1. Introduction
In primates, infants spend early developmental periods in close contact with caregivers and often maintain close social relationships with caregivers throughout development and into adulthood. Not surprisingly, social separation in young monkeys reliably produces increases in hypothalamic-pituitary-adrenal (HPA) activity (and its primary output, cortisol) and anxiety-like behavior, and has been a standard laboratory method of inducing stress responses in primates (Coe et al., 1983; Dettling et al., 2002; French et al., 2012; Gunnar et al., 1981). Despite these rapid separation-induced changes in behavior and HPA function, reunion with caregivers quickly alleviates the effects of separation. Reunion with caregivers results in cessation of stressor-induced behavior, and a return to baseline HPA function and social behavior (Dettling et al., 2002, 1998; French et al., 2012). The speed of this return to baseline is dependent on the severity of the stressor. For example, capture and brief handling usually produces an increase in cortisol in young squirrel monkeys, but if a juvenile is captured, handled, and then immediately returned to the mother, this cortisol response is blunted (Levine, Coe, Smotherman, & Kaplan, 1978; Mendoza, Smotherman, Miner, Kaplan, & Levine, 1978; Wiener, Johnson, & Levine, 1987). If the separation is prolonged though, cortisol can remain elevated for 30 minutes to 12 hours after reunion with the caregiver (French et al., 2012; Levine et al., 1978), returning to normal levels within two days (Cinini et al., 2014). Additionally, reunion with a caregiver causes reductions in stressor-induced behavior, and increases in novelty exploration and peer-directed social behavior (Dettling, Feldon, & Pryce, 2002; Dettling, Pryce, Martin, & Döbeli, 1998; Erickson et al., 2005). Clearly there are properties of the reunion experience that serve to terminate stress activation and begin behavioral and physiological regulation.
Parents and peers can serve as powerful social buffers during stressful situations, and they can also serve as regulators of physiological function following a stressor (DeVries et al., 2003; Hostinar et al., 2014). Maintaining some contact with a caregiver can help to buffer the effects of social separation. For example, moving young monkeys from group housing to single housing causes increases in cortisol, but if the mother is relocated with the infant, this response is blunted (Wiener et al., 1987). Similarly, compared to total social separation, the cortisol response is blunted when young monkeys are removed from the home cage and housed adjacent to the mother (Bayart, Hayashi, Faull, Barchas, & Levine, 1990; Levine, Johnson, & Gonzalez, 1985). Even limited communication (olfactory and auditory) between a mother-infant pair partly attenuates the cortisol response to separation in young squirrel monkeys (Levine, Wiener, & Coe, 1993). Behaviorally, infants engage in more time in contact with the mother following exposure to novelty stress if exposed to novelty alone, relative to when exposed to novelty with the mother present (Parker et al., 2006). There are also effects of early social environment on social buffering. When mother-reared macaques are separated from the group with a partner, they engage in more social contact behavior, and have lower cortisol than nursery reared macaques (Winslow et al., 2003). Similarly, infant squirrel monkeys exposed to an early stress-inoculation paradigm, in which young monkeys are repeatedly exposed to a mild separation stressor followed by maternal reunion, are less neophobic than normally-reared monkeys when exposed to novelty with the mother present (Parker et al., 2006).
In addition to these social effects on HPA activity and behavior during exposure to stressors, social interactions with a caregiver that follow a stressor can also modulate HPA activity. In squirrel monkeys, longer nursing duration immediately following separation is associated with lower plasma cortisol and ACTH (Parker et al., 2006). Behaviorally, young monkeys seek out contact with the caregiver upon reunion, and monkeys that exhibit greater behavioral agitation during the stressor have longer durations in contact with the mother (Dettling et al., 1998; Gunnar et al., 1981). Thus it is clear that contact with the caregiver during and after a stressor can alter behavioral and HPA responses to stressors in young primates.
Infants develop a strong social bond with the primary caregiver, and in most nonhuman primates, the primary caregiver is the mother. In contrast to macaques and squirrel monkeys, marmosets develop within a family unit in which they can interact with caregivers of varied ages and sexes (French, 1997; Nunes, Fite, Patera, & French, 2001; Tardif, 1997). Additionally, the HPA system in developing marmosets is highly sensitive to familial social influences (Birnie et al., 2013; Dettling et al., 2002; Mustoe et al., 2014). The purpose of the current study was to examine relationships among affiliative sociality, HPA activity and behavior along a shorter timescale. We separated young marmosets from their family groups and evaluated whether HPA reactivity during the stressor was related to affiliative reunion behavior. We also assessed whether marmosets who engaged in more affiliative behavior during reunion subsequently returned to baseline HPA functioning in the hours following the stressor. Specifically, we predicted that if engagement in affiliative social interactions with the family is a normative response to stressors, then marmosets that exhibit higher cortisol responses to separation stress would engage in higher rates of grooming and play with family members upon reunion. We also predicted that if social interactions with the family serve an important regulatory role in HPA function following the cessation of a stressor, then marmosets that participated in more grooming and play during reunion would display cortisol values closer to baseline levels after 24 hours post-separation.
