Abstract
Objective
Obesity is a known risk factor for various metabolic disorders and cardiovascular diseases. Recently we demonstrated that angiotensin female AT2 receptor (AT2R) knockout mice on high-fat diet (HFD) had higher body weight and adiposity with a parallel reduction in estrogen (17,β-estradiol/E2). The present study investigated whether the anti-adiposity effects of the AT2R are estrogen-dependent in female mice.
Methods
Female C57BL/6 ovary-intact (Ovi) mice were treated with an AT2R agonist (C21, 0.3 mg/kg, daily i.p.). Ovariectomized (Ovx) mice, supplemented with E2 (5 μg/day, pellets implanted subcutaneously), were treated with an AT2R agonist (C21, 0.3 mg/kg, daily i.p.) or vehicle. After 4-days of pre-treatment with C21, Ovi and Ovx mice were placed on either normal diet (ND) or HFD while the C21 treatment continued for the next 10 days. For a long-term study, Ovi mice were placed on HFD and treated with C21 for 12 weeks.
Results
Ovi mice fed the HFD had increased parametrial white adipose tissue (pWAT) weight, plasma free fatty acid and triglycerides compared to Ovi mice on ND. Ovariectomy alone caused similar changes in these parameters which were further increased by HFD-feeding. C21 treatment attenuated these HFD-induced changes in Ovi as well as Ovx mice. HFD also, increased the liver/body-weight ratio and decreased the liver expression of the β-oxidation enzyme, carnitine palmitoyltransferase 1 (CPT1-A). C21 treatment attenuated these changes as well. The long-term C21 treatment of Ovi mice lowered the HFD-induced body weight gain, increase in pWAT weight, parametrial adipocyte size and hyperinsulinemia induced by HFD. Finally, HFD drastically reduced urinary estrogen and the beneficial metabolic changes in response to C21-treatment occurred without significantly increasing urinary estrogen.
Conclusion
We suggest that the pharmacological activation of AT2R by the agonist C21 reduces adiposity and body weight gain independent of estrogen in female mice. Improvement in fatty acid metabolism is a potential mechanism by which the AT2R exerts anti-adiposity effects.
Keywords: Obesity, adiposity, AT2R, high-fat diet, estrogen
1. Introduction
Obesity is a major risk factor for various metabolic disorders and cardiovascular diseases (CVD) namely diabetes, hypertension, atherosclerosis, etc. A positive balance in energy intake vs. energy expenditure is believed to be a primary cause for the development and maintenance of obesity, defined as the storing of fat in adipocytes resulting in enlargement of adipocyte size and mass (adiposity). The incidence of extreme obesity is much higher in women as compared to men [1]. Also, the relative risk of CVD in obese females is higher than in obese males [1]. In addition to obesity as an independent risk factor for CVD, menopause provides an added risk factor for such diseases. Estrogen, the female sex hormone, regulates adiposity/obesity and after menopause the absence of estrogen triggers adiposity in women.
Storing excessive fat by adipocytes is a normal physiological phenomenon. In addition to storing, the adipose also mobilizes fatty acids (FA) to generate heat by activating mitochondrial uncoupling protein 1 (UCP1). The activated UCP1 causes shunting of the proton away from the ATP synthase [2]. In obesity, increased adipocyte size and free fatty acids (FFA) are associated with non-alcoholic liver injury progression [3]. In liver cells, fatty acids undergo β-oxidation providing metabolites for gluconeogenesis and other metabolic pathways [4]. The enzyme, carnitine palmitoyl transferase, is the rate-limiting step of mitochondrial fatty-acid β-oxidation pathway [5] and the activity of this enzyme is used as an index of liver fatty acid β-oxidation. During adiposity and obesity, these metabolic markers are dysregulated further affecting the metabolism ensuing into dyslipidemia and fatty liver.
The renin-angiotensin system (RAS) regulates blood pressure and impacts various aspects of CVD [6]. Recently a local RAS in adipose tissue has been implicated in the pathophysiology of metabolic syndromes and adiposity [7-9]. Angiotensin II (AngII), a major agonist peptide of the RAS, exerts its physiological effects via two receptor subtypes, namely the angiotensin type 1 receptor (AT1R) and the angiotensin type 2 receptor (AT2R). Evidence suggests that activation of the AT1R contributes to adiposity and weight gain in male animal models [10,11]. The role of AT2R in obesity remains unclear. Male AT2R knockout mice were protected from high fat diet (HFD)-induced weight gain [12,13], while the double AT2R along with apolipoprotein-E knockout (a model of atherosclerosis) mice exhibited greater adiposity in response to HFD compared with single apolipoprotein-E knockout mice [14]. Further, the pharmacological stimulation of the AT2R in male mice reduces adiposity and improves insulin resistance [15,16]. On the other hand, the significance of AT2R in female obesity is suggested by the studies showing that AT2R polymorphism is associated with increased body mass index in Japanese women [17]. Recently, we observed that female AT2R-KO mice fed a HFD had increased body weight gain and adiposity, accompanied by a decrease in urinary 17,β-estradiol (E2) [13]. Although levels of AT2R expression and estrogen levels are positively correlated [18,19], and estrogen is a known regulator of adiposity [19], it is unknown whether the decrease in estrogen levels in female AT2R-KO mice contributes to the increased adiposity observed. To address this issue in the present study, we utilized bilateral ovariectomized and ovary-intact mice to assess the role of estrogen in AT2R-mediated effects on adiposity/obesity. Pharmacological activation of AT2R was achieved by treating the mice with the orally active preferential AT2R agonist C21.
