Abstract
Objective(s):
Mesenchymal stem cells (MSCs) derived from Wharton’s jelly (WJ-MSCs) are now much more appealing for cell-based infertility therapy. Hence, WJ-MSCs differentiation toward germ layer cells for cell therapy purposes is currently under intensive study.
Materials and Methods:
MSCs were isolated from human Wharton’s jelly and treated with BMP4, retinoic acid (RA) or co-cultured on human amniotic epithelial (HAE) and chorionic plate (HCP) placenta feeder cells. profile of POU5F1, Fragilis, Plzf, DDX4, Piwil2, Stra8, Dazl, β1- and α6-integrins (ITΒ1, ITA6) genes expression as germ cell markers were analyzed using RT-PCR and real-time PCR. Immunocytochemistry of surface markers were conducted.
Results:
After 3 weeks treatment with different reagents and co-culture system, morphology of WJ-MSCs changed to shiny clusters and germ cell specific markers in mRNA were up-regulated in both placental feeder + RA and BMP4 + RA. Induction of hWJ-MSCs with BMP4 in presence of RA resulted in significant up-regulation (P≤0.05) of all germ cell specific genes (c-Kit; 2.84±0.59, DDX4; 1.69±0.39, Piwil2; 1.14±0.21, Dazl; 0.65±0.25, α6 integrin; 1.26±0.53, β1 integrins; 1.18±0.65) compared to control and placental feeder cells + RA. Our results indicated that HAE and HCP followed by RA treatment were involved in human germ cell development.
Conclusion:
We demonstrated that under the right conditions, hWJ-MSCs have the ability to differentiate to germ cells and this provides an excellent pattern to study infertility cause and treatment.
Keywords: Mesenchymalstem cell, PGC, Placenta, Spermatogonial stem cell, Wharton’s jelly
Introduction
Efficient derivation of germ cells from different sources of stem cells in vitro, has been challenging in the treatment of male infertility (1-3). Researchers have attempted to produce germ cells in vitro by inducing different sources of stem cells, mostly iPSCs and embryonic stem cells, for germ cell transplantation (4, 5). Wharton’s jelly (WJ), has recently become the preferred source of stem cells, including mesenchymal stem cells (MSCs) (6), due to their rapid availability with a massive donor source, non-invasive collection with no risk or discomfort for the donor, no ethical restrictions, high in vitro proliferation rates and immunomodulatory effects for allogeneic cell transplantation (7). WJ-MSCs possess multipotent properties between embryonic and adult stem cells differentiating into adipogenic, osteogenic and chondrogenic progeny (8), however their relatively higher CFU-F and proliferative potential, higher telomerase activity, shorter population doubling times, and longer times to senescence, without loss of stem cell potency represent their more primitive stage than those derived from adult tissues (9).
The ability to differentiate WJ-MSCs selectively depends in part on secreted growth and differentiation factors that mimic the environment of a particular cell lineage. Bone Morphogenetic Protein 4 (BMP4) and retinoic acid (RA) play the most important role in this pathway (10). In vitro Bmp4 treatment enables bone marrow derived pluripotent stem cells to become primordial germ cells (PGCs) (11). In mice, PGCs differentiate at 7.25-E7.5, and are marked by expression of a germ cell specific gene called stella (Dppa3) (12). Also, Researchers have found that fetal male germ cells can respond to the presence of exogenously added RA in their medium to alter their sex-specific pathway (13).
One of the strategies for manipulating stem cell differentiation is using feeder cell layers which are utilized in co-culture to mimic the effects of gonadal somatic cells and control PGC’s differentiation from meiosis in the females to mitotic arrest in males (14). Co-culturing is assumed to be the most effective and also a safe strategy to prepare stem cells for clinical trials. Mitomycin-C-deactivated placental cells (as an alternative to irradiation to inhibit the feeder layer growth) are the perfect choice for feeder layer adapted from available aborted fetal tissues (15, 16). Human placenta feeder layers are considered a step forward strategy in clinical trials compared to the most common mouse embryonic fibroblast (MEF) feeders, excluding the risk of zoonosis from animal feeders (17). In this study, we examine germ-like cell differentiation potential of hWJ-MSCs co-cultured with placental cells in comparison with BMP4 or RA treatment. Our findings can improve germ cell differentiation from stem cells and make a new approach to male infertility treatment based on cell therapy.
Materials and Methods
Isolation, characterization and expansion of hWJ-MSCs
Fresh human umbilical cords (n=10) were obtained from full-term male babies after cesarean section delivery with informed consent using the guidelines approved by Tehran University of Medical Sciences’ Ethical Committee. Pregnant women with specific cases, such as intrauterine fetal death, maternal pre-eclampsia, infections, sexually transmitted diseases or hepatitis were excluded.