2. Method
2.1 Subjects
A total of 52 marmosets (Callithrix geoffroyi; 30 males, 22 females) were tested at three developmental time points (6, 12, and 18 months of age). These time points were chosen as distinct developmental stages: juvenile, sub-adult, and young adult (Yamamoto, 1993). Subject attrition from the study lead to different samples sizes at each age in which complete behavioral and endocrine data were available: 6 months (n=44), 12 months (n=40), and 18 months (n=29). Marmosets that were removed from the study did not differ from those that remained on any behavioral or endocrine measures (t’s < ± 1.62, p’s >.11) Marmosets were housed in family groups composed of a breeding pair and their offspring (mean number of caregivers = 4.1) at the Callitrichid Research Center (CRC) at the University of Nebraska at Omaha. The CRC maintains large enclosures (minimum of 0.8 m3 per individual), and branches, rope vines, and enrichment devices are rotated regularly. Marmosets at the CRC are provided with commercial marmoset food (Zupreme) at approximately 0800h, and fruit and invertebrate protein at approximately 1400 hours. All procedures were approved by UNMC/UNO Institutional Animal Care and Use Committee (IACUC 07-033-05-FC).
2.2 Standardized Psychosocial Stressor Procedure
At 6, 12, and 18 months of age, marmosets were removed from the family group, placed into a small cage (50 × 50 × 50 cm), and removed to a quiet location with only limited auditory and olfactory contact with other marmosets in the colony (including their family group). Marmosets were provided with food and water, and left relatively undisturbed for 8 hours from 0900h to 1700h. At the end of 8 hours, marmosets were then released back into the family’s home cage. Marmosets that were members of twin litters experienced the separation procedure on the same day, and returned to the home cage at the same time. Further details on the standardized psychosocial stressor procedure can be found in Taylor, Mustoe, and French (2014). The social separation procedures at the three time points we sampled represented the first exposures to experimental stressors in this sample of marmosets.
2.3 Behavioral Observations
Baseline levels of affiliative social behavior of marmoset offspring with family members were determined via observations conducted on undisturbed marmoset family groups (20-min observations three times per week) during the 30 days immediately prior to each social separation. Rates of affiliative social behavior were also recorded in the first 20 minutes after each separated marmoset was returned to its home cage. We recorded two indices of affiliative social behavior in both sets of observations. Allogrooming and a cumulative measure of social play (see ethogram and definitions in Agmo et al. 2012; Birnie et al. 2012) were recorded, and we differentiated bouts of grooming and play that were initiated by the reunited marmoset and bouts that were received by the reunited marmosets. Two extreme outliers (>20 SD above the mean for play behavior, twins from the same litter), were removed from analyses.
2.4 Urine Collection and Cortisol Assay
First-void urine samples were collected the morning before, the morning of but prior to, and the morning after the separation using a non-invasive collection procedure. During the separation procedure, urine was collected hourly from plastic sheeting placed under the cage. Urinary cortisol (CORT) was measured using an enzyme immunoassay. Details on the assay and its validation in marmosets can be found in Smith and French (1997). Intra- and inter-assay coefficients of variation (CV) for high and low concentration quality control pools were acceptable (intra-assay: 8.9% and 9.8%; inter-assay: 12.3% and 14.9%, respectively). To control for variable fluid intake and output, cortisol concentrations were divided by creatinine concentration to give a value of μg cortisol/mg creatinine. Baseline CORT was determined by averaging the two first-void samples prior to the separation procedure, CORT levels during the separation procedure reflect the hourly samples collected during separation, and Post-separation CORT reflects levels in first-void samples collected the day following the separation procedure. Two indices of HPA function were derived from these values: CORT reactivity (Highest CORT during separation minus Baseline CORT) and CORT regulation (Post-separation CORT minus Baseline CORT). For CORT reactivity, a higher value indicates a larger glucocorticoid response to separation, and for CORT regulation, a higher value indicates poorer negative feedback regulation of cortisol following the cessation of social separation. CORT reactivity shows the most relative consistency within individuals across development, but changes significantly in absolute values. In contrast, CORT regulation shows very little intra-individual consistency but changes very little across development in absolute values (French et al., 2012).
2.5.1 Analytic Strategy
In order to characterize the effects of separation and subsequent reunion on marmosets’ social behavior, we compared rates of social behavior upon reunion with rates of these behavioral patterns during undisturbed home-cage observations in the month prior to each separation using a 2(social condition) × 3(age) × 2(sex) mixed factorial ANOVA. Post-hoc comparisons were done using t-tests.
