Abstract
Purpose: To establish a new mouse model for wound healing studies on hemophilia A. Methods: Total 54 male mice with different genotypes including wild-type nude mice, heterozygous mice (FVIII-/-/Nu) and FVIII deficient mice (FVIII-/-) were generated and verified by PCR. Mice were subjected to wound healing research by making a 5 mm-thickness wound on mice skin and applying recombinant human epidermal growth factor (EGF, 10 μg/g) ointment, FVIII ointment (30 IU) or the ointment base to heal the wounds. Furthermore, keratinocytