google Scholar]
- Pace KE, Hahn HP, Baum LG. Preparation of recombinant human galectin-1 and use in T-cell death assays. Methods Enzymol. 2003;363:499–518. doi: 10.1016/S0076-6879(03)01075-9. [DOI] [PubMed] [Google Scholar]
- Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29:3595–3602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier I, Sato S. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J Biol Chem. 2002;277:17663–17670. doi: 10.1074/jbc.M201562200. [DOI] [PubMed] [Google Scholar]
- Pelletier I, Hashidate T, Urashima T, Nishi N, Nakamura T, Futai M, et al. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J Biol Chem. 2003;278:22223–22230. doi: 10.1074/jbc.M302693200. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–317. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Gruppi A. Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of the disease. Parasite Immunol. 2005;27:103–114. doi: 10.1111/j.1365-3024.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Reimer T, Ulfig N, Friese K. Antibiotics: treatment of preterm labor. J Perinat Med. 1999;27:35–40. doi: 10.1515/JPM.1999.004. [DOI] [PubMed] [Google Scholar]
- Saussez S, Kiss R. Galectin-7. Cell Mol Life Sci. 2006;63:686–697. doi: 10.1007/s00018-005-5458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Filho FC, Ortega-Lopez J, Arroyo R. YIGSR is the preferential laminin-1 residing adhesion sequence for Trichomonas vaginalis. Exp Parasitol. 1998;88:240–242. doi: 10.1006/expr.1998.4227. [DOI] [PubMed] [Google Scholar]
- Singh BN. The existence of lipophosphoglycan-like molecules in Trichomonads. Parasitol Today. 1994;10:152–154. doi: 10.1016/0169-4758(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Singh BN, Beach DH, Lindmark DG, Costello CE. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch Biochem Biophys. 1994;309:273–280. doi: 10.1006/abbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Smith L, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis. 2001;7:927–932. doi: 10.3201/eid0706.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath GF, Garraway LA, Turco SJ, Beverley SM. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci USA. 2003a;100:9536–9541. doi: 10.1073/pnas.1530604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003b;301:1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- Vicovac L, Jankovic M, Cuperlovic M. Galectin-1 and -3 in cells of the first trimester placental bed. Hum Reprod. 1998;13:730–735. doi: 10.1093/humrep/13.3.730. [DOI] [PubMed] [Google Scholar]
- Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- Walzel H, Neels P, Bremer H, Kohler H, Raab N, Barten M, Brock J. Immunohistochemical and glycohistochemical localization of the beta-galactoside-binding S-type lectin in human placenta. Acta Histochem. 1995;97:33–42. doi: 10.1016/s0065-1281(11)80204-7. [DOI] [PubMed] [Google Scholar]
- WHO. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections Overview and Estimates. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- von Wolff M, Wang X, Gabius HJ, Strowitzki T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod. 2005;11:189–194. doi: 10.1093/molehr/gah144. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. 1994;23:682–690. doi: 10.1093/ije/23.4.682. [DOI] [PubMed] [Google Scholar]