For the main hypotheses in our paper, the fundamental analytical questions were as follows: (1) does the magnitude of the cortisol response during social separation predict the nature of offspring-caregiver social interactions upon reunion, and (2) does the ability of marmosets to regulate the stress response and return to baseline the day following social separation depend upon the nature and quality of social behavior upon reunion? In order to maximize data use and control for within-individual and within-family nonindependence, these relationships between HPA activity and reunion behaviors were analyzed using multilevel modeling (MLM) analyses (HLM; Raudenbush, Bryk, Cheong, Congdon, & du Toit, 2000). A three level model was employed, that first included within-subject variables, reunion behavior and CORT at 6, 12, and 18 months (Level-1). Next we controlled for the between subject variables sex and early parental care (Level-2). Finally, because marmosets produce twin litters that develop in families of differing sizes, we controlled for both litter and family size (Level-3). Reunion behaviors were modeled by first controlling for age, baseline cortisol, and family size at birth, and then by adding cortisol reactivity as a predictor. CORT regulation was modeled by controlling for age, baseline cortisol, and family size at birth, and then adding subject- and family-initiated social behavior at reunion. Due to constraints on degrees of freedom, grooming and play behavior were examined as separate models, and only family-initiated social behavior (grooming or play) was entered as a random effect. Neither initiated nor received grooming was significantly correlated with initiated or received play (r’s < ± .23, p’s > .23). Model significance was assessed by the change in Δχ2 compared to the previous model, and effect sizes are expressed as percent of reduced prediction error (PRPE) compared to the previous model.
2.5.2 Model Justification
The unconditional model intraclass correlation for total grooming behavior, total play behavior, and CORT regulation indicated that 70.5-86.4% of the variability was within individuals (Level 1), that 0.02-1.5% of the variability was between individuals (Level 2), and that 13.6-29.4% of the variability was between families (Level 3). This indicates that most of the variability in total grooming behavior varies within individuals. There was still considerable variability in grooming behavior between families, but very little variability to be explained between individuals (i.e. there were no effects of sex or early care). Because of the small proportion of variance to be explained at Level 2, only effects at Level 1 and Level 3 were examined.
3. Results
3.1 Social separation affects social behavior at reunion
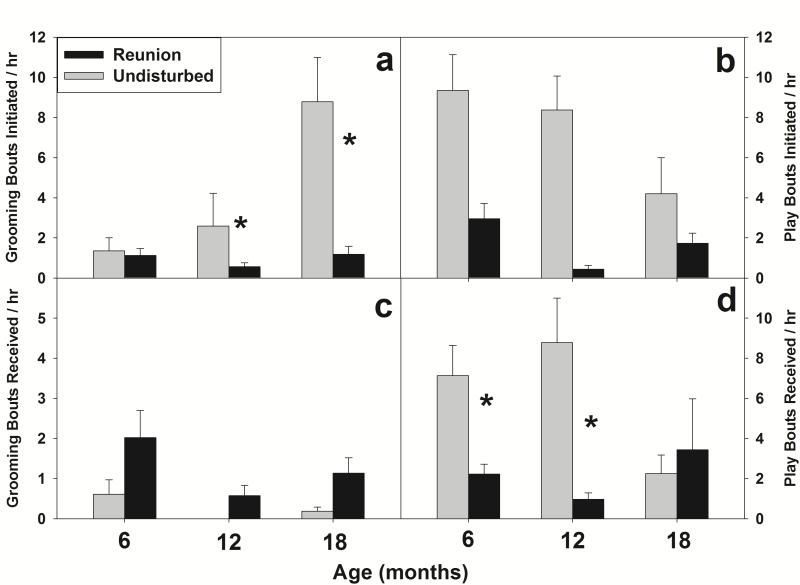
Social separation disrupted social behavior upon reunion relative to normative social behavior within the family (Figure 1). Rates of grooming initiated by the marmoset under undisturbed conditions increased across development, but initiated grooming was inhibited during reunion (F(2,74) = 5.19, p = .008; Figure 1a). Similarly, young marmosets initiated less play behavior than they did under undisturbed conditions (F(1,37) = 16.87, p < .001; Figure 1b). Young marmosets also received less play behavior during reunion than under undisturbed conditions, but this difference was only apparent at 6 and 12 months of age (F(2,74) = 4.96, p = .009; Figure 1d). In contrast to play behavior and initiated grooming, separated marmosets received more grooming from the family during reunion than they did under undisturbed conditions (F(1,37) = 6.80, p = .013;Figure 1c). Males and females did not differ in rates of received or initiated social behavior (all F’s (1,37) < 0.853, n.s.)
Figure 1.
Mean (±SEM) frequencies of grooming and play during undisturbed home cage observations (gray) and post-separation reunion observations (black). Grooming initiation (a) and play bouts initiated and received (b,d) were significantly higher during home cage observations than following reunion, while received grooming from family members (c) was significantly higher following reunion than during home cage observations. Asterisks indicate differences at specific ages in the case of significant age × social condition interaction effects.