The umbilical cords were processed for isolation of WJ-MSCs using previous studies (18) with slight modifications as follows: Briefly, after rinsing in normal saline (0.9% w/v sodium chloride), the cords were aseptically stored at 4 °C in sterile saline until processing. Next, the umbilical cord vessels were removed manually from cord segments, and the exposed Wharton’s jelly tissue was cut into very small pieces or explants, approximately 2–3 mm, before placing them in a tissue culture dish. The explants were inserted into a drop of Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) containing 10% fetal bovine serum (FBS; Sigma-Aldrich), 2 mm L-glutamine (Invitrogen, USA), 100 u/ml penicillin (Sigma-Aldrich), and 100 u/ml streptomycin (Sigma-Aldrich) and 1 µg/ml amphotericin B for 5 min at room temperature. Afterward, they were plated in 25 cm2 culture flasks and placed in 37°C humidified incubator with 5% CO2 to migrate cells from Wharton’s jelly tissue explants. The medium was changed after 2 days and replaced every 2 days. After 8 days, the tissue was inspected under phase contrast light microscope to monitor cell migration. The whole adherent fraction was detached by trypsinization and replated using a 1:2 dilution factor. Spindle-shaped cells were used from the fourth passage for characterization.
hWJ-MSCs Surface marker profiling by flow cytometry
Passage 3 hWJ-MSCs were analyzed by flow cytometry to determine the pluripotent cell characteristics. After culture media removal, the cells were rinsed with PBS and trypsinized; cells were incubated with CD34-PE (phycoerythrin conjugated), CD45-FITC (fluorescein isothiocyanate), CD14-PE, CD73-PE, CD90-FITC, CD105-FITC and HLA-DR-FITC monoclonal antibodies (Abcam, UK) in dark for 30 min at 4 °C. Negative control samples were incubated with FITC/PE-conjugated mouse IgG1 isotype antibodies to help differentiate non-specific background signals from specific antibody signals. Cells were washed with PBS to remove unbound antibody and resuspended to analyze on a Partec PAS III flow cytometer (Partec GmbH, Münster, Germany).
hWJ-MSCs differentiation to osteogenesis and adipogenesis lineages
The differentiation potential of cells was examined on third passage of the hWJ-MSCs. For induction of osteogenic differentiation, the hWJ-MSCs were plated in six-well plates at a density of 10000 cells/cm2 in triplicates. After 48 hr, osteogenic differentiation medium (Sigma-Aldrich) was added to the cells, and the supplemented DMEM culture medium was added to the control cells. Medium refreshment was performed every 3–4 days for 21 days. Finally, the cells were washed with PBS, fixed with 4% paraformaldehyde and stained with alizarin red (Sigma-Aldrich) to detect the presence of calcium deposition in osteocytes.
For induction of adipogenic differentiation, the MSCs were plated in six-well plates at a density of 10000 cells/cm2 in triplicates. After 48 hr, adipocyte differentiation medium (Sigma-Aldrich) was added to the cells. In case of controls, the supplemented DMEM culture medium was added to the cells (used as control group). The culture media were replaced every 3 days for 21 days. Finally, the cells were washed with PBS, fixed with 4% paraformaldehyde and stained with Oil Red (Sigma-Aldrich) to detect the presence of neutral lipid vacuoles in adipocytes.
hWJ-MSCs differentiation to male germ cells by BMP4 treatment
hWJ-MSCs at passages 3 and 4 were treated with DMEM supplemented with 10% FBS, 1% pen/strep and BMP4 (10 ng/ml; Sigma-Aldrich) to differentiate into PGCs. These cells were cultured for 21 days in BMP4 containing media as described previously and harvested for upcoming assays.
hWJ-MSCs differentiation to male germ cells by BMP4/RA treatment
BMP4 was removed from the culture of 4 day BMP4 treated hWJ-MSCs. The cells were washed three times with serum-free medium before resuspension in RA treatment medium.
BMP4 treated hWJ-MSCs passaged with 10% FBS and 1% pen/strep DMEM medium containing RA (1 µM; Sigma-Aldrich) for 17 days to induce sex-specific pathway.
Human amniotic epithelial (HAE) feeder cell and human chorionic plate (HCP) feeder cell preparation
Human placenta feeder layers were prepared according to previous studies (19), with some modifications. Placentas used in accord with Tehran University of Medical Sciences’ Ethical Committee principles, were obtained from HIV and hepatitis B negative pregnant women with male baby after obtaining written informed consent.
