Abstract
Context:
Periodontal disease and oxidative stress (OS) are part of a vicious cycle with each causing a deleterious effect on the other causing changes in the levels of antioxidants, and enzymes of antioxidant defense. Biomarkers and methods used for measuring OS are very expensive.
Aims:
To see how gamma-glutamyltransferase (GGT) fares, as a biomarker for OS in periodontits along with other routinely used biomarkers.
Design:
A cross-sectional study involving 300 people of which 150 were cases and 150 were controls.
Setting:
Candidates enrolled were patients visiting the OPD of MGV's Dental College and Hospital, Nasik, India between January 2011 and December 2012.
Materials and Methods:
Serum samples of patients with periodontitis, and controls were analyzed for malondialdehyde, superoxide dismutase (SOD), glutathione peroxidase (GPx), uric acid, and GGT.
Statistical Analysis Used:
Analysis was performed using Student's t test. P <0.05 were considered to be significant.
Results:
Malondialdehyde values were found to be significantly higher cases, while SOD, GPx and uric acid levels were found to be lower than controls. GGT levels were significantly higher in cases as compared to controls.
Conclusions:
GGT may be used as a cheap, quick, easy and precise marker for measuring OS.
Keywords: Gamma-glutamyltransferase, oxidative stress, periodontitis
INTRODUCTION
A lot of interest has been generated among scientists to discover various markers of oxidative stress (OS), ever since, evidence first began to trickle down, that OS is a culprit in the development and exacerbation of many diseases. Experimental and clinical studies have provided increasing substantial evidence to implicate OS both as cause as well as effect of many diseases and conditions such as cardiovascular diseases, aging, diabetes, Parkinson's disease, Alzheimer's disease, cancer, schizophrenia, maturity onset cataract and autism.[1,2,3,4]
Periodontitis is an inflammatory disease of the supporting tissues of the teeth, caused by specific microorganisms or groups of specific microorganisms; resulting in progressive destruction of the periodontal ligament and alveolar bone with pocket formation, recession, or both.[5] On an average 10-15% of the population worldwide suffers from periodontitis.[6] A recent prevalence study on periodontitis in the adult US population, puts this figure at over 47%.[7] Though the tissue destruction in periodontitis is caused by the interaction between microorganisms and the host response, many studies in the recent past have proved beyond doubt that periodontitis is associated with increased OS.[8,9]
Measurement of OS in cell is dependent on extremely sensitive assays and accurate quantification. Free radicals are so reactive and short-lived that direct measurement is usually not possible. Reactive oxygen species (ROS) is mainly measured indirectly, following formation of oxidative by-products of lipids, proteins and nucleic acids. Various biomarkers have been used to show the presence of OS. The markers, which have always been traditionally used and continue to be popular are estimation of malondialdehyde (TBARS assay), total antioxidant capacity or total antioxidant power, nitric oxide, antioxidant enzymes superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione reductase, Glutathione-S-Transferase, and estimation nonenzymatic markers like Vitamin C, Vitamin E, coenzyme Q, uric acid, bilirubin etc.[8,10,11,12,13,14,15,16]
In the case of patients with periodontitis, these markers have been estimated in serum, saliva and gingival crevicular fluid (GCF).[17,18] Since, the past decade, isoprostanes are being increasingly used as a biomarker of oxidative DNA damage, and have slowly but steadily become a well-recognized gold standard of oxidant status. In dentistry too, this marker has been used extensively in saliva and serum samples.[19] Many new techniques are now available for measuring ROS directly e.g., electron paramagnetic resonance, histochemical staining methods, Mass Spectroscopy, cytochemical methods, ROS tracer dyes, etc.[20] Noninvasive techniques using real time imaging of redox changes have been used in animal studies.[21] Choice of assay depends on ease, instrumentation, kits available and of course funds. One problem routinely encountered, especially in smaller cities of India is the lack of expertise, appropriate instrumentation and storage facilities and also nonavailability of kits. Hence, there is an urgent need to standardize and promote alternate, quick, cheap and precise markers for measuring OS.