2. Methods
2.1. Animals and experimental protocols
Eight-week old ovary-intact (Ovi) and bilateral-ovariectomized (Ovx) C57BL/6 female mice were obtained from Harlan Inc. (Indianapolis, IN). Another batch of 4-week old C57BL/6 Ovi female mice were used for a long-term study, as outlined below. The surgery for ovariectomy was performed at Harlan, Inc. when the mice were 5 weeks old. Following 2 weeks to recovery from the surgery, the animals were shipped to the University of Houston's animal care facility. The animals were housed with free access to food and tap water and maintained under a 12-hr light/dark cycle. The experimental protocols herein were approved by the Institutional Animal Care and Use Committee of the University of Houston. These protocols were:
-
a)
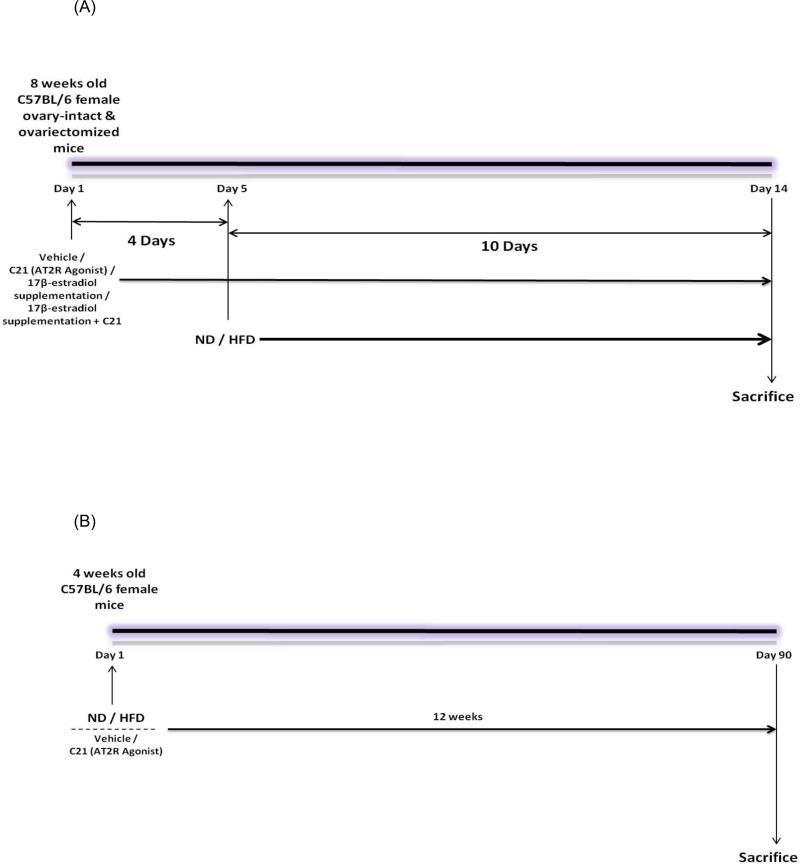
Two week protocol: Starting on day 1, 8-week old Ovi and Ovx mice were pre-treated with vehicle or the AT2R agonist ‘Compound 21’ (C21) at a dose of 0.3 mg/kg, daily i.p. for 4 days (Fig. 1A) In two additional groups of Ovx mice, supplementation with 17β-estradiol (E2) (5 μg/day) was provided by implanting E2-pellets subcutaneously. These Ovx E2 supplemented mice also were treated with C21 (0.3 mg/kg, daily i.p.) or vehicle for 4 day. On day 5 the mice were continued on a normal diet (ND) or placed on an iso-Kcal high-fat diet (HFD) while the treatments with C21 and/or E2 continued for 10 more days. Each treatment group had n=8 mice. On day 15, in a non-fasting state, animals were euthanized by cervical dislocation under isoflurane anesthesia. Plasma was collected and stored at -80°C until further use. The livers were removed, patted dry, weighed, snap-frozen and preserved at -80°C. Parametrial adipose tissue (pWAT) also was removed, patted dried and weighed. Part of this pWAT was preserved in buffered formalin for histological examination while the remaining was snap-frozen and stored at -80°C.
-
b)
Twelve week protocol: Four week old female C57BL/6 mice were placed on ND or HFD and simultaneously treated with C21, 0.3 mg/kg, daily i.p., or vehicle for 12 weeks (Fig. 1B). Each treatment group had n=9 mice. At the end of 12 weeks, the mice were sacrificed. Plasma was collected and stored at -80°C until further use. Parametrial WAT was removed, patted dry and weighed. Part of this pWAT was preserved in buffered formalin for histological examination while the remaining was snap-frozen and stored at -80°C.
Fig. 1.
(A) Treatment protocol for two weeks. (B) Treatment protocol for twelve weeks.
2.2. Food intake and body weight
Food intake and body weight of the mice were measured as reported in our previous article [13].
2.3. Histological analysis
Parametrial white adipose tissue (pWAT) samples, preserved in buffered formalin solution, were used to prepare 5μM thick paraffin-embedded sections. After staining the sections with hematoxylin and eosin (H&E), adipocyte size was measured in three microscopic fields separated by at least 100μM (in single-blinded condition) using Nikon eclipse TS 100 light microscope (10X) fitted with a Nikon (Infinity 1) camera with NIS-Elements D 3.2 software (Nikon Instruments Inc., Melville, NY). The adipocyte size was expressed in μm2.
2.4. Free fatty acid (FFA) and triglyceride (TAG) estimation
Plasma FFA and TAG levels were measured using quantification kits, as we reported earlier [13].
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) for plasma leptin and insulin
Plasma leptin and plasma insulin levels were determined using an ELISA kit following the manufacturer's instructions. The absorbance was measured on a micro-plate reader (BMG LABTECH, Inc. Cary, NC).
2.6. Urinary 17,β-estradiol (E2) extraction and Enzyme Immunoassay (EIA) for E2
Twenty four-hour urine samples were collected after placing individual mice in metabolic cages during the last 5 days of the treatment period. The five 24-hour urine samples were pooled as one sample for each mouse. E2 was extracted from the pooled samples and used to determine the urinary E2 concentration, which provided an average of E2 during the estrous cycle. The E2 extraction and measurement were performed using EIA as we reported earlier [13].
2.7. Western blotting
Proteins were extracted from adipose and hepatic tissues and resolved by 4-12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, MA). The membranes were blocked with 5% fat-free milk and incubated with appropriate primary antibodies. For the loading controls, the membranes were stripped and re-probed with an anti-β-actin antibody. For detection of protein bands, horseradish peroxidase (HRP)-conjugated anti-IgG secondary antibody and enhanced chemiluminescence substrate were used. The signals were recorded and analyzed by Quantity One Analysis Software (Bio-Rad, CA) for the densitometry of the bands.
2.8. Chemicals
The diets used in the study were a Teklad custom research diet (catalog # TD.06414) with adjusted calorie diet (60/Fat) containing 18.4% protein, 21.3% carbohydrate and 60.3% fat (referred to herein as high fat diet, HFD), and an isocaloric normal diet 7022 (29% protein, 56% carbohydrate and 15% fat) (referred to herein as normal diet, ND).. Both diets were purchased from Harlan, Indianapolis, IN and contained similar levels of mineral mix AIN-93G-MX (94046) and vitamin mix AIN93-VX (94047). Compound C21 (97% pure) was custom synthesized. The following reagents were purchased from Abcam, MA: plasma free fatty acid quantification assay kit (catalog # ab65341); leptin mouse ELISA kit (catalog # ab100718); CPT-1A antibody (catalog # ab128568); uncoupling protein-1 antibody (catalog # ab10983). The triglyceride quantification assay kit catalog # K622-100 was purchased from Biovision and ultra-sensitive mouse insulin measuring ELISA kit was purchased from Crystal Chem Inc., Downers Grove, IL. The Estradiol EIA kit (catalog # 582251) was purchased from Cayman Chemical, Ann Arbor, MI. The antibodies for AT2R were custom raised from EZ Biolab, Carmel, IN. The anti-β-actin antibody (catalog # 4970) was purchased from Cell Signaling Technology, Danvers, MA.
2.9. Statistical Analysis
The data were analyzed using GraphPad Prism 4 (GraphPad software, San Diego, CA) and subjected to one-way ANOVA with Newman-Keuls post-hoc test and unpaired Student's t test. A p value of less than 0.05 was considered statistically significant. Data are presented as mean±SEM.