First, the decidua parietalis was removed by careful scraping. The amnion and chorion were then manually separated and washed extensively in phosphate-buffered saline (PBS; Sigma), before being cut into small pieces (2 × 2 cm). Amnion fragments were incubated for 7 min at 37 °C in PBS containing 2.4 U/ml dispase (Roche, Mannheim, Germany). After a resting period (5–10 min) at room temperature in DMEM medium (Gibco) supplemented with 10% fetal bovine serum (FBS; Sigma), the fragments were digested with 0.75 mg/ml collagenase (Roche) and 20 µg/ml DNase (Roche) for approximately 3 hr at 37 °C. Amnion fragments were then removed and mobilized cells were passed through a 100 µm cell strainer (BD Falcon, Bedford, MA) and the cells were collected by centrifugation at 200 × g for 10 min. We refer to these cells as amniotic mesenchymal cells (AMCs). The collagenase-undigested amnion fragments were incubated with 0.25% trypsin (Sigma) at 37 °C for 2 min in order to obtain amniotic epithelial cells (AECs), which were collected by filtration and centrifugation as described above. Chorion fragments were subjected to two 8 min incubations in PBS containing 2.4 U/ml dispase at 37 °C, separated by a resting period of 5–10 min in DMEM medium containing 10% FBS. The stromal and trophoblastic layers of the chorion were then separated from each other and digested separately with collagenase and DNase as described above. Mobilized cells from the stromal layer, called chorionic mesenchymal cells (CMCs), were then collected as above. The trophoblastic part of the chorion was further treated with 0.25% trypsin for 2 min in order to detach the chorionic trophoblastic cells (CTCs), which were also collected. HAE or HCP cells were placed in 25 cm2 tissue culture flasks containing 10% FBS and 1% Penicillin-Streptomycin DMEM. After 7 days of incubation at 37 °C in 5% CO2, cultured monolayers of HAE and HCP cells were treated with mitomycin-C (Sigma-Aldrich) for 3 hr to inhibit feeder cells mitosis. Then, the cells were trypsinized by 0.25% trypsin-EDTA to produce single-cell suspensions of feeder cells, which were cultured in sterile 3 cm tissue culture dishes for 3 days. The full-seated HAE and HCP cells were used for feeder layers.
hWJ-MSCs differentiation to male germ cells by co-culturing system of HAE and HCP
Initially, hWJ-MSCs at passages 3 and 4 were treated with 1 µM RA for 7 days and then seeded onto mitomycin C-treated HAE/HCP feeder layer for 14 days with the 10% FBS and 1% pen/strep DMEM low glucose culture medium. Finally, the 21 day co-cultured cells were harvested out for the future assays. For morphological cell assessment after treating with RA, the cells were transduced by virus before co-culturing on HAE or HCP. Briefly, 5×106 293T cells were transfected by a mixture consisting of pLox-EWGFP, PAX and PMD vectors. After passing 24, 48 and 72 hr post transfection, pooling the collected supernatants, centrifuging for 5 min at 1500 rpm to remove cell debris and passing through a 0.45 μm or 0.22 μm filter, the resulting supernatant, containing virus particles, was used for transduction.
RNA extraction and RT-PCR analysis of hWJ-MSC derived cells
Total RNA was extracted from treated or co-cultured hWJ-MSC derived cells using TRIpure reagent (Roche, Germany) according to manufacturer instructions. Afterwards, cDNA was generated using 1 μg of the isolated total RNA by a cDNA synthesis kit (Invitrogen, USA) according to the kit instructions.
Appropriated primer sets for PGC and spermatogonial specific markers, which are listed in Table 1, including POU5F1, Fragilis, DDX4, Plzf and Piwil2 (Mili), Stra8, Dazl, β1- and α6-integrins (ITΒ1, ITA6) were designed using Primer3 software. PCR reactions were performed using Taq DNA polymerase (Roche, Germany). The PCR mixture contained 1 µl template cDNA, 0.4 µM of each primer (1 µl), 0.2 mM of dNTPs (0.5 µl), 0.625 unit/25 µl reaction of Taq DNA polymerase (0.125 µl), 1.5 mM MgCl2 (0.75 µl) and 1X PCR Buffer (2.5 µl) in a total volume of 25 µl with distilled water. In an Eppendorf thermal cycler (Mastercycler® 5330) PCR reactions were performed as follows: 94°C for 3 min, 35 cycles of PCR at 94°C for 30 sec, 60 °C for 30 sec, 72 °C for 1 min, and 72 °C for 5 min. GAPDH was used as a control housekeeping gene.
Table 1.
Genes, primers, and sizes of amplification products (bp) for quantification of gene expression by real-time quantitative polymerase chain reaction
| Gene | Primer sequence | Length | Code number | Tm |
|---|---|---|---|---|
| Itα6 | F:5´-CCCTGATGTTGCTGTTGGTTC-3´ R:5´- TGGCGGAGGTCAATTCTGTTAG-3´ | 114 | NM_000210.2 | 59 |
| Itβ1 | F:5´-TAGCAAAGGAACAGCAGAGAAG-3´ R:5´-AGGTAGTAGAGGTCAATGGGATAG-3´ | 150 | NM_002211.3 | 58 |
| Stra8 | F:5´- AAGGACAGCGGCGTGGAC -3´ R:5´- CTGGCAAGCACTGAACTGGAG -3´ | 149 | NM_182489.1 | 61 |
| Plzf | F:5´-AAGTTCAGCCTCAAGCATCAG- 3′ R:5´- CGTTGTGCGTTCTCAGGTG- 3′ | 133 | NM_00101801 | 59 |
| Ddx4 | F:5´- CTTAGACCCAGACGAATGTATGC-3´ R:5´- GTTCACTTCCACTGCCACTTC-3´ | 119 | NM_001166533.1 | 58 |
| Pou5f1 | F:5´- CCATCTGCCGCTTTGAGG-3´ R:5´- ACGAGGGTTTCTGCTTTGC-3´ | 133 | NM_001285987.1 | 58 |
| Dazl | F:5´- GCTCGCCTGACGCCATCTTTG-3´ R:5´- GCTGATGAGGACTGGGTGCTG-3´ | 98 | NM_001190811.1 | 62 |
| Piwil2 | F:5´-TGGTTGGAGTAGGACGCTTG-3´ R:5´- GGGACGGTGTGCTGAAGG-3´ | 122 | NM_001135721.1 | 59 |
| Fragilis | F :5´- GCACCCTCTACCTGAATCTG-3´ R:5´- AGGATGTTGTAGCACTTGGC-3´ | 136 | NM_001025295.2 | 58 |
Gel electrophoresis of PCR products
PCR products were separated by electrophoresis on 2% agarose gels (Merck, Germany) with TAE buffer, stained in ethidium bromide 0.5 mg/l aqueous solution for 10–15 min. Images of the gels were made using a White/Ultraviolet Transilluminator (UVP).