Gamma-glutamyltranspeptidase/transferase (GGT) is an enzyme that is a routinely used diagnostic marker to detect diseases of the liver and bile ducts. Its normal range is 0-51 IU/L, though the levels may vary slightly among laboratories.[22] GGT is present in the cell membranes of many tissues including the kidneys, bile duct, pancreas, gall bladder, spleen, heart, brain and seminal vesicles.[23] Lee et al. concluded that serum GGT levels within its normal range predicted both oxidative damage products of arachidonic acid (F2-isoprostanes) and markers of inflammation (fibrinogen and C-reactive protein) in dose response manner.[24] It is becoming increasingly clear that GGT is much more than just a marker for liver diseases and has the potential role as a marker in other inflammatory conditions.
In the past decade, studies have found that elevated serum GGT levels can be correlated to cardiovascular diseases, and it is now being investigated as a potential marker for predicting increased risk for such diseases.[25] Gradually accumulating evidence seems to point that GGT has much more potential than previously thought. A study carried out in 2007 concludes that GGT may be an early predictor for the development of chronic kidney disease.[26] GGT has, of late, also been used as a biomarker for metabolic syndrome.[27,28] Another interesting aspect of GGT has been proposed in a study done in 1997, which says that the enzyme is located on the outer cell envelope of Treponema denticola; the anaerobic pathogen that plays an important role in the progression of periodontal disease. The authors go on to suggest that GGT may play a role in the propagation of the organism within the inflamed periodontal tissues.[29] In spite of such promising scope, the benefits of this marker have somehow been underutilized.
There is some evidence to say that periodontitis is associated with systemic diseases.[30,31,32] Hence, the aim of our study was to investigate, whether serum GGT could be used as a reliable marker for showing the presence of OS in patients with periodontitis. The objectives were a comparison of serum GGT as an OS marker with other traditional markers like serum malondialdehyde (MDA), SOD, GPx and uric acid in subjects with and without periodontitis.
MATERIALS AND METHODS
The protocol for this cross-sectional study was approved by the Institutional Ethics Committee of MGV's KBH Dental College and Hospital, Nasik and the Human Ethics Committee (MUHS/UDIRT/138/2012) at UDIRT, Maharashtra University of Health Sciences, Nashik. Prior to enrollment in the study, candidates who fulfilled the inclusion criteria were given detailed oral information about the study and invited to participate. A patient information sheet was handed over to them and written informed consent obtained.
Totally 150 subjects in the age group of 18-65 years with chronic periodontitis and 150 healthy controls (age and sex matched) fulfilling the inclusion and exclusion criteria were included in the study. They were recruited from the OPD, Department of Periodontics; MGV's KBH Dental College and Hospital, Nashik.
Inclusion criteria
-
Control Group (CG)
- At least 14 teeth in the mouth,
- Bleeding on probing (BOP) in less than 30% of periodontal sites with probing pocket depth (PPD) of 1-3 mm
- No BOP and clinical attachment loss (CAL) of 3 mm or more at less than 30% of all sites in the mouth
- Not barred by exclusion criteria.
-
Study Group (SG) Patients with periodontitis
- At least 14 teeth in the mouth,
- BOP in more than 30% of periodontal sites with PPD of 1-3 mm
- BOP and CAL of 3 mm or more at more than 30% of all sites in the mouth
- Not barred by exclusion criteria.
Exclusion criteria
Unfavorable systemic conditions (rheumatic fever, heart problems, hypertension, diabetes, liver or kidney diseases, etc
Any infection requiring prophylactic antibiotic treatment
Pregnant and lactating women, women on hormonal contraceptives or on hormone replacement therapy
Patients taking steroids or NSAID (for previous 3 months) or vitamin supplements
Subjects who smoke and regularly consume alcohol
Persons who have undergone scaling/root planing in the past 6 months.