3.2 HPA reactivity does not predict reunion behaviors
3.2.1 Grooming Behavior
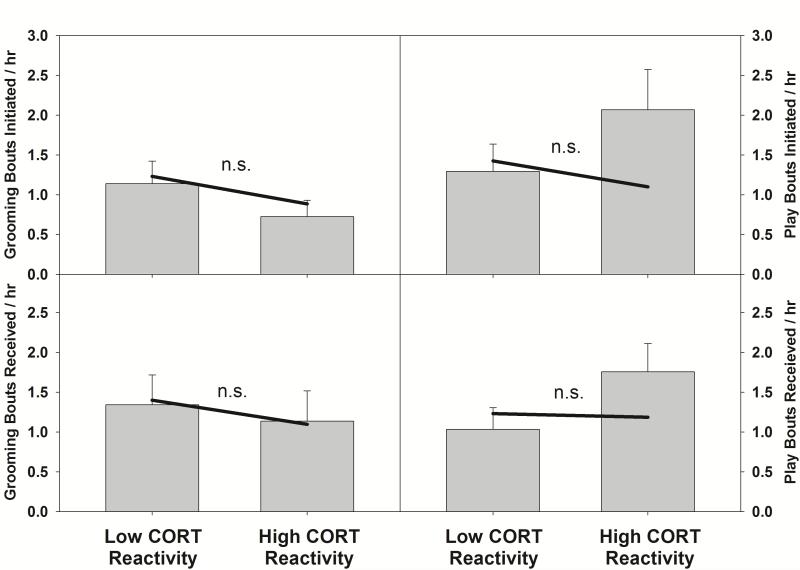
The magnitude of the stress response during separation did not predict variation in frequencies of play and grooming upon reunion. Peak CORT reactivity in marmosets during separation was not associated with grooming behavior at reunion (Δχ2 (7)=4.14, PRPE= 5.1%, p=.901; Supplementary Table S1). Additionally, when grooming behavior was split into grooming initiated by, and received by the separated marmoset, CORT reactivity was not associated with either initiated or received grooming (initiate: Δχ2(7)= 8.58, PRPE= 15.4%, p=.284; receive: Δχ2(7)= 4.31, PRPE= 21.7%, p=. 743; Figure 2a-b). Thus the degree of HPA activation that a separated marmoset exhibits has no relationship to subsequent grooming behavior upon reunion.
Figure 2.
Mean (±SEM) frequencies of social behavior collapsed across ages. Solid lines represent model predictions at CORT reactivity values at the 25th and 75th percentiles. For illustrative purposes, data were split at the median for CORT reactivity. n.s. indicates that the regression coefficient in the MLM for CORT reactivity was not significant.
3.2.2 Play Behavior
Similarly, peak CORT reactivity during separation in marmosets was not associated with total play behavior at reunion (Δχ2(7)=2.82, PRPE= 3.4%, p=.901; Supplementary Table S2). Additionally, CORT reactivity was not associated with play either initiated or received by the reunited marmoset (initiate: Δχ2(7) = 5.28, PRPE= 6.2%, p=.626; receive: Δχ2(7)= 3.84, PRPE= 13.0%, p=. 798; Figure 2c-d). As with grooming behavior, the degree of physiological perturbation that a separated marmoset exhibits had no relationship to subsequent play behavior upon reunion.
3.3 Reunion behaviors are associated with HPA recovery
3.3.1 Grooming behavior
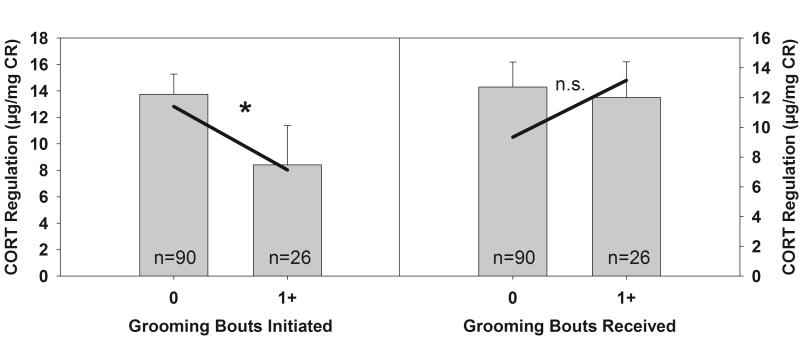
Grooming behavior during reunion was significantly associated with CORT regulation in reunited marmosets (Δχ2(13) =54.00, PRPE=19.5%, p<.001). As shown in Fig. 3, young marmosets who initiated grooming with family members more frequently had better CORT regulation (i.e., lower scores) than marmosets who initiated less frequent grooming with the family (t(22)=−2.27, p=.033). Grooming initiated by the family toward the reunited marmoset, however, was not associated with CORT regulation (t(22) = 1.89, p= .072; Supplementary Table S3). Family size did not moderate the effect of grooming on CORT regulation.
Figure 3.
Mean (±SEM) cortisol regulation collapsed across ages. Solid lines represent model predictions at 0 and 1 grooming bouts. For illustrative purposes, data were split by individuals who initiated no grooming bouts with family members upon reunion, and individuals who initiated at least one grooming bout with a family member upon reunion. Asterisks indicate a significant regression coefficient in the MLM. n.s. indicates regression coefficient in the MLM was not significant.