Quantitative real-time PCR of hWJ-MSC-derived cells after treatment
The extracted RNAs were subjected to quantitative real-time PCR analysis to evaluate the expression level of pluripotency markers. First-strand cDNA was generated as described above. Quantitative real-time PCR was performed in triplicate by SYBR green real-time master mix (Takara, Japan) in Rotor gene 3000 system (Corbett, Germany). SYBR Green master mix was added to each well of the PCR reactions (10 μl of SYBR Green, 6 μl of water, 1 μl of primers and 2 μl of cDNA). Real-time PCR reactions were as follows, 40 cycles at 95°C for 10 sec and 60°C for 60 sec. Real-time PCR data and relative quantification were analyzed using the Bio-Rad CFX Manager. Following the DC method, the cycle threshold (Ct) was calculated automatically and normalization was carried out against human Gapdh Ct value.
Immunocytochemistry (ICC)
Cells were passaged to coverslips and fixed with 4% (vol/vol) paraformaldehyde in 0.01 M PBS to perform immunocytochemistry. The samples were incubated with blocking buffer containing 10% normal goat serum and 0.3% Triton® X-100 for 1 hr at room temperature. Then, the samples were incubated with anti-SSEA4 primary antibody (1:100; Abcam, UK), DDX4 (1:100; Abcam, UK), c-Kit (1:100; Abcam, UK) at 4 °C overnight. After washing three times with PBS, the samples were incubated with secondary anti-rabbit IgG-FITC and anti-rabbit IgG-PE (Abcam, UK) antibodies. Finally, the samples were washed 3 times with PBS and observed under fluorescence microscope. Images were captured using a Zeiss LSM 5 fluorescent microscope.
Statistical analyses
Data were presented as mean±SD (standard deviation) and were analyzed using one-way repeated measure analysis of variance (ANOVA) followed by Tukey’s post hoc test. P-values < 0.05 were considered statistically significant.
Results
hWJ-MSC displayed fibroblastic morphology and expressed MSCs specific non-hematopoietic surface markers
Following disruption of umbilical cord tissue and isolation of hWJ-MSCs, their morphological characters were inspected under inverted microscope at passages 1, 3 and 10 (21st day). Microscopic observations confirmed the fibroblastic-like appearance of the isolated cells which was similar to other MSCs (Figure 1).
Figure 1.
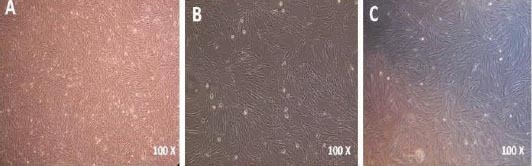
Morphology of human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs). passage 1; fibroblast-like hWJ-MSCs (A), passage 3 (B), and passage 4 (21st day) (C)
Identification of hWJ-MSC
hWJ-MSCs were labeled with PE- or FITC-conjugated antibodies and examined by flow cytometric analysis, which confirmed the presence of MSC markers (including CD105, CD90 and CD73) and the absence of hematopoietic stem cell markers (including CD34 and CD45) and also leukocytes marker (CD14) and human leukocyte antigen (HLA)-DR. Taken together, flow cytometry confirmed the MSC characteristics of the hWJ-MSCs at the third passage (Figure 2).
Figure 2.