Periodontal parameters
A full-mouth periodontal examination was performed by a single examiner (N.D.). The following periodontal parameters were assessed using a graduated UNC15 probe at 6 sites per tooth, that mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual and disto-lingual:
PPD
CAL
BOP.
Biochemical parameters
Blood samples were collected by venipuncture from the median cubital vein in plain bulbs, and immediately processed (centrifuged at 2000 rpm for 15 min). The serum obtained was analyzed at the same day for the following parameters:
Statistical analysis
To detect a small difference in means of 2 (±0.2) units, when the assumed variation is of 16-25 units (estimation based on pilot studies), the minimum sample size needed was 130. The power of the test was set at 95%. During the period of the study, we were able to enroll 150 consenting individuals in each group. Data were analyzed using computer software, IBM Statistical Package for Social Sciences (SPSS) Version 20 (Bangalore, Karnataka, India). Analysis was done using Student's t test and data were expressed as mean ± standard deviation. P < 0.05 were considered to be statistically significant.
RESULTS
Subjects were recruited in the study, when they came to the college OPD for a dental examination. Hence, it was not possible to obtain age and sex matched subjects in the two groups.
All subjects were Indians, with a predominance of lower-middle class Maharashtrian population. The subjects in the SG are found to have higher mean age as compared to controls. Tables 1 and 2 shows the comparison of the mean values of the biochemical parameters between the CG and SG.
Table 1.
Demographic characteristics of controls and cases

Table 2.
Comparison of values between control group and study group
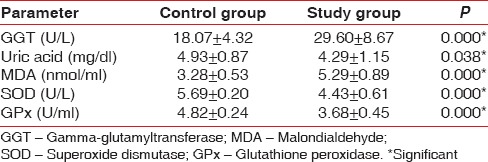
Mean MDA values in the SG is found to be significantly higher than that of controls, while mean SOD, GPx and Uric acid values are significantly lower than that of controls. Within reference range, GGT levels in the SG are found to be significantly higher than controls.
DISCUSSION
Mean age of patients with periodontitis is higher than that of controls indicating that periodontitis is associated with increasing age. However, OS in periodontitis is independent of age and gender.[9] It comes as no surprise that the levels of serum MDA are higher in periodontitis patients as compared to healthy controls. Periodontitis is an inflammatory condition leading to increased OS. Increased OS leads to increased lipid peroxidation which explains the increased levels of MDA in patients as compared to controls. Our results are consistent with the results obtained by Canakci et al.[36]
Uric acid is the most important endogenous antioxidant in the human plasma and acts as a potent free-radical scavenger.[37] Some of the scientists support the view that serum antioxidants increase in response to increased OS. The study of Barnes et al. concludes that the purine degradation pathway, a major biochemical source for ROS production, is significantly accelerated at the periodontal sites.[38] Uric acid is the end product of purine metabolism, and this may offer another explanation to the increase in uric acid levels in patients with periodontitis. Persons with weak antioxidant defense may have a lower level of antioxidant enzymes that makes them more susceptible to infections in the first place.[39,40] This can be correlated with our results where controls were found to have significantly higher concentrations of the enzymes of antioxidant defense namely, SOD and GPx as well as uric acid. GPx are a major group of enzymes that remove hydrogen peroxide generated by SOD in the cytosol and mitochondria by oxidizing reduced glutathione to its oxidized form. Increased oxygen metabolism results in overproduction of superoxide radicals that are known to inhibit GPx. SOD catalyzes the dismutation of superoxide radicals that are produced by various mechanisms.[41]
In OS, there is excessive production of free radicals like superoxide anions which disturbs the subtle balance in favor of free radicals.[42,43] To detoxify these free superoxide radicals, large amounts of SOD are utilized. This may also account for the decreased concentrations of GPx and SOD in patients with periodontitis when compared to controls. Our results are consistent with results obtained by other studies.[17,44,45]
But what is most heartening is that our results seem to indicate that GGT can be an extremely good indicator of OS in periodontitis. Even within the reference range, there is significant variation in the mean GGT levels in controls and patients with periodontitis with controls showing lower mean values of the enzyme as compared to patients with periodontitis. GGT seems to be useful in studying OS-related issues in both epidemiological and clinical settings.[24,46] Elevated levels of this enzyme have already been used as a marker for OS in Tuberculosis, Menopause, Alzeimer's disease, hypertension, etc.[47,48,49,50] So we can say that elevated levels of this enzyme in patients with periodontitis is a marker of OS.