3.3.2 Play behavior
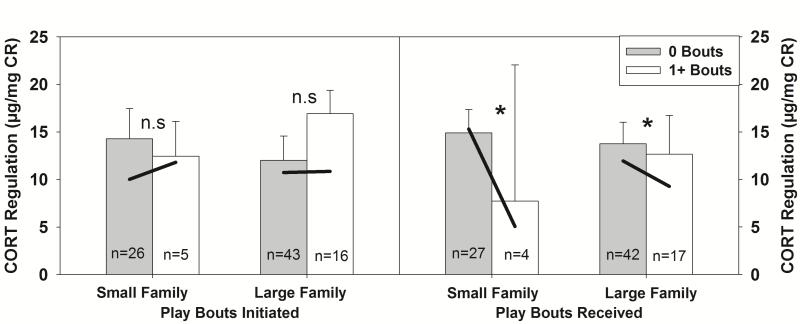
Play behavior during reunion was significantly associated with CORT regulation in reunited marmosets (Δχ2(13)=48.27, PRPE=15.6% p<.001). Marmosets that received higher rates of play from family members exhibited enhanced CORT regulation (t(22) = −2.37, p= .027; Supplementary Table S4), and this effect was strongest in small families (t(22) = 3.03, p= .006; Figure 4). In contrast, rates of play by reunited marmosets did not predict CORT regulation (t(22) = 0.35, p= .198).
Figure 4.
Raw mean (±SEM) cortisol regulation collapsed across ages. Solid lines represent model predictions at 0 and 1 bouts of play. For illustrative purposes, data were split by individuals who received no play from family members upon reunion, and individuals who received at least one play bout from a family member upon reunion. . Small family = 2 caregivers, Large family = 3+ caregivers. Asterisks indicate a significant regression coefficient in the MLM. n.s. indicates regression coefficient in the MLM was not significant.
3.4 Reunion behavior predicts HPA regulation better than HPA reactivity does
Six- and 12-month old marmosets with higher CORT reactivity exhibited higher CORT regulation, while marmosets at 18 months of age did not show an association between HPA reactivity and regulation (6 months, r(50) = .358, p =.011; 12 months, r(41)= .429, p =.005; 18 months, r(33) = .110, p =.541). Because of a potential link between HPA reactivity and regulation, we wanted to explore the possibility that CORT reactivity alone could account for variability in CORT regulation better than affiliative social reunion behavior did. To examine this, we used a model comparison approach. First, CORT reactivity was added to the model that controlled for age, baseline CORT and family size. Consistent with the relationships found from pairwise correlations, CORT reactivity in marmosets during separation was significantly associated with CORT regulation above and beyond the control variables (Δχ2 (7)= 32.27, p = .014; t(22) = 3.07, p = .006). Then we compared the total amount of variability that was accounted for by this model to the amount of variability accounted for by play or grooming in the models above (supplementary tables S3-4). Play and grooming each accounted for significantly more variability in CORT regulation than CORT reactivity did (CORT reactivity vs play: Δχ2 (7) = 16.00, p = .025; CORT reactivity vs grooming: Δχ2 (7) = 21.73, p = .003, respectively). Thus, these findings suggest that a marmoset’s HPA recovery is more strongly associated with their interactions with the family following a stressor than with their physiological responses during the stressor.
4. Discussion
Primates are highly social and live in close-knit social groups. Consequently, a social separation from the social group for the duration of a day serves as a significant stressor for a developing primate (French et al., 2012; Gunnar et al., 1981). We have shown here that separation impacts social interactions with the family in marmoset monkeys. Specifically, the overall frequencies of social behavior were reduced during reunion compared to baseline levels of social behavior, and levels of cortisol during separation were independent of social behavior during reunion. Importantly, we also show that despite relatively low levels of social behavior at reunion, affiliative behaviors upon reunion predicted HPA regulation. Marmosets that initiated more grooming and received more play during reunion with their families showed cortisol levels closer to baseline the following morning. This suggests that affiliative behavior is an important modulator in how marmosets respond and adapt to social stressors, and that the family exerts a powerful social influence on young monkeys. In sum, the relationship between affiliative behavior during reunion and physiological recovery highlights the importance of quality social relationships in developing mature and resilient recovery from stress.
4.1 Affiliative Behavior and HPA regulation
We used two reunion behaviors as our measure of affiliative behavior, allogrooming and play. Allogrooming among primates is largely regarded as a mechanism to strengthen social bonds (Dunbar, 2010), and both initiating and receiving grooming are associated with decreased cortisol (Gust et al., 1993; Shutt et al., 2007). Willingness to engage in grooming, and its effect on HPA activity, depends in part on social context and life experience. Mother-reared monkeys engage in more grooming and social contact behavior with a partner during separation from the group than peer-reared monkeys do, and also have smaller increases in cortisol (Winslow et al., 2003). Additionally, increased grooming was associated with decreased cortisol in separated and reunited marmosets, but this was only true in related males (Galvão-Coelho et al., 2012). These studies inform our results, by illustrating that the relationship between affiliative behavior and HPA activity are mediated by both social history (as in comparisons between rearing conditions) and relationship quality (as in comparisons between related and unrelated males). We found that young marmosets recovering from social separation had cortisol levels on the morning following social separation that were closer to baseline if they had initiated more grooming with the family. This result emerged in spite of the fact that overall rates -initiated grooming is markedly lower following separation than it is during typical home cage conditions. From the current study and those referenced here, it is clear that grooming is an important component of both the HPA system and the development of social bonds.
