Abstract
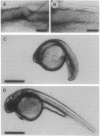
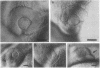
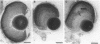
In the embryonic zebrafish retina, as in other vertebrates, retinoic acid is synthesized from retinaldehyde by two different dehydrogenases, one localized dorsally, the other primarily ventrally. Early in eye development only the ventral enzyme is present. Citral competitively inhibits the ventral enzyme in vitro and decreases the production of retinoic acid in the ventral retina in vivo. Treatment of neurula-stage zebrafish embryos with citral during the formation of the eye primordia results in eyes lacking a ventral retina. This defect can be partially rescued by retinoic acid. The results demonstrate that synthesis of retinoic acid can be selectively inhibited in vivo and suggest that retinoic acid is necessary for the proper development of the ventral retina.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connor M. J. Oxidation of retinol to retinoic acid as a requirement for biological activity in mouse epidermis. Cancer Res. 1988 Dec 15;48(24 Pt 1):7038–7040. [PubMed] [Google Scholar]
- Connor M. J., Smit M. H. Terminal-group oxidation of retinol by mouse epidermis. Inhibition in vitro and in vivo. Biochem J. 1987 Jun 1;244(2):489–492. doi: 10.1042/bj2440489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M., Blum A. S., Mendez-Otero R., Barnstable C. J. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature. 1986 Dec 4;324(6096):459–462. doi: 10.1038/324459a0. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Epstein D. J., St-Jacques B., Shen L., Mohler J., McMahon J. A., McMahon A. P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993 Dec 31;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Holder N., Hill J. Retinoic acid modifies development of the midbrain-hindbrain border and affects cranial ganglion formation in zebrafish embryos. Development. 1991 Dec;113(4):1159–1170. doi: 10.1242/dev.113.4.1159. [DOI] [PubMed] [Google Scholar]
- Hyatt G. A., Schmitt E. A., Marsh-Armstrong N. R., Dowling J. E. Retinoic acid-induced duplication of the zebrafish retina. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8293–8297. doi: 10.1073/pnas.89.17.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Tickle C., Dollé P., Wolpert L., Duboule D. Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. Nature. 1991 Apr 18;350(6319):585–589. doi: 10.1038/350585a0. [DOI] [PubMed] [Google Scholar]
- Krauss S., Concordet J. P., Ingham P. W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993 Dec 31;75(7):1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- McCaffery P., Lee M. O., Wagner M. A., Sladek N. E., Dräger U. C. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development. 1992 Jun;115(2):371–382. doi: 10.1242/dev.115.2.371. [DOI] [PubMed] [Google Scholar]
- McCaffery P., Neve R. L., Dräger U. C. A dorso-ventral asymmetry in the embryonic retina defined by protein conformation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8570–8574. doi: 10.1073/pnas.87.21.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrery P., Posch K. C., Napoli J. L., Gudas L., Dräger U. C. Changing patterns of the retinoic acid system in the developing retina. Dev Biol. 1993 Aug;158(2):390–399. doi: 10.1006/dbio.1993.1197. [DOI] [PubMed] [Google Scholar]
- McGinnis N., Kuziora M. A., McGinnis W. Human Hox-4.2 and Drosophila deformed encode similar regulatory specificities in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):969–976. doi: 10.1016/0092-8674(90)90500-e. [DOI] [PubMed] [Google Scholar]
- Morgan B. A., Izpisúa-Belmonte J. C., Duboule D., Tabin C. J. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992 Jul 16;358(6383):236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- Nohno T., Noji S., Koyama E., Ohyama K., Myokai F., Kuroiwa A., Saito T., Taniguchi S. Involvement of the Chox-4 chicken homeobox genes in determination of anteroposterior axial polarity during limb development. Cell. 1991 Mar 22;64(6):1197–1205. doi: 10.1016/0092-8674(91)90274-3. [DOI] [PubMed] [Google Scholar]
- Oro A. E., McKeown M., Evans R. M. The Drosophila retinoid X receptor homolog ultraspiracle functions in both female reproduction and eye morphogenesis. Development. 1992 Jun;115(2):449–462. doi: 10.1242/dev.115.2.449. [DOI] [PubMed] [Google Scholar]
- Raymond P. A. Movement of retinal terminals in goldfish optic tectum predicted by analysis of neuronal proliferation. J Neurosci. 1986 Sep;6(9):2479–2488. doi: 10.1523/JNEUROSCI.06-09-02479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E., Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993 Dec 31;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. Retinoic acid modifies mesodermal patterning in early Xenopus embryos. Genes Dev. 1991 Feb;5(2):175–187. doi: 10.1101/gad.5.2.175. [DOI] [PubMed] [Google Scholar]
- Schuh T. J., Hall B. L., Kraft J. C., Privalsky M. L., Kimelman D. v-erbA and citral reduce the teratogenic effects of all-trans retinoic acid and retinol, respectively, in Xenopus embryogenesis. Development. 1993 Nov;119(3):785–798. doi: 10.1242/dev.119.3.785. [DOI] [PubMed] [Google Scholar]
- Sharma S. C., Hollyfield J. G. Specification of retinotectal connexions during development of the toad Xenopus laevis. J Embryol Exp Morphol. 1980 Feb;55:77–92. [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990 Jun;4(6):932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982 Apr 8;296(5857):564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Trisler D. Cell recognition and pattern formation in the developing nervous system. J Exp Biol. 1990 Oct;153:11–27. doi: 10.1242/jeb.153.1.11. [DOI] [PubMed] [Google Scholar]
- Wagner M., Han B., Jessell T. M. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992 Sep;116(1):55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]