One of the most important molecules involved in the antioxidant defense of our body happens to be glutathione, which exerts its action by shunting between oxidized (GSSG) and reduced state (GSH). Changes in the levels of reduced Glutathione are a major indicator of OS conditions. GGT is an enzyme catalyzing GSH breakdown.[51,52]
Traditional diagnosis of periodontal disease is based on clinical (GI, PD, CAL, BOP) and radiographic parameters. Advances in periodontal research is now moving toward methods wherein periodontal disease risk and active periodontal destruction is quantified by biomarkers in GCF like aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, creatine kinase, GGT, etc.[53] Studies have shown a significant increase in salivary GGT levels in patients with periodontal disease that decrease with periodontal treatment.[54] Periodontal health seems to have a bearing on other organs such as heart, kidneys, liver, pancreas, etc.[55,56,57] Since, this enzyme is produced in these organs, it is clear that systemic health is compromised at the outset, even though the patient appears to be systemically healthy.
Gamma-glutamyltransferase is a very stable enzyme whose in-vitro assays can be carried out as 25°C, 30°C or 37°C. Measurement of GGT using automated analyzers is quick, cheap and precise. Most laboratories routinely perform this enzyme assay.
Further understanding and evidence would be required to say whether stratification of periodontal patients can be done using GGT. Future studies may also look at correlation studies between GGT and clinical parameters like plaque index (PI), BOP and CAL. Longitudinal studies are necessary to determine, whether elevated levels of GGT alone may predict the development of unfavorable systemic conditions. But until then, it really seems as though this is one biomarker that cannot be ignored or overlooked.
CONCLUSIONS
Scientists have been obtaining increasing evidence about OS being a culprit in the development of adverse systemic conditions. Modern science develops new sophisticated technologies to detect free radicals. Newer markers of OS are being used. GGT seems to be one enzyme that has not got its due credit in this important arena. With larger studies, this may just prove to be a simple, easy to use, quick, cheap and precise predictor of OS in periodontitis and adverse systemic outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Serra JA, Domínguez RO, Marschoff ER, Guareschi EM, Famulari AL, Boveris A. Systemic oxidative stress associated with the neurological diseases of aging. Neurochem Res. 2009;34:2122–32. doi: 10.1007/s11064-009-9997-5. [DOI] [PubMed] [Google Scholar]
- 2.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 3.Puertas MC, Martínez-Martos JM, Cobo MP, Carrera MP, Mayas MD, Ramírez-Expósito MJ. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol. 2012;47:625–30. doi: 10.1016/j.exger.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Novak MJ. Classification of diseases and conditions affecting the periodontium. In: Newman MG, Takei H, Klokkevold PR, Carranza FA, editors. Carranza's Clinical Periodontology. 10th ed. New Delhi: Elsevier (South-Asia Edition); 2006. pp. 103–4. [Google Scholar]
- 6.Baelum V, Lopez R. Periodontal epidemiology: Towards social science or molecular biology? Community Dent Oral Epidemiol. 2004;32:239–49. doi: 10.1111/j.1600-0528.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 8.Borges I, Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm 2007. 2007 doi: 10.1155/2007/45794. 45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89:1241–6. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti E, Monaco A, Procaccini L, Mummolo S, Gatto R, Tetè S, et al. Periodontal disease: The influence of metabolic syndrome. Nutr Metab (Lond) 2012;25(9):88. doi: 10.1186/1743-7075-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miricescu D, Totan A, Calenic B, Mocanu B, Didilescu A, Mohora M, et al. Salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol Scand. 2014;72:42–7. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 12.Omeh YS, Uzoegwu PN. Oxidative stress markers in periodontal disease patients. Niger J Biochem Mol Biol. 2010;25:50–4. [Google Scholar]