Social play is an important affiliative behavior for both developing and adult primates, and the functions and nature of play may vary depending on age, species, and sex. In young infant and juvenile primates play appears to facilitate behavioral flexibility and development (Poirier and Smith, 1974). In adolescents and adults social play is often tied to forms of social assessment, especially in primates that have more rigid social hierarchies (Pellis and Iwaniuk, 2000). Less is understood about the role of play in response to social stressors. Marmosets exposed to early social deprivation show reduced levels of infant social play (Pryce et al., 2011). Furthermore, adult marmosets that engage in increased levels of social play showed less behavioral indicators of stress (Norscia and Palagi, 2011). This effect of stress and adult play is limited and unidirectional insofar as social play attenuates later stress responses rather than stressful situations influencing later play. Our findings in marmosets align with this view because the level of HPA activation during the stressor was not associated with subsequent play during reunion, but instead, social play during reunion was associated with an enhanced HPA recovery the day following the stressor. The effects of play on HPA activity is not restricted to acute stressors and regulation; long-term associations between increased early-life social play and later-life HPA regulation during social stressors have been shown in both rats and marmosets (Mustoe et al., 2014). Also, when play is deprived during adolescence, there is a long-term effect on overall social behavior as adults, and poorer glucocorticoid regulation and recovery following social stressors in rodents (van den Berg et al., 1999). These studies show that social play not only influences later-life HPA activation but, more interestingly, play can alleviate the negative impacts from early-life stress and prenatal glucocorticoid exposure on later-life HPA activation during social stressors (Mustoe et al., 2014). Taken together, these findings highlight that social play during key developmental stages is an important social activity for both the immediate and long-term regulation of HPA and behavioral responses to significant social stressors.
4.2 Development of HPA Activation in Response to Stressors
It is important to consider the role of maturation and developmental stability when characterizing the way in which young primates respond to stressful situations. Generally, young monkeys are affected by social separation more so than older monkeys; younger monkeys have larger increases in HPA activity and exhibit higher rates of behavioral responses during separation than adult monkeys (Capitanio et al., 2005; French et al., 2012; Taylor et al., 2014). Behavioral responses to stressors tend to be more variable within individuals and across ages, and more subject to habituation, while cortisol responses to stressors tend to be more stable (Coe et al., 1983; French et al., 2012; Taylor et al., 2014). By 6 months of age, marmosets exhibit basal cortisol levels and diurnal variation typical of adults (Pryce et al., 2002), but the development of the HPA system is flexible during critical periods in early life; early environmental influences tend to result in stable, trait-like responses to stress in adulthood (Suomi, 1997). Increased parental maltreatment during infancy is associated with increased cortisol reactivity in young adulthood (Birnie et al., 2013), and experimental manipulations show that this enhanced reactivity may be mediated by low levels of glucocorticoid receptor expression in the hippocampus of marmosets exposed to early life stressors (Arabadzisz et al., 2010). If physiological responses to stressors are a trait-like characteristic, it would be expected that juvenile marmosets that show a high degree of HPA activation in response to a social separation stressor are also likely to show a high degree of HPA activation as young adults. It appears, though, that the developmental stability of HPA activity depends on which HPA parameter is being examined. For instance, marmosets show high stability across age in HPA reactivity during a stressor, but individual differences in baseline cortisol levels and HPA regulation across age exhibit lower stability and greater variability (French et al., 2012). It is worth noting that CORT reactivity, which is relatively consistent across development, was not associated with reunion behavior. In contrast, CORT regulation, which is not stable across development, was in fact, related to reunion behavior. Even in adult macaques, cortisol measured during stressors is more consistent across time than are basal measures of cortisol (Capitanio et al., 1998). This highlights that while physiological responses during a stressor in primates appear to be trait-like, there is still considerable room for social context to modulate physiological responses following a stressor. The expression of affiliative behavior with the family following stressful situations provides an important behavioral and physiological support for a young and developing primate.