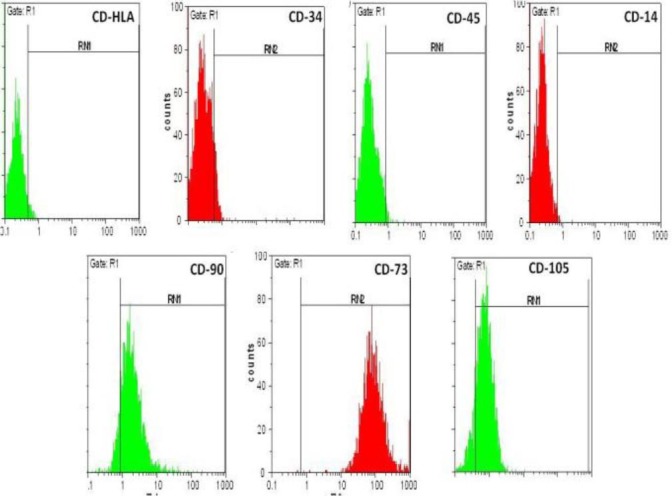
Identification of human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) passage 4. These cells were labeled with PE or FITC conjugated antibodies and examined by flow cytometry. The cells were positive for CD-90, CD-105 and CD-73 and negative for CD-34, CD-45, CD-14 and HLA. Data are representative of three independent experiments
hWJ-MSCs were able to differentiate to osteocytes and adipocytes
After isolation and proliferation of the hWJ-MSCs, osteogenic differentiation was induced. Twenty one days after induction, the adipogenic differentiation capacity of the isolated cells was confirmed following staining of intra cytoplasmic lipid droplets by Red Oil stain (Figure 3-A). In addition, the alizarin red S staining of the cells confirmed their osteogenic differentiation (Figure 3-B).
Figure 3.
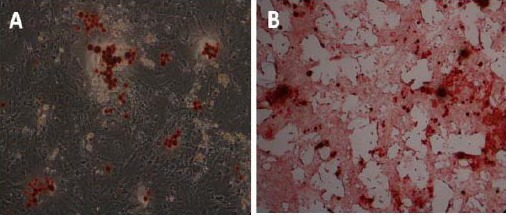
Differentiation of human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) to osteogenesis and adipogenesis lineage; (A) Adipogenic lineages derived from hWJ-MSCs (Oil Red-O staining); Accumulation of intracellular lipid droplets, (B) Osteogenic lineages derived from hWJ-MSCs (Alizarin Red-S staining); Calcium deposits in differentiation cells
Morphological changes in hWJ-MSCs after induction
To induce hWJ-MSCs into germ cells, hWJ-MSCs were treated with BMP4, RA or co-cultured on HAE and HCP during 21 days. Morphological changes were observed every day under a phase contrast microscope. After passing 7 days, the cells typically appeared as slender spindles and formed a tadpole-like shape (Figure 1 A). However, the flat, wide or polygonal cells in the initial induction (Figure 4 A) almost disappeared at the 21st incubation day, and their density was looser in induced experimental groups compared with untreated cells cultured in basic culture medium (Figure 4 B-D). The morphology of these tadpole-like cells did not change significantly up to day 7 of treatment in any of the experimental groups (Figure 4 B-D). All groups containing treated and untreated hUMSCs were 100% confluent on day 14 and changed morphologically from slender spindles to shiny clusters at the 21st treatment day (Figure 4 A-D). Indeed, the transfected cells for tracing morphology of cell on placental feeder layers were apoptosed after RA induction and removed (Figure 5).
Figure 4.
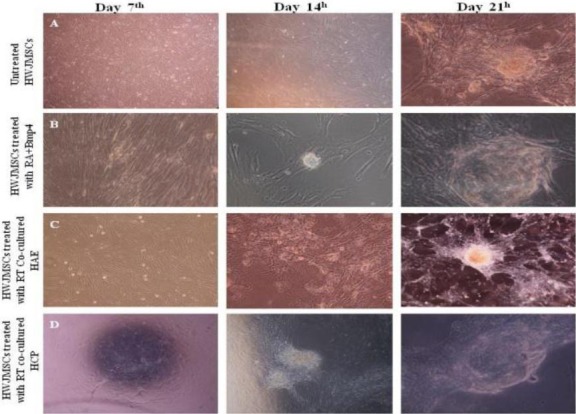
Comparison of morphological changes in differentiated human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) (B–D) with undifferentiated hWJ-MSCs, (A) at different times (days 7, 14, 21). RA; Retinoic acid, BMP4; Bone morphogenic protein 4, HAE; Human Amniotic Epithelium, HCP; Human Chorionic Plate
Figure 5.
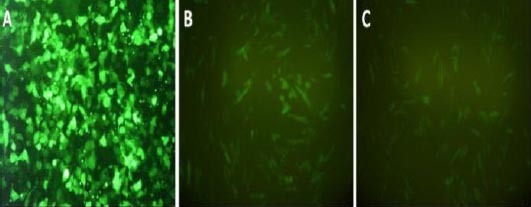
Comparison of morphological changes in transduced human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) after retinoic acid treatment (B), and placental cells co-culturing (C), with undifferentiated hWJ-MSCs (A), on day 21, passage 4. HEK 293 was used to produce viruses for transduction of hWJ-MSCs
Immunocytochemistry staining of specific germ cell markers
The expression of germ cell-specific markers, the SSEA4, DDX4 and cKit, was analyzed by immunofluorescence staining at 21st day of differentiation stage. All markers were expressed in hWJ-MSCs treated with BMP4/RA or co-cultured on HAE and HCP at 21st day (Figure 6).
Figure 6.