- 13.Battino M, Ferreiro MS, Quiles JL, Bompadre S, Leone L, Bullon P. Alterations in the oxidation products, antioxidant markers, antioxidant capacity and lipid patterns in plasma of patients affected by Papillon-Lefèvre syndrome. Free Radic Res. 2003;37:603–9. doi: 10.1080/1071576031000083116. [DOI] [PubMed] [Google Scholar]
- 14.Pendyala G, Thomas B, Kumari S. The challenge of antioxidants to free radicals in periodontitis. J Indian Soc Periodontol. 2008;12:79–83. doi: 10.4103/0972-124X.44100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapple IL, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. 2007;137:657–64. doi: 10.1093/jn/137.3.657. [DOI] [PubMed] [Google Scholar]
- 16.Dhotre PS, Suryakar AN, Bhogade RB. Oxidative stress in periodontitis. Eur J Gen Med. 2012;9:81–4. [Google Scholar]
- 17.Canakci V, Yildirim A, Canakci CF, Eltas A, Cicek Y, Canakci H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J Periodontol. 2007;78:1602–11. doi: 10.1902/jop.2007.060469. [DOI] [PubMed] [Google Scholar]
- 18.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55:70–8. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 19.Takane M, Sugano N, Iwasaki H, Iwano Y, Shimizu N, Ito K. New biomarker evidence of oxidative DNA damage in whole saliva from clinically healthy and periodontally diseased individuals. J Periodontol. 2002;73:551–4. doi: 10.1902/jop.2002.73.5.551. [DOI] [PubMed] [Google Scholar]
- 20.Shulaev V, Oliver DJ. Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant Physiol. 2006;141:367–72. doi: 10.1104/pp.106.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–93. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 22.Gamma-Glutamyl Trans Peptidase: Medline Plus Medical Encyclopedia. [Last updated on 2013 Jan 21; Last cited on 2013 Jan 24]. Available from: http://www.nlm.nih/gov/medlineplus/ency/article/003458.htm .
- 23.Goldberg DM. Structural, functional, and clinical aspects of gamma-glutamyltransferase. CRC Crit Rev Clin Lab Sci. 1980;12:1–58. doi: 10.3109/10408368009108725. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Blomhoff R, Jacobs DR., Jr Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535–9. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 25.Pompella A, Emdin M, Passino C, Paolicchi A. The significance of serum gamma-glutamyltransferase in cardiovascular diseases. Clin Chem Lab Med. 2004;42:1085–91. doi: 10.1515/CCLM.2004.224. [DOI] [PubMed] [Google Scholar]
- 26.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. Gamma-glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–7. doi: 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 27.Simão AN, Dichi JB, Barbosa DS, Cecchini R, Dichi I. Influence of uric acid and gamma-glutamyltransferase on total antioxidant capacity and oxidative stress in patients with metabolic syndrome. Nutrition. 2008;24:675–81. doi: 10.1016/j.nut.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Kasapoglu B, Turkay C, Bayram Y, Koca C. Role of GGT in diagnosis of metabolic syndrome: A clinic-based cross-sectional survey. Indian J Med Res. 2010;132:56–61. [PubMed] [Google Scholar]
- 29.Mäkinen PL, Mäkinen KK. Gamma-glutamyltransferase from the outer cell envelope of Treponema denticola ATCC 35405. Infect Immun. 1997;65:685–91. doi: 10.1128/iai.65.2.685-691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher MA, Taylor GW, West BT, McCarthy ET. Bidirectional relationship between chronic kidney and periodontal disease: A study using structural equation modeling. Kidney Int. 2011;79:347–55. doi: 10.1038/ki.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorn BR, Dunn WA, Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–8. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 34.Das K, Samanta L, Chainy GB. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys. 2000;37:201–4. [Google Scholar]