3. Results
3.1. Effect of C21 on food Intake
Food intake was calculated in total kilo calories (Kcal) consumed over the treatment period. In the ‘two week protocol’ (Table 1), the total Kcal intake was significantly higher in the HFD group as compared to ND group of Ovi mice. The Kcal intake also was significantly higher in HFD-fed Ovx mice compared with Ovx on ND. Treatment with C21 did not change the Kcal intake in either the Ovi or Ovx mice fed a HFD. However, E2 supplementation alone significantly reduced the total Kcal intake in Ovx mice fed a HFD. This was not affected by C21 treatment.
Table 1.
Metabolic and hormonal parameters of ovary-intact (Ovi) and ovariectomized (Ovx) C57BL/6 mice on ND and HFD (2-week protocol)
| Ovi |
Ovx |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameters/Mice groups | ND | HFD | HFD-C21 | ND | HFD | HFD-C21 | HFD-E | HFD-E+C21 |
| Total Kcal intake, in 2 weeks | 98±5.0 | 132±5.8* | 137±4.8 | 120±7.1 | 153±5.3$ | 141±10.1 | 125±3.0@ | 123±4.9@ |
| Plasma leptin (non-fasting), ng/mL | 1.66±0.51 | 8.69±1.20* | 6.59±0.80 | 3.44±0.35 | 8.43±0.10$ | 7.44±1.21 | 1.55±0.77@ | 1.69±0.46@ |
| Change in body weight in 2 weeks, g | 3.49±0.55 | 4.22±0.51* | 3.54±0.30 | 5.35±0.33 | 9.16±0.38$ | 7.80±0.90 | 6.06±0.43@ | 5.58±0.43@ |
| pWAT weight / body weight, mg/g | 10.9±0.4 | 29.1±4.0* | 20.9±0.7# | 28.1±2.8 | 45.5±2.3$ | 28.8±4.2@ | 12.7±1.5@ | 12.0±0.6@ |
| Liver weight / body weight, mg/g | 47.3±1.6 | 62.1±4.9* | 51.0±1.6# | 56.4±3.8 | 67.8±2.6$ | 54.1±1.7@ | 36.0±2.0@ | 35.1±2.5@ |
| Plasma FFA, nmol/μL | 3.99±0.30 | 6.90±0.23* | 4.96±0.25# | 8.54±0.38 | 10.52±0.75$ | 8.01±0.38@ | 6.91±0.54@ | 6.70±0.31@ |
| Plasma TAG, nmol/μL | 1.63±0.12 | 4.37±0.8* | 2.41±0.15# | 4.98±0.16 | 8.36±0.37$ | 5.51±0.41@ | 3.12±0.19@ | 2.58±0.24@ |
| Plasma insulin, ng/mL | 0.69±0.02 | 2.34±0.16* | 0.89±0.22# | 0.82±0.04 | 2.37±0.16$ | 1.38±0.09@ | 0.58±0.20@ | 0.50±0.06@ |
| Urinary E2, pg/24 h urine | 41.04+2.4 | 12.22+1.6*,ev | 13.75+1.6ev | nd | nd | nd | 71.41+9.7 | 76.73+9.3 |
ND - Normal Diet, HFD - High-Fat Diet, HFD-C21 - High-Fat Diet on C21, HFD-E - H igh-Fat Diet on E2, HFD-E+C21 - High-Fat Diet on E2+C21
Significantly different from Ovi on ND
Significantly different from Ovi on HFD
Significantly different from Ovx on ND
Significantly different from Ovx on HFD. Data analyzed using one-way ANOVA with Newman-keuls post hoc test and Student t test (p<0.05); n = 4-9.
Extrapolated value, nd - not detectable.
In the ‘twelve week protocol’, the total Kcal intake over 12 weeks (Table 2) was significantly higher in the HFD-fed mice. Treatment with C21 had no effect on Kcal intake in either the ND or HFD group.
Table 2.
Metabolic and hormonal parameters of vehicle-treated and C21-untreated female Ovi C57BL/6 mice fed on ND and HFD (12-week protocol)
| Parameters/Mice groups | ND | HFD | ND-C21 | HFD-C21 |
|---|---|---|---|---|
| Total Kcal intake, in 12 weeks | 722±23.6 | 933±25.8* | 716±27.2 | 866±33.3 |
| Change in body weight in 12 weeks, g | 14.0±0.29 | 27.3±1.21* | 14.7±0.32 | 22.49±1.10# |
| pWAT weight / body weight, mg/g | 49.1±4.9 | 141.9±21.4* | 59.7±3.5 | 99.1±17.7# |
| Plasma Insulin (fasting), ng/mL | 0.13±0.02 | 0.69±0.05* | 0.16±0.02 | 0.37±0.06# |
| Plasma Insulin (non-fasting), ng/mL | 0.24±0.03 | 2.86±0.53* | 0.21±0.03 | 1.28±0.06# |
| Urinary E2, pg/24 h urine | 38.82±2.6 | 10.5±1.3*,ev | 40.6±2.0 | 14.6 ±0.7 #,ev |
ND - Normal Diet, HFD - High-Fat Diet, ND-C21 - Normal Diet on C21, HFD-C21 High-Fat Diet on C21
Significantly different from ND group
significantly different from HFD. Data analyzed using one-way ANOVA with Newman-keuls post hoc test and Student t test (p<0.05); n = 4-9.
Extrapolated value.
3.2. Effect of C21 on body weight gain
In the ‘two week protocol’ (Table 1), as expected, total body weight was significantly increased by placing the Ovi mice on HFD. Similarly, Ovx mice had a significant increase in body weight gain with HFD feeding. The increase in body weight gain in HFD-fed Ovx mice was not affected by C21 treatment. However, in Ovx mice supplementation with E2 reduced the body weight gain caused by HFD. The addition of C21 treatment to E2 supplementation in Ovx mice had no additional effect on body weight gain.
In the “12 weeks protocol” HFD caused a significant increase in the body weight gain of Ovi mice compared to ND Ovi mice. Treatment with C21 significantly attenuated this increase in body weight gain (Table 2).
3.3. Effect of C21 on parametrial white adipose tissue (pWAT) weight
In the ‘two week protocol’ the pWAT weight was significantly increased by HFD feeding in both the Ovi mice and Ovx mice (Table 1). C21 treatment attenuated the pWAT weight increase in both the Ovi and Ovx mice fed with HFD. E2 supplementation in Ovx mice caused a remarkable reduction in pWAT weight, which was not affected by the C21 co-treatment.
In the ‘twelve weeks protocol’, similar to the body weight gain, the pWAT weight in HFD-fed Ovi mice was significantly increased, which was attenuated by the C21 treatment (Table 2).