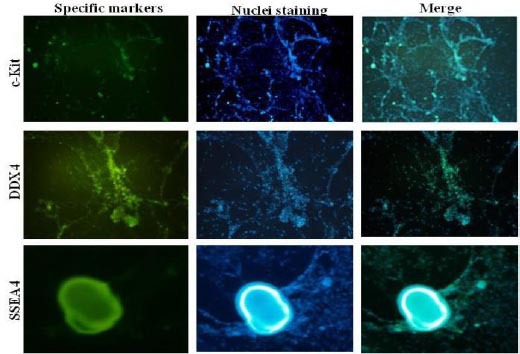
Immunofluorescence of male germ cell markers, DDX4, c-Kit and SSEA4 in differentiated human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) treated with BMP4 + retinoic acid 21 days post induction. Nuclei were stained with DAPI (4´, 6´-diamidino-2-phenylindole) staining. Magnification; 100X
Expression of germ cell specific genes in hWJ-MSC-derived cells
hWJ-MSC-derived cells from BMP4/RA or placenta feeder layer co-culture were checked for markers of PGC and spermatogonia, as follows: POU5F1, Fragilis, DDX4, Plzf and Piwil2 (Mili), Stra8,Dazl, Itβ1 and ITA6. RNA isolated from human testicular tissue served as a positive control. All the cells and also the testis sample were positive for POU5F1 gene. All cells were positive for POU5F1, fragilis, Dazl, ITA6 and Itβ1 with slight expression differences. However, expression of STRA8 was negative in all detected samples. DDX4 was highly expressed in the testis sample, although it was rarely seen in control, BMP4/RA and treated cells, except placenta feeder cell co-cultured hWJ-MSCs which did not express this marker. Piwil2 expression was significant in testis and BMP4/RA treated hWJ-MSCs compared to its lower expression in control hWJ-MSCs and no expression in HCP co-cultured hWJ-MSCs as well as Plzf (Figure 7).
Figure 7.
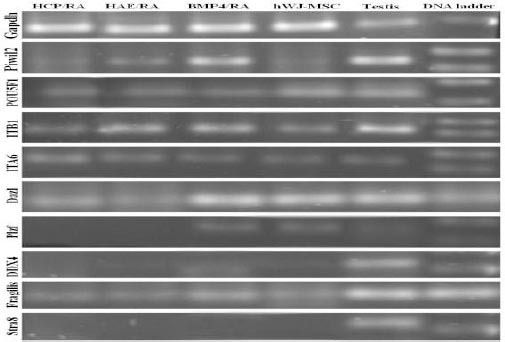
RT PCR analysis. Differentiation stages initiated 4 passage human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) and ended in spermatogonial colony like cells. GAPDH was chosen as an internal control and adult testis was positive control. RA; Retinoic acid, hzBMP4; Bone morphogenic protein 4, HAE; Human Amniotic Epithelium, HCP; Human Chorionic Plate
Real-time PCR expression analysis of control hWJ-MSCs and treated cells genes profile
Quantitative real-time PCR analysis of hWJ-MSCs, BMP4/RA treated hWJ-MSCs and placenta feeder layer co-cultured hWJ-MSCs indicated high expression level of cKit marker compared to hWJ-MSCs and hWJ-MSCs and placenta feeder layer co-cultured hWJ-MSCs. This increase was significant (P≤0.05). The results showed expression of DDX4, ITA6, Piwil2 and Dazl was increased significantly (P≤0.05) compared to other groups. The ITΒ1 gene was over expressed (P≤0.05) in hWJ-MSCs, BMP4/RA treated hWJ-MSCs compared to placenta feeder layer co-cultured hWJ-MSCs. All of experimental groups expressed the POU5F1 gene (P≤0.05) (Figure 8).
Figure 8.
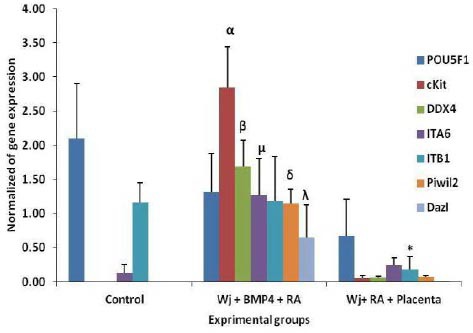
Real-time PCR analysis. mRNA levels were normalized with respect to GAPDH, chosen as an internal control. Differentiation stages initiated 4 passage human Wharton’s jelly Mesenchymal Stem Cells (hWJ-MSCs) and ended in spermatogonial colony like cells. Histograms show mean expression values (± SD, n=3; p ≤ 0.05).α, β, μ, δ and λ: significant difference with other groups in the same gene; *: significant difference with control (hWJMSC) and Wj+BMP4+RA groups in ITΒ1 gene. RA; Retinoic acid, BMP4; Bone morphogenic protein 4
Discussion
In vitro derived germ cells are very useful tools for understanding molecular and cellular mechanisms of male infertility, establishment of therapeutic approaches for male infertility and provide testing systems to examine toxicological effects of drugs on human germ cells (20). Although several studies have shown that germ cells can be differentiated from mouse and human embryonic and adult stem cells, human germ cells differentiated in these studies generally did not develop beyond the earliest stages (21). In the present work, human Wharton’s jelly was used; our reasons for looking at Wharton’s jelly as a source of primitive cell types were easy proliferation and expansion, low immunogenicity, high differentiation capacity to other cell lineages and treating neural, spinal and somatic diseases, absence of moral and ethical problems, high immune tolerance in transplantations, and interestingly, harvesting without any pain or discomfort. It illustrates the ability of WJCs to differentiate in osteogenic and adipogenic lineages further evidencing their pluripotency. These cells had spindle-shaped elongated morphology similar to fibroblastoid cells. This characteristic was visible up to the end of culturing. A positive response for CD105, CD73, CD90 mesenchymal markers and a negative response for CD34, CD45 and CD14 hematopoietic markers were detected in hWJCs in fourth passage. Overall, the data reported in this study strongly indicate that the cells derived from Wharton’s jelly belong to the mesenchymal stem cell population. Wharton’s jelly cells have been cultured for more than 80 population doublings with no indications of senescence, alterations in shape, improved growth rate, or change in capability to