- 35.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 36.Canakci CF, Cicek Y, Yildirim A, Sezer U, Canakci V. Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur J Dent. 2009;3:100–6. [PMC free article] [PubMed] [Google Scholar]
- 37.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes VM, Teles R, Trivedi HM, Devizio W, Xu T, Mitchell MW, et al. Acceleration of purine degradation by periodontal diseases. J Dent Res. 2009;88:851–5. doi: 10.1177/0022034509341967. [DOI] [PubMed] [Google Scholar]
- 39.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 40.Puertollano MA, Puertollano E, de Cienfuegos GÁ, de Pablo MA. Dietary antioxidants: Immunity and host defense. Curr Top Med Chem. 2011;11:1752–66. doi: 10.2174/156802611796235107. [DOI] [PubMed] [Google Scholar]
- 41.Said NS, Hadoud KM, Nada WM, Tarhouny SA. Superoxide dismutase, glutathione peroxidase and Vitamin E in patients with diabetic retinopathy. Life Sci J. 2013;10:1851–6. [Google Scholar]
- 42.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thangaswamy S, Bridenbaugh EA, Gashev AA. Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol. 2012;10:53–62. doi: 10.1089/lrb.2011.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redón J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096–101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 45.Mathur A, Mathur L, Manohar B, Mathur H, Shankarapillai R, Shetty N, et al. Antioxidant therapy as monotherapy or as an adjunct to treatment of periodontal diseases. J Indian Soc Periodontol. 2013;17:21–4. doi: 10.4103/0972-124X.107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR, Jr, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004;37:1018–23. doi: 10.1016/j.freeradbiomed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Mokondjimobe E, Longo-Mbenza B, Akiana J, Ndalla UO, Dossou-Yovo R, Mboussa J, et al. Biomarkers of oxidative stress and personalized treatment of pulmonary tuberculosis: Emerging role of gamma-glutamyltransferase. Adv Pharmacol Sci 2012. 2012 doi: 10.1155/2012/465634. 465634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdul-Rasheed OF, Al-Shamma GA, Zillo BH. Serum γ-glutamyltransferase as Oxidative Stress Marker in Pre-and Postmenopausal Iraqi Women. Oman Med J. 2010;25:286–8. doi: 10.5001/omj.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yavuz BB, Yavuz B, Halil M, Cankurtaran M, Ulger Z, Cankurtaran ES, et al. Serum elevated gamma glutamyltransferase levels may be a marker for oxidative stress in Alzheimer's disease. Int Psychogeriatr. 2008;20:815–23. doi: 10.1017/S1041610208006790. [DOI] [PubMed] [Google Scholar]
- 50.Karakurt O, Cagirci G, Eryasar NE. Gamma-glutamyl transferase activity increases in prehypertensive patients. Turk J Med Sci. 2011;41:975–80. [Google Scholar]
- 51.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 52.Paolicchi A, Sotiropuolou M, Perego P, Daubeuf S, Visvikis A, Lorenzini E, et al. Gamma-glutamyl transpeptidase catalyses the extracellular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney. Eur J Cancer. 2003;39:996–1003. doi: 10.1016/s0959-8049(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 53.Todorovic T, Dozic I, Vicente-Barrero M, Ljuskovic B, Pejovic J, Marjanovic M, et al. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. 2006;11:E115–9. [PubMed] [Google Scholar]
- 54.Dabra S, China K, Kaushik A. Salivary enzymes as diagnostic markers for detection of gingival/periodontal disease and their correlation with the severity of the disease. J Indian Soc Periodontol. 2012;16:358–64. doi: 10.4103/0972-124X.100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–58. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171–5. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 57.Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–6. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]