3.4. Effect of C21 on parametrial adipocyte size
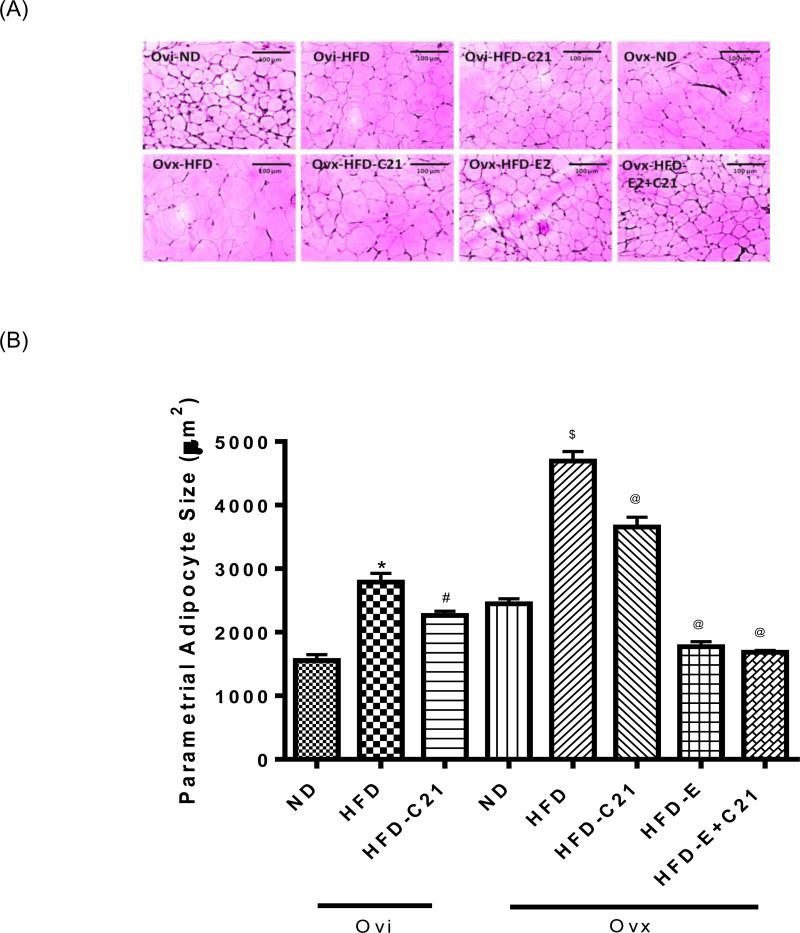
In the ‘two weeks protocol’, similar to the pWAT weight, HFD-feeding significantly increased the pWAT adipocyte size in Ovi as well as Ovx mice (Fig. 2A, 2B). This increase in the adipocyte size in both the Ovi and Ovx mice was significantly attenuated by C21 treatment. In Ovx mice, E2 supplementation reduced the HFD-induced increase in the adipocyte size. This was not affected further by the C21 co-treatment.
Fig. 2. The parametrial adipose tissue (pWAT) adipocyte size in different treatment groups of ovary-intact (Ovi) and ovariectomized (Ovx) mice fed with ND or HFD (2-week protocol).
(A) Representative micrograph of pWAT adipocyte (magnification, 10X). (B) Bar graphs represent the adipocyte size expressed as cell area (μM2). Results are means ± SEM; * -significantly different from Ovi on ND, # - significantly different from Ovi on HFD, $ - significantly different from Ovx on ND, @ - significantly different from Ovx on HFD. Data were analyzed using one-way ANOVA with Newman-keuls post hoc test and Student's t test (p<0.05); N=3 (3 sections per slide from 3 mice). (ND - Normal Diet, HFD - High-Fat Diet, HFD-C21 - High-Fat Diet on C21, HFD-E - High-Fat Diet on E2, HFD-E+C21 - High-Fat Diet on E2+C21).
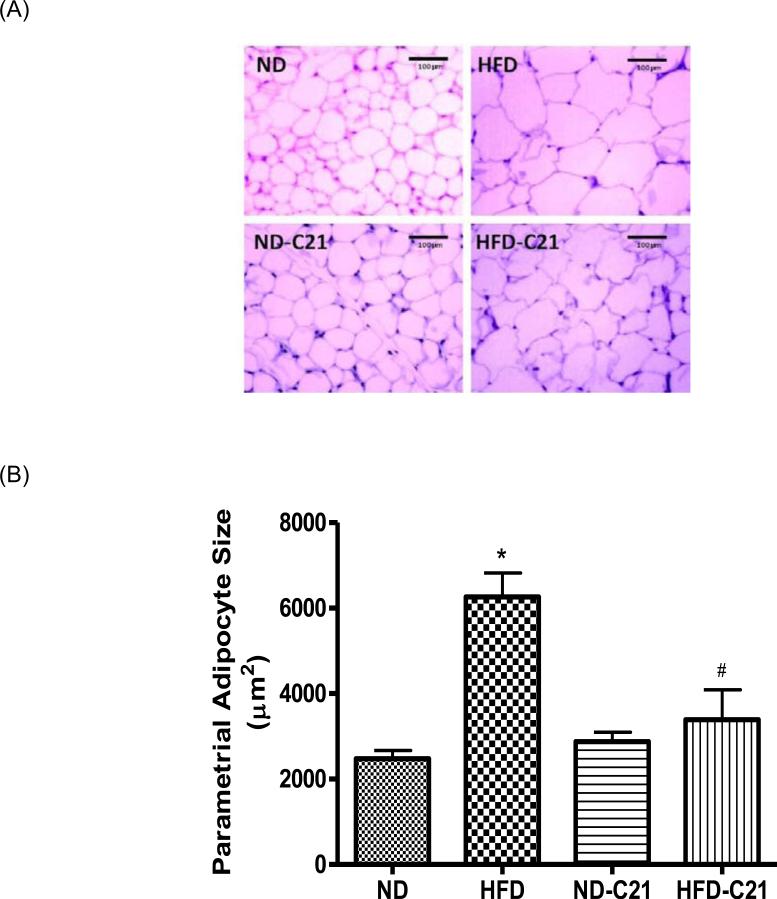
In the ‘twelve weeks protocol’ (Fig. 3A and B), HFD caused an increase in the parametrial adipocyte size in Ovi mice, which was reduced by the C21 treatment.
Fig. 3. The parametrial white adipose tissue (pWAT) adipocyte size in different treatment groups of ovary-intact mice fed with ND or HFD for 12 weeks.
(A) Representative micrograph of pWAT adipocyte (magnification, 10X). (B) Bar graphs represent the adipocyte size expressed as cell area (μM2). Results are means ± SEM; * - significantly different from ND, # - significantly different from HFD. Data were analyzed using one-way ANOVA with Newman-keuls post hoc test and Student's t test (p<0.05); N=3 (3 sections per slide from 3 mice). (ND -Normal Diet, HFD - High-Fat Diet, ND-C21 - Normal Diet on C21, HFD-C21 - High-Fat Diet on C21).
3.5. Effect of C21 on urinary 17β-estradiol (E2)
In the ‘two weeks protocol’ (Table 1), Ovi mice fed a HFD excreted significantly lower amounts of urinary E2. This was not influenced by C21 treatment. In Ovx-ND and Ovx-HFD mice the urinary levels of E2 were not in the detectable range, except in the groups supplemented with E2 pellets.
In the ‘twelve weeks protocol’ (Table 2), the urinary E2 levels in Ovi mice fed a HFD were markedly reduced compare to mice fed a ND. C21 treatment significantly increased urinary E2 levels in the HFD group.