differentiate into neurons. Wharton’s jelly cells have telomerase activity discovered in human embryonic stem cells (22). hUMSCs in Wharton’s Jelly of the umbilical cord seem to be a favorable source of stem cells for conversion into male germ like cells, because of large potential donor source, immediate accessibility, lack of discomfort to the donor, and low risk of rejection, as shown in this study, as a negative HLA marker. Therefore, hUMSCs in Wharton’s Jelly of the umbilical cord have the potential to become an excellent candidate in cell replacement therapy of infertility (23). Our data revealed that the 21 days of hWJ-MSCs differentiation in vitro, transmembrane tyrosine kinase receptor KIT (c-KIT) /DDX4/Piwil2/alfa6 and beta 1 integrin genes level increased in RA + BMP4 and RA+ placental feeder cells groups compared to control group. We observed that treatment with RA and placental feeder cells decreased all examined germ cell makers compared to RA + BMP4 group. RA, an active derivative of vitamin A, influences germ cell differentiation and is required for the transition into meiosis for both female and male germ cells. RA receptors (RARs) are expressed in both Sertoli cells and germ cells, which can be stimulated by RA. Retinoids are involved in the regulation of testicular functions, which appear to be necessary for spermatogenesis and the development of spermatocytes through early stages of meiosis. RA promotes differentiation of ESCs and bone marrow cells into germ (24), Furthermore, BMP4 leads to expression of PGCs specific genes such the Dpp3a (Stella), Fragilis, mouse vase homologue (Mvh) gene (25, 26). In the present study, we found hUMSCs changed morphologically to shiny clusters after incubation in this culture system, although we could not observe the round cell type that had been reported by other groups. Perhaps long-term culture is needed for the round cell shape. However, the expression of germ-cell specific genes such as c-Kit/DDX4/Piwil2/alfa6 and beta 1 integrin in the differentiated hUMSCs confirmed that hUMSCs can differentiate into germ cells. To analyze germ cell characteristics of induced hUMSCs we examined the expression of mRNA and protein markers diagnostic of germ cell development at different stages of HUMSC differentiation. C-Kit is a germ-cell enriched gene highly expressed in PGCs. It is expressed in early spermatogenic cells and in later stages of spermatogenesis, particularly in the acrosomal particles of the round spermatids and the acrosomal region of testicular spermatozoa (27).Intergrin a6 is the surface marker of spermatogonial stem cells (28). Integrins such as αv, α6, and β1 are also expressed on adult spermatogonia (28, 29). However, not only prospermatogonia and adult undifferentiated spermatogonia but also somatic cells in the testis express all of these markers (30).
Vasa mRNA and protein levels are abundant and specific in germ cells of both sexes throughout development. In humans, Vasa protein is present in migrating primordial germ cells. During normal spermatogenesis, Vasa expression is relatively weak to intermediate in spermatogonia, intensive in spermatocytes/spermatids, and absent in spermatozoa (30).
In this study, we observed Vasa expressed in RA + BMP4 and RA + placental feeder cells treated hUMSCs Piwil2 is sharply expressed in spermatids and partially in primary spermatocytes on day 14 post-fertilization, and acts as a germline–sertoli–germline signaling cycle. Piwil2 knockout mice have uncompleted spermiogenesis initiation (31-33). In both male and female mice and humans, Dazl is expressed primarily by PGCs in the fetal gonads (34) and throughout gametogenesis (35). In the male, Dazl is expressed during spermatogenesis in gonocytes, spermatogonia and primary spermatocytes (36). During meiosis, Dazl is translocated from the nucleus of the spermatogonia into the cytoplasm of secondary spermatocytes, spermatids and spermatozoa (36, 37). Overall, in the present study, we concluded that RA is able to differentiate mesenchymal stem cells into male germ like cells. This support should be followed by BMP4 or feeder cells to achieve the best results in vitro male germ cell differentiation. We introduced an efficient model to access male germ cells based on mRNA level of germ specific markers.
Conclusion
Our findings provide a novel effective approach for generation of germ cells in vitro and studying the interaction of germ cells with specific inducers and the feeder layers. Our work represents an essential step towards gaining knowledge of the molecular properties of hWJ-MSCs in the field of cell therapy and infertility. We demonstrated that under appropriate conditions, hWJ-MSCs have the ability to differentiate into germ cells.