4.3 Parallels with human studies
The presence of a conspecific, whether it is a parent or a partner, can be a powerful social buffer during stressful situations for rodents, nonhuman primates, and humans alike (Kikusui et al., 2006). Our data supplement this body of work regarding social influences on stress physiology by showing that affiliative behavior after a stressor can also enhance recovery from separation stress. Parker and colleagues (Parker et al., 2006) showed that monkeys that engaged in more time nursing immediately following exposure to stress had lower basal and post-stressor cortisol; we have extended this finding to grooming and play behavior, and also found that it was consistent throughout development. Though research examining the relationship between reunion behavior and HPA regulation is limited in nonhuman primates, it is abundant in the study of human children, and parallels can be drawn between the current study and studies in human children that make use of the Strange Situation Paradigm. In the current study, proximity-seeking behaviors at reunion are used as a measure of affiliative behavior. Similarly, in humans attachment styles are assigned according to the child’s proximity-seeking affiliative responses to both separation and reunion. Evidence that supports the hypothesis that highly affiliative (i.e. securely attached) children have smaller cortisol responses to separation than low affiliative (i.e. insecurely attached) children is mixed, but it seems that as the duration between reunion and sampling increases (i.e. the more similar a measure gets to CORT regulation and less similar to CORT reactivity), so do the differences between high affiliative and low affiliative children’s cortisol levels, with the high affiliative children returning to baseline, and the low affiliative children sustaining high cortisol levels (Gunnar et al., 1989; Hertsgaard et al., 1995; Smider et al., 2002; Spangler and Grossmann, 1993). Additionally, school-age children who are characteristically outgoing and well-liked tend to exhibit rapid HPA adaptation to separation from the parents, while those children who tend to exhibit social expression problems also experience high afternoon cortisol and a flattened cortisol awakening response (both indices of atypical HPA regulation) (Gunnar et al., 1997; Laurent et al., 2014). In both young nonhuman primates and human children, engaging in affiliative behavior has a positive impact on the HPA system’s recovery from stress.
4.4 Concluding remarks
In this study we detailed the relationships between affiliative social behaviors and HPA regulation across three developmental time points. One of the strengths of the current study is that we were able to examine relationships between reunion behavior and HPA activity across distinct developmental periods. We were also able to statistically control for social and developmental variables, such as age, family size, and baseline cortisol. Interestingly, family size had a moderating effect on the relationship between play and cortisol regulation. This demonstrates the remarkable complexity and adaptability of the developing HPA system, and there are likely many more variables that influence relationships between social behavior and HPA activity. Additionally, we found that relationships between behavior and HPA activity depended on which partner initiated the activity. This raises additional questions about the development of actor/recipient relationships, reciprocity between parents, infants, and other social partners, and the relationships between these complex multi-partner family interactions and HPA development. It is abundantly clear though, that the presence of a conspecific during a stressful encounter buffers peak HPA reactivity (Kikusui et al., 2006), and we have shown that interactions with the family after separation is associated with enhanced HPA recovery. We suggest that among marmosets, cortisol reactivity appears to be trait-like and stable across development, whereas cortisol regulation is state-like, and appears to be subject to modification via social interactions. This highlights the important role of intra-family social interactions in shaping the development of some components of the HPA axis.
Supplementary Material
Highlights.
Social behavior was lower during reunion compared with undisturbed conditions.
Levels of cortisol during separation did not predict subsequent reunion behavior.
Social behavior upon reunion with the family predicted more complete HPA recovery.
Acknowledgements
We would like to thank Heather Jensen and her team of volunteers for providing excellent care of all the marmosets used in this project. This research was supported by a grant from the NIH awarded to JAF (HD042882).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Feldon J, Law AJ, Harrison PJ, Pryce CR. Primate Early Life Stress Leads to Long-Term Mild Hippocampal Decreases in Corticosteroid Receptor Expression. Biol. Psychiatry, Synaptic Development in Mood Disorders. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. doi:10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta) Behav. Neurosci. 1990;104:98. [PubMed] [Google Scholar]
- Birnie AK, Taylor JH, Cavanaugh J, French JA. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi) Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.08.011. doi:10.1016/j.psyneuen.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): Evidence for temporal and situational consistency. Am. J. Primatol. 1998;44:29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. doi:10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev. Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. doi:10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Cinini SM, Barnabe GF, Galvao-Coelho N, Medeiros MA, Perez-Mendes P, Sousa MB, Covolan L, Mello LE. Social isolation disrupts hippocampal neurogenesis in young non-human primates. Front. Neurosci. 2014;8:45. doi: 10.3389/fnins.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol. Biochem. Behav. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Pryce CR, Martin RD, Döbeli M. Physiological responses to parental separation and a strange situation are related to parental care received in juvenile Goeldi’s monkeys (Callimico goeldii) Dev. Psychobiol. 1998;33:21–31. doi: 10.1002/(sici)1098-2302(199807)33:1<21::aid-dev3>3.0.co;2-u. doi:10.1002/(SICI)1098-2302(199807)33:1<21::AID-DEV3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. doi:10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Erickson K, Gabry KE, Schulkin J, Gold P, Lindell S, Higley JD, Champoux M, Suomi SJ. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Dev. Psychobiol. 2005;46:331–339. doi: 10.1002/dev.20061. doi:10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- French JA. Proximate regulation of singular breeding in callitrichid primates. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; New York, NY, US: 1997. pp. 34–75. [Google Scholar]