3.6. Effect of C21 on plasma leptin
Plasma leptin levels were measured in the ‘two weeks protocol’ to assess the influence of C21 treatment on appetite (Table 1). Feeding mice the HFD caused a significant increase in the plasma leptin levels in both the Ovi and Ovx mice compared to mice fed the ND. The C21 treatment did not affect the plasma leptin levels in either Ovi or Ovx mice placed on HFD. On the other hand, E2 supplementation in Ovx mice fed a HFD caused a significant reduction in the plasma leptin levels (Table 1), which was not further influenced by the C21 co-administration.
3.7. Effect of C21 on plasma free fatty acid (FFA) and triglyceride (TAG)
Feeding the mice a HFD caused a significant increase in the plasma levels of FFA and TAG in Ovi as well as Ovx mice, compared to mice fed a ND. (Table 1). The increases in FFA and TAG in Ovi and Ovx mice receiving the HFD were attenuated by C21 treatment. In Ovx mice receiving E2 supplementation the HFD did not increase in the plasma FFA and TAG levels, and C21 co-administration had no effect on plasma FFA or TAG levels.
3.8. Effect of C21 on plasma insulin
In the “two week protocol”, feeding Ovi and Ovx mice a HFD caused a significant increase in the plasma insulin levels compared to mice fed a ND (Table 1). This increase in insulin was attenuated by C21 treatment in both the Ovi and Ovx mice. The increase in the plasma insulin levels caused by the HFD was attenuated by C21 treatment in both the Ovi and Ovx mice (Table 1). In Ovx mice fed a HFD, E2 supplementation prevented the increase in the plasma insulin levels and C21 co-administration had no effect on plasma insulin levels in Ovx mice receiving E2.
Similarly to the “2 week protocol”, Ovi mice fed a HFD for 12-weeks also exhibited enhanced plasma insulin levels, regardless of whether insulin was measured during the fasting or non- fasting state. Treatment with C21 reduced plasma insulin levels irrespective of the fasting state in HFD fed mice (Table 2).
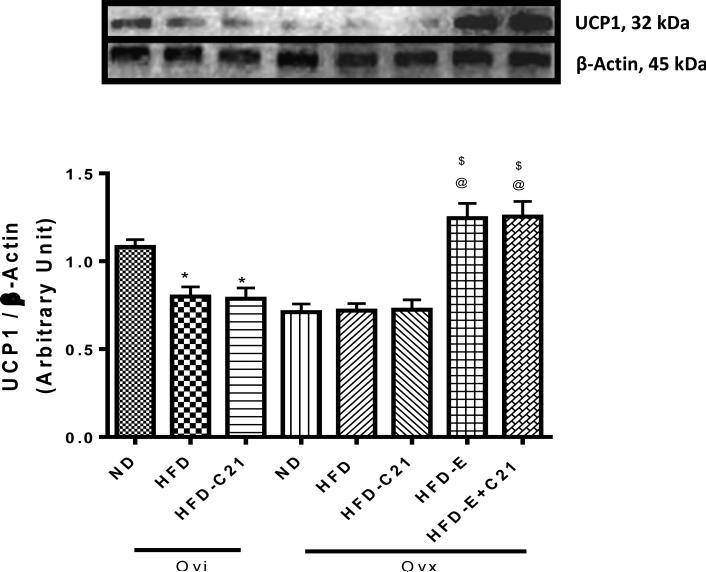
3.9. Effect of C21 on uncoupling protein-1 (UCP1) expression in pWAT
The expression levels of the UCP1 (32 KDa) protein in the pWAT were determined by Western blotting. The data suggest that HFD-feeding in Ovi and Ovx mice decreased UCP1 expression compared to levels in comparable mice fed a ND (Fig. 4). The C21-treatment did not affect the UCP1 expression in either the Ovi or Ovx mice fed a HFD. However, E2 supplementation in Ovx HFD-fed mice caused a significant increase in the UCP-1 expression. Treatment with C21 in these mice had no additional effect.
Fig. 4. Protein expression of uncoupling protein-1 (UCP1) in the pWAT of ovary-intact (Ovi) and ovariectomized (Ovx) mice fed with ND or HFD (2-week protocol).
Upper panel: Representative western blots for UCP1 with loading control β-actin. Bar graphs represent the ratios of densities of UCP1 and β-Actin protein bands i.e. UCP1/β-Actin. Results are means ± SEM; * - significantly different from Ovi on ND, $ - significantly different from Ovx on ND, @ -significantly different from Ovx on HFD. Data were analyzed using one-way ANOVA with Newman-keuls post hoc test and Student's t test (p<0.05); N=3 in each group. (ND - Normal Diet, HFD - High-Fat Diet, HFD-C21 - High-Fat Diet on C21, HFD-E - High-Fat Diet on E2, HFDE+C21 - High-Fat Diet on E2+C21).
3.10. Effect of C21 on liver weight and CPT1-A protein expression
As evidenced by the liver weight-to-body weight ratio, liver weight was significantly increased in the Ovi and Ovx mice fed a HFD compared to their respective ND-fed controls (Table 1). C21 treatment attenuated the increase in liver weight in both the Ovi and Ovx mice fed a HFD. E2 supplementation in Ovx mice fed a HFD prevented any increase in the liver weight. C21 co-administration to these mice had no additional effect on liver weight.
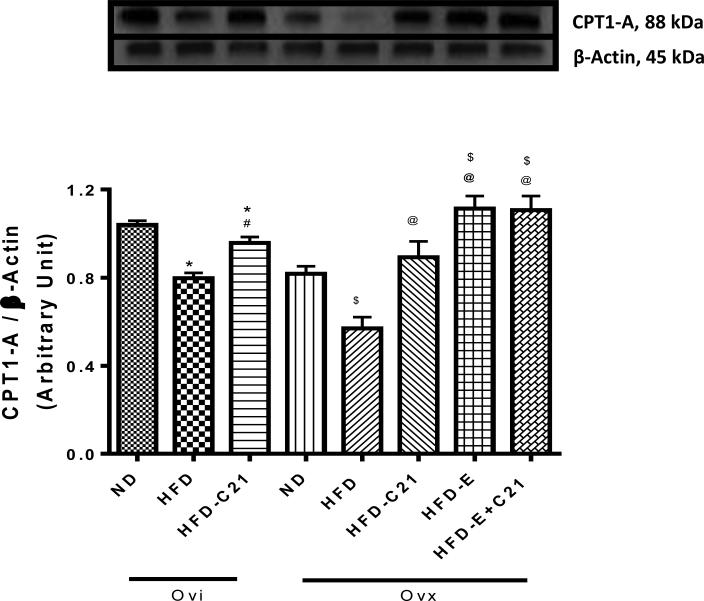
In addition to liver weight, the levels of CPT1-A (88 KDa) protein expression were quantified in liver samples by western blotting (Fig. 5). The data suggest that HFD feeding reduced the expression of CPT1-A in both Ovi and Ovx mice compared to their respective ND-fed controls. Treatment with C21 prevented the reduction in CPT1-A expression in both the Ovi and Ovx mice fed a HFD. E2 supplementation in Ovx mice fed a HFD caused a significant increase in the CPT1-A expression, which was not further affected by the C21 co-treatment.
Fig. 5. Protein expressions of CPT1-A in the liver of ovary-intact (Ovi) and ovariectomized (Ovx) mice fed with ND or HFD (2-week protocol).