Acknowledgment
We thank Mrs Ramezan Ali, Danesh Payeh, Ghasemi and Dr Gheysari for technical assistance. The results reported in this paper were part of a student thesis and supported by Tehran University of Medical Sciences, Tehran, Iran.
References
- 1.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Nat Acad Sci. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kee K, Gonsalves JM, Clark AT, Pera RAR. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 3.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 4.Easley IV CA, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Reports. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panula S, Medrano JV, Kee K, Bergström R, Nguyen HN, Byers B, et al. Human germ cell differentiation from fetal-and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bongso A, Fong C-Y. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 7.Troyer DL, Weiss ML. Concise review: wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cell. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010;6:499. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlin R, Davis D, Weiss M, Schultz B, Troyer D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8. doi: 10.1186/1477-7827-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewen-Campen B, Schwager EE, Extavour CG. The molecular machinery of germ line specification. Mol Reprod Dev. 2010;77:3–18. doi: 10.1002/mrd.21091. [DOI] [PubMed] [Google Scholar]
- 11.Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Kashani IR. BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int. 2012;36:1185–1193. doi: 10.1042/CBI20110651. [DOI] [PubMed] [Google Scholar]
- 12.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta K, Lin Y, Hogg N, Yamamoto M, Yamazaki Y. Direct effects of retinoic acid on entry of fetal male germ cells into meiosis in mice. Biol Reprod. 2010;83:1056–1063. doi: 10.1095/biolreprod.110.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwahashi K, Yoshioka H, Low EW, McCarrey JR, Yanagimachi R, Yamazaki Y. Autonomous regulation of sex-specific developmental programming in mouse fetal germ cells. Biol Reprod. 2007;77:697–706. doi: 10.1095/biolreprod.107.062851. [DOI] [PubMed] [Google Scholar]
- 15.Morishita T, Yokoyama M, Nozaki M, Sano M, Nakano H. Improvement in blastocyst hatching of mouse embryos cocultured with human placental cells. J Assist Reprod Genet. 1993;10:463–467. doi: 10.1007/BF01212934. [DOI] [PubMed] [Google Scholar]
- 16.Suşman S, Rus-Ciucă D, Soriţău O, Tomuleasa C, Buigă R, Mihu D, et al. Pancreatic exocrine adult cells and placental stem cells co-culture. Working together is always the best way to go. Rom J Morphol Embryol. 2011;52:999–1004. [PubMed] [Google Scholar]
- 17.Miyamoto K, Hayashi K, Suzuki T, Ichihara S, Yamada T, Kano Y, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22:433–440. doi: 10.1634/stemcells.22-4-433. [DOI] [PubMed] [Google Scholar]
- 18.Venugopal P, Balasubramanian S, Majumdar AS, Ta M. Isolation, characterization, and gene expression analysis of Wharton’s jelly-derived mesenchymal stem cells under xeno-free culture conditions. Stem Cells Cloning. 2011;4:39. doi: 10.2147/SCCAA.S17548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Lako M, Armstrong L. Derivation of Human Sperm from Embryonic Stem Cells Dev. 2009;18:1111–1112. doi: 10.1089/scd.2009.0063. [DOI] [PubMed] [Google Scholar]
- 21.Kee K, Angeles VT, Flores M, Nguyen HN, Pera RAR. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 23.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang P, Lin LM, Wu XY, Tang QL, Feng XY, Lin GY, et al. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem. 2010;109:747–754. doi: 10.1002/jcb.22453. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Nat Acad Sci. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagano MC. In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod. 2007;76:546–551. doi: 10.1095/biolreprod.106.058271. [DOI] [PubMed] [Google Scholar]
- 27.Sandlow JI, Feng H-L, Sandra A. Localization and expression of the <i>c-Kit</i> receptor protein in human and rodent testis and sperm. Urology. 1997;49:494–500. doi: 10.1016/S0090-4295(96)00494-3. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Nat Acad Sci. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinohara T, Avarbock MR, Brinster RL. β1-and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc Nat Acad Sci. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice. Dev Biol. 2003;258:209–225. doi: 10.1016/s0012-1606(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 31.Cox DN, Chao A, Lin H. Piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 32.Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 35.Kerr CL, Cheng L. The dazzle in germ cell differentiation. J Mol Cell Biol. 2010;2:26–29. doi: 10.1093/jmcb/mjp041. [DOI] [PubMed] [Google Scholar]
- 36.Ruggiu M, Saunders PT, Cooke HJ. Dynamic subcellular distribution of the DAZL protein is confined to primate male germ cells. J Androl. 2000;21:470–477. [PubMed] [Google Scholar]
- 37.Lin YM, Chen CW, Sun HS, Tsai SJ, Lin JSN, Kuo PL. Presence of DAZL transcript and protein in mature human spermatozoa. Fertil Steril. 2002;77:626–629. doi: 10.1016/s0015-0282(01)03226-5. [DOI] [PubMed] [Google Scholar]