- French JA, Smith AS, Gleason AM, Birnie AK, Mustoe A, Korgan A. Stress reactivity in young marmosets (Callithrix geoffroyi): Ontogeny, stability, and lack of concordance among co-twins. Horm. Behav. 2012;61:196–203. doi: 10.1016/j.yhbeh.2011.12.006. doi:10.1016/j.yhbeh.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão-Coelho NL, Silva HPA, De Sousa MBC. The Influence of Sex and Relatedness on Stress Response in Common Marmosets (Callithrix jacchus) Am. J. Primatol. 2012;74:819–827. doi: 10.1002/ajp.22032. doi:10.1002/ajp.22032. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Gonzalez CA, Goodlin BL, Levine S. Behavioral and pituitary-adrenal responses during a prolonged separation period in infant rhesus macaques. Psychoneuroendocrinology. 1981;6:65–75. doi: 10.1016/0306-4530(81)90049-4. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Dev. Psychol. 1989;25:355. [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stanbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Dev. Psychobiol. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. doi:10.1002/(SICI)1098-2302(199707)31:1<65::AID-DEV6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between Social Factors and Pituitary-Adrenocortical Activity in Female Rhesus Monkeys (Macaca mulatta) Horm. Behav. 1993;27:318–331. doi: 10.1006/hbeh.1993.1024. doi:10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical Responses to the Strange Situation in Infants with Disorganized/Disoriented Attachment Relationships. Child Dev. 1995;66:1100–1106. doi:10.2307/1131801. [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychol. Bull. 2014;140:256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. B Biol. Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. doi:10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, Leve LD. Stress System Development from Age 4.5 to 6: Family Environment Predictors and Adjustment Implications of HPA Activity Stability versus Change. Dev. Psychobiol. 2014;56:340–354. doi: 10.1002/dev.21103. doi:10.1002/dev.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Coe CL, Smotherman WP, Kaplan JN. Prolonged cortisol elevation in the infant squirrel monkey after reunion with mother. Physiol. Behav. 1978;20:7–10. doi: 10.1016/0031-9384(78)90194-4. doi:10.1016/0031-9384(78)90194-4. [DOI] [PubMed] [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behav. Neurosci. 1985;99:399–410. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Levine S, Wiener SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;18:297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Smotherman WP, Miner MT, Kaplan J, Levine S. Pituitary-adrenal response to separation in mother and infant squirrel monkeys. Dev. Psychobiol. 1978;11:169–175. doi: 10.1002/dev.420110209. doi:10.1002/dev.420110209. [DOI] [PubMed] [Google Scholar]
- Mustoe AC, Taylor JH, Birnie AK, Huffman MC, French JA. Gestational cortisol and social play shape development of marmosets’ HPA functioning and behavioral responses to stressors: GCE, Social Play, and HPA Activity. Dev. Psychobiol. 2014:n/a–n/a. doi: 10.1002/dev.21203. doi:10.1002/dev.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norscia I, Palagi E. When play is a family business: adult play, hierarchy, and possible stress reduction in common marmosets. Primates J. Primatol. 2011;52:101–104. doi: 10.1007/s10329-010-0228-0. doi:10.1007/s10329-010-0228-0. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among Paternal Behavior, Steroid Hormones, and Parental Experience in Male Marmosets (Callithrix kuhlii) Horm. Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. doi:10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, Iwaniuk AN. Adult–Adult Play in Primates: Comparative Analyses of its Origin, Distribution and Evolution. Ethology. 2000;106:1083–1104. doi:10.1046/j.1439-0310.2000.00627.×. [Google Scholar]
- Poirier FE, Smith EO. Socializing functions of primate play. Am. Zool. 1974;14:275–287. [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology (Berl.) 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. doi:10.1007/s00213-010- 1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Palme R, Feldon J. Development of pituitary-adrenal endocrine function in the marmoset monkey: infant hypercortisolism is the norm. J. Clin. Endocrinol. Metab. 2002;87:691–699. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong YF, Congdon R, du Toit M. Hierarchical Linear and Nonlinear Modeling-HLM5. Lincolnwood IL SSI Sci. Softw. 2000 [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than to receive? Biol. Lett. 2007;3:231–233. doi: 10.1098/rsbl.2007.0052. doi:10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Dev. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiol. Behav. 1997;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossmann KE. Biobehavioral Organization in Securely and Insecurely Attached Infants. Child Dev. 1993;64:1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. doi:10.2307/1131544. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br. Med. Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Tardif SD. The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins. Coop. Breed. Mamm. Camb. Univ. Press Camb. 1997:11–33. [Google Scholar]
- Taylor JH, Mustoe AC, French JA. Behavioral responses to social separation stressor change across development and are dynamically related to HPA activity in marmosets. Am. J. Primatol. 2014:n/a–n/a. doi: 10.1002/ajp.22228. doi:10.1002/ajp.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- Wiener SG, Johnson DF, Levine S. Influence of postnatal rearing conditions on the response of squirrel monkey infants to brief perturbations in mother-infant relationships. Physiol. Behav. 1987;39:21–26. doi: 10.1016/0031-9384(87)90339-8. doi:10.1016/0031-9384(87)90339-8. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. From dependence to sexual maturity: the behavioural ontogeny of Callitrichidae. Marmosets Tamarins Syst. Behav. Ecol. Oxf. Univ. Press Oxf. 1993:235–254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.