Upper panel: Representative western blots for CPT1-A with loading control β-actin. Bar graphs represent the ratios of densities of CPT1-A and β-Actin protein bands i.e. CPT1-A /β-Actin. Results are means ± SEM; * - significantly different from Ovi on ND, # - significantly different from Ovi on HFD, $ - significantly different from Ovx on ND, @ - significantly different from Ovx on HFD. Data were analyzed using one-way ANOVA with Newman-keuls post hoc test and Student's t test (p<0.05); N=3-4 in each group. (ND - Normal Diet, HFD - High-Fat Diet, HFD-C21 - High-Fat Diet on C21, HFD-E - High-Fat Diet on E2, HFD-E+C21 - High-Fat Diet on E2+C21).
3.11. Effect of C21 on pWAT AT2R protein expression
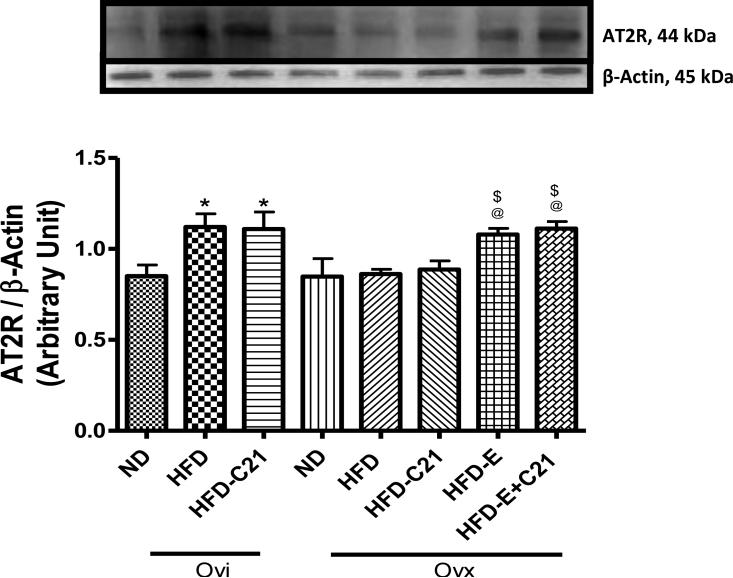
Expression of the AT2R was detected by western blotting in pWAT as indicated by a distinct band at approximately 44 KDa (Fig. 6). The data suggest that HFD feeding in Ovi mice caused an increase in the AT2R (32%) expression, but had no effect in Ovx mice. Treatment with C21 had no effect on the expression of the AT2R in either Ovi or Ovx mice. However, E2 supplementation in Ovx mice fed a HFD caused an increase in the AT2R expression.
Fig. 6. Protein expression of angiotensin II type 2 receptor (AT2R) in the pWAT of ovary-intact (Ovi) and ovariectomized (Ovx) mice fed with ND or HFD (2-week protocol).
Upper panel: Representative western blots for AT2R with loading control β-actin. Bar graphs represent the ratios of densities of AT2R and β-actin protein bands. Results are means ± SEM; * -significantly different from Ovi on ND, $ - significantly different from Ovx on ND, @ - significantly different from Ovx on HFD. Data were analyzed using one-way ANOVA with Newman-keuls post hoc test and Student's t test (p<0.05); N=3-5 in each group. (ND - Normal Diet, HFD -High-Fat Diet, HFD-C21 - High-Fat Diet on C21, HFD-E - High-Fat Diet on E2, HFD-E+C21 -High-Fat Diet on E2+C21).
4. Discussion
Recently we reported that AT2R knock-out female mice fed a HFD exhibited increased body weight, increased gonadal or parametrial WAT (pWAT), and reduced urinary estrogen [13] compared to WT female mice fed a HFD. This study indicated a protective role of the AT2R on adiposity. The present study was undertaken to further investigate the effects of AT2R activation on obesity and the role of estrogen by pharmacologically activating the AT2R in female mice with and without ovaries, the major organs producing estrogen. Our results suggest that the anti-adiposity effects of AT2R activation are likely independent of the levels of estrogen. Specifically, we observed that in ovary-intact (Ovi) female mice fed a HFD, treatment with the AT2R agonist C21 caused a reduction in adiposity (as measured by pWAT weight and parametrial adipocyte size) and that a similar effect was observed in ovariectomized (Ovx) mice fed a HFD.
The results of the present study support our conclusion that the anti-obesity and anti-adiposity effects of AT2R activation are independent of estrogen levels. First, we observed that short-term treatment with AT2R agonist had no significant effects on estrogen levels in Ovi mice fed a HFD. Nevertheless, C21 treatment reduced both body weight gain and increased adiposity in these mice. Second, we observed that C21 was able to reduce body weight gain and increased adiposity in Ovx mice fed a HFD. A potential concern in interpreting this data is the fact that the expression AT2R was increased by HFD feeding in Ovi mice and in Ovx mice supplemented with E2 only. Therefore, it could be argued that the increase in expression of the AT2R under HFD conditions contributed to the effects of C21 on weight gain and increased adiposity. However, in Ovx fed with HFD, C21 treatment was able to exert both the anti-obesity and anti-adiposity effects in the absence of an increase in the expression of the AT2R during HFD feeding. Moreover, independent anti-obesity effects of estrogen and AT2R activation seem to occur via different mechanisms. Specifically, we observed that ovariectomized mice had increased Kcal intake associated with increased levels of plasma leptin; both the parameters were decreased by E2 supplementation. Consistent to our observations, ovariectomy has been shown to induce hyperphagia and body weight gain while exogenous administration of estrogen (E2) prevents this phenomenon [20]. In summary, our results strongly suggest that C21, via AT2R activation, can exert potent anti-obesity and anti-adiposity effects independent of effects on either estrogen levels or modification of the action of estrogen.
Another potential mechanism whereby there could be an overlap between the effects of estrogen and the effects of AT2R activation involves the reported interaction between E2 and leptin [21]. High-fat diet has been reported to reduce E2 levels [22] and the deficiency of E2 then causes leptin resistance [21] which is a known aftermath of obesity [23]. In keeping with these observations, low levels of E2 could lead to the accumulation of fat mass and the development of obesity and hyperlipidemia [22, 24]. Further, E2 supplementation in Ovx mice reduces adipocyte size [25]. However, unlike E2 supplementation, we observed that AT2R agonist C21 treatment prevented HFD-induced adiposity without impacting the Kcal intake and plasma leptin levels in Ovi as well as in Ovx mice. These findings suggest that C21 does not impact adiposity via the leptin-hyperphagia axis. This provides additional evidence separating the anti-adiposity action of AT2R from those of estrogen and suggests that AT2R activation and E2 supplementation exert anti-adiposity effects via different mechanisms. One argument that could be made against this hypothesis is that an additive effect of C21 and E2 might have been expected when these agents were simultaneously administered to Ovx mice fed a HFD. However, C21 treatment caused no further changes beyond the changes that E2 alone produced in our study. A likely explanation for the lack of additivity may be that the E2 dose used in our study was sufficiently high to cause the maximal changes in adiposity and metabolic indices such as plasma insulin levels, liver CPT1-A expression, and fatty-liver. This issue might be clarified by future studies with E2 supplementation at levels which only restore E2 levels to those observed in Ovi female mice.
Another potential mechanism by which AT2R activation might interact with the hyperphagia-leptin axis to produce its anti-obesity and anti-adiposity effects is by modifying the action of leptin on insulin and insulin resistance. Although leptin levels fluctuate over a 24 hour cycle [26], chronic elevation of leptin levels during adiposity is known to be associated with hyperinsulinemia [27]. The hyperinsulinemia is also an indicator of insulin resistance [27] and we observed hyperinsulinemia under increased adiposity in this study. In the present study, higher Kcal intake in the form of HFD could be considered a factor which would drive both hyperleptinemia and hyperinsulinemia, culminating in impaired metabolism. Since estrogen is linked to the hyperphagia-leptin axis, E2 supplementation led to reduced hyperinsulinemia and improved fatty acid metabolism (as induced by a reduction in plasma FFA and TAG) in Ovx mice. However, C21 treatment did not affect hyperphagia-leptin axis, but improved the indices of adiposity and metabolism in both the Ovi and Ovx mice fed on HFD. Clearly, activation of the AT2R by C21 seems to separate leptin from the insulin-fatty acid metabolism axis. The improvement in hyperinsulinemia and lipid profile by C21 is consistent with recent reports, including ours in the AT2R knockout mice [13, 15-16]. Pharmacological activation of AT2R with C21 improves insulin sensitivity in male mice [15, 16]. Moreover, we previously reported that reduced levels of AT2R expression in HFD-fed female mice led to hyperinsulinemia, increases in plasma FFA and hepatic TAG without affecting Kcal intake [13]. In summary, these observations clearly indicate that the pharmacological activation of the AT2R reduces adiposity and improves insulin sensitivity without impacting the leptin-hyperphagia axis.
The molecular mechanism(s) of AT2R-mediated reduction in adiposity and improvement in metabolic disorder are not clear. However, some of the data collected in this study indicate enhanced fatty acid (FA) oxidation or β-oxidation and energy utilization in the mice treated with the AT2R agonist. We observed an increase in CPT1A protein expression in the liver of HFD-fed Ovi as well as Ovx mice treated with C21. CPT1 is a mitochondrial enzyme, which is critical for the regulation of mitochondrial FA β-oxidation [28, 29]. The changes in CPT1 expression/activity correlates with the rate of FA β-oxidation in liver and other fatty acid utilizing tissues [30-32]. Decreased oxidation of fatty acids to generate energy is associated with adiposity and obesity [33] while increased β-oxidation has been shown to prevent adiposity and obesity [34]. Therefore, the increased levels of CPT1A support an increase in FA oxidation or β-oxidation after AT2R agonist treatment. Likewise, we observed a marked reduction in the liver to body weight ratio in HFD-fed Ovi as well as Ovx mice treated with C21. This finding is congruous with an earlier report where treatment with CGP42112A (another AT2R agonist) caused a reduction in liver to body weight ratio in a mouse model of colorectal cancer liver metastases [35]. Collectively, these findings suggest that AT2R activation by treatment with C21 reduces liver fat accumulation caused by HFD via increased β-oxidation in liver, as indicated by the increased liver CPT1A expression, contributing to a reduction in adiposity and hyperinsulinemia.
An increase in the heat-generating capacity of WAT is another mechanism by which treatment with the AT2R agonist C21 could reduce adiposity without affecting Kcal intake. In order to address this possibility, in pWAT we measured a well-established index of heat-generating capacity in adipose tissue, namely UCP-1. When expression of UCP-1 is increased it promotes proton leakage across the inner membrane of the mitochondria, reducing oxidative efficiency and this results in increased hear generation. UCP-1 expression decreases in adipose tissue during estrogen deficiency and UCP-1 expression is restored/increased by estrogen supplementation [36-38]. Consistent with these reports, we observed that Ovx caused a reduction in pWAT UCP-1 expression, which was increased by estrogen treatment. Treatment with C21 caused no changes in the expression of UCP1 protein in Ovi or Ovx mice. Moreover, a recent report indicates that stimulation of AT2R in adipose tissue suppresses UCP-1 expression [39]. Therefore, we suggest that AT2R activation by treatment with C21 may not promote the use of the fatty acids for heat generation. However, we have not assessed the body temperature of the mice in our studies.
Finally, an important objective of the present study was to address the relevance of short term (2 week) reductions in adiposity and weight gain to the potential long-term beneficial effects of AT2R activation by C21 administration. Since 2 week feeding of mice with a HFD does not cause significant changes in body weight gain, we fed Ovi mice with a HFD for 12 weeks (long-term) and assessed the effect of C21 treatment. Long-term activation of AT2R by C21 treatment attenuates the body weight gain caused by feeding with HFD by ~25%, reduces pWAT weight, and reduces adipocyte size. Reducing body weight by 20-30% is therapeutically significant [40, 41]. In Ovi mice fed a ND, 12 week treatment with C21 does not affect urinary E2 levels. However, in Ovi mice fed a HFD, urinary E2 is markedly reduced. Combining HFD with treatment with C21 causes a modest but significant increase in urinary E2 levels, compared to feeding with a HFD alone. However, it's unlikely that such a modest increase in E2 might have contributed to the anti-obesity/anti-adiposity effects of AT2R agonist treatment.
Overall, this study provides clear evidence that AT2R activation with preferential agonist C21 reduces adiposity and obesity independent of the estrogen status, in the short-term as well as in the long-term. The improvement in adiposity/obesity is further substantiated by the changes in lipidemia and plasma insulin. As mentioned above, however, this study fell short in providing evidence whether the beneficial effects of AT2R agonist and estrogen could be additive. Lower doses of estrogen with and without AT2 agonist treatment may provide an answer to this question of metabolic additivity, if any, between AT2R and estrogen. Moreover, inasmuch as this study clearly provides evidence suggesting a direct anti-adiposity role for AT2R activation, the underlying molecular mechanisms that may potentially link AT2R activation with these metabolic changes are yet to be identified.
In conclusion, pharmacological activation of the AT2R by a novel agonist C21 attenuates the increase in adiposity and the long-term body weight gain caused by HFD feeding in female mice. This effect is independent of estrogen. Moreover, the anti-adiposity effect of AT2R agonist treatment is accompanied by improvement in fatty acid metabolism and reduction in hyperinsulinemia, suggesting a reduction of insulin resistance. These findings support a translational significance of AT2R as a potential therapeutic target to control obesity and improve associated metabolic disorders in females irrespective of their estrogen hormone status.
Acknowledgement
The authors would like to thank Dr. Douglas Eikenburg for carefully reading the manuscript and his editorial comments.
Funding
This study was supported by National Institutes of Health grant R0I DK-61578 and institutional funds.
Abbreviations
- AT1R
Angiotensin Type 1 Receptor
- AT2R
Angiotensin Type 2 Receptor
- AngII
Angiotensin II
- CPT1A
Carnitine Palmitoyltransferase-1A
- CVD
Cardiovascular Diseases
- C21
Compound 21
- E2
17,β-estradiol or estrogen
- FA
Fatty Acid
- FFA
Free Fatty Acid
- HFD
High-Fat Diet
- i.p.
Intraperitoneal
- Kcal
Kilo Calorie
- KO
Knock Out
- ND
Normal Diet
- Ovi
Ovary-intact
- Ovx
Ovariectomized
- pWAT
Parametrial White Adipose Tissue
- RAS
Renin Angiotensin System
- TAG
Tri-acyl Glycerol or Triglyceride
- UCP1
Uncoupling Protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Authors have no conflict of interest to disclose.
Author Contributions
Conceived and designed the experiments: SN, TH. Performed the experiments and data collection: SN, MAK, PS, QA. Analysis and interpretation of the data: SN, MAK, TH. Contributed reagents/materials/analysis tools: TH. Wrote the manuscript: SN, TH.
REFERENCES
- 1.Pan L, Freedman DS, Gillespie C, et al. Incidences of obesity and extreme obesity among US adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Popul Health Metr. 2011;9:56. doi: 10.1186/1478-7954-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richard D, Picard F. Brown fat biology and thermogenesis. Front Biosci (Landmark Ed) 2011;16:1233–60. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 3.Wree A, Schlattjan M, Bechmann LP, et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism. 2014;63(12):1542–52. doi: 10.1016/j.metabol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Chow JC, Jesse BW. Interactions between gluconeogenesis and fatty acid oxidation in isolated sheep hepatocytes. J Dairy Sci. 1992;75(8):2142–8. doi: 10.3168/jds.S0022-0302(92)77974-0. [DOI] [PubMed] [Google Scholar]
- 5.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–43. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–19. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 7.Schling P, Mallow H, Trindl A, et al. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Int J Obes Relat Metab Disord. 1999;23:336–41. doi: 10.1038/sj.ijo.0800821. [DOI] [PubMed] [Google Scholar]
- 8.Putnam K, Shoemaker R, Yiannikouris F, et al. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–30. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney int. 2011;79:162–8. doi: 10.1038/ki.2010.391. [DOI] [PubMed] [Google Scholar]
- 10.Araki K, Masaki T, Katsuragi I, et al. Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice. Hypertension. 2006;48:51–7. doi: 10.1161/01.HYP.0000225402.69580.1d. [DOI] [PubMed] [Google Scholar]
- 11.Kouyama R, Suganami T, Nishida J, et al. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinol. 2005;146:3481–9. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- 12.Yvan-Charvet L, Even P, Bloch-Faure M, et al. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–9. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 13.Samuel P, Khan MA, Nag S, et al. Angiotensin AT(2) receptor contributes towards gender bias in weight gain. PloS One. 2013;8:e48425. doi: 10.1371/journal.pone.0048425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwai M, Tomono Y, Inaba S, Kanno H, Senba I, Mogi M, et al. AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am J Hypertens. 2009;22:784–91. doi: 10.1038/ajh.2009.85. [DOI] [PubMed] [Google Scholar]
- 15.Ohshima K, Mogi M, Jing F, et al. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARgamma activation. PloS One. 2012;7:e48387. doi: 10.1371/journal.pone.0048387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shum M, Pinard S, Guimond MO, et al. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high-fat/high-fructose diet-induced insulin resistance in rats. Am J Physiol Endocrinol Metab. 2013;304:E197–210. doi: 10.1152/ajpendo.00149.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotani K, Sakane N, Saiga K, et al. The angiotensin II type 2 receptor gene polymorphism and body mass index in healthy Japanese women. Ann Clin Biochem. 2007 Jan;44(Pt 1):83–5. doi: 10.1258/000456307779595959. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura Y, Karube M, Aoki H, et al. Angiotensin II induces ovulation and oocyte maturation in rabbit ovaries via the AT2 receptor subtype. Endocrinol. 1996;137:1204–11. doi: 10.1210/endo.137.4.8625890. [DOI] [PubMed] [Google Scholar]
- 19.Armando I, Jezova M, Juorio AV, et al. Estrogen upregulates renal angiotensin II AT(2) receptors. Am J Physiol Renal Physiol. 2002;283(5):F934–43. doi: 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- 20.Wade GN, Zucker I. Development of hormonal control over food intake and body weight in female rats. J Comp Physiol Psychol. 1970;70:213–20. doi: 10.1037/h0028713. [DOI] [PubMed] [Google Scholar]
- 21.Ainslie DA, Morris MJ, Wittert G, et al. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–8. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 22.Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295:E904–12. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riant E, Waget A, Cogo H, et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinol. 2009;150:2109–17. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 25.D'Eon TM, Souza SC, Aronovitz M, et al. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–91. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 26.Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 27.Ceddia RB, Koistinen HA, Zierath JR, et al. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J. 2002;16:1163–76. doi: 10.1096/fj.02-0158rev. [DOI] [PubMed] [Google Scholar]
- 28.Fiamoncini J, Turner N, Hirabara SM, et al. Enhanced peroxisomal beta-oxidation is associated with prevention of obesity and glucose intolerance by fish oil-enriched diets. Obesity (Silver Spring) 2013;21:1200–7. doi: 10.1002/oby.20132. [DOI] [PubMed] [Google Scholar]
- 29.Gyamfi D, Everitt HE, Tewfik I, et al. Hepatic mitochondrial dysfunction induced by fatty acids and ethanol. Free Radic Biol Med. 2012;53:2131–45. doi: 10.1016/j.freeradbiomed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Stephens FB, Wall BT, Marimuthu K, et al. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J Physiol. 2013;591:4655–66. doi: 10.1113/jphysiol.2013.255364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010;11:380–8. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 32.Bruce CR, Hoy AJ, Turner N, et al. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–8. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempf AM, Strother ML, Li C, et al. Leptin as a marker of body fat and hyperinsulinemia in college students. J Am Coll Health. 2006;55:175–80. doi: 10.3200/JACH.55.3.175-180. [DOI] [PubMed] [Google Scholar]
- 34.Koves TR, Noland RC, Bates AL, et al. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–82. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ager EI, Chong WW, Wen SW, et al. Targeting the antiogensin II type 2 receptor (AT2R) in colorectal liver metastases. Cancer Cell Int. 2010;10:19. doi: 10.1186/1475-2867-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 37.Lizcano F, Guzman G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed Res Int. 2014;2014:757461. doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grobe JL, Park S, Littlejohn NK, et al. HBPR, American Heart Association's Meeting. Hypertension; New Orleans, LA: 2013. Angiotensin suppresses thermogenic capacity through adipose AT2 receptors. [Google Scholar]
- 40.Bloom SR, Kuhajda FP, Laher I, et al. The obesity epidemic: pharmacological challenges. Mol Interv. 2008;8:82–98. doi: 10.1124/mi.8.2.6. [DOI] [PubMed] [Google Scholar]
- 41.Jansson SP, Engfeldt P, Magnuson A, et al. Interventions for lifestyle changes to promote weight reduction, a randomized controlled trial in primary health care. BMC Res Notes. 2013;6:213. doi: 10.1186/1756-0500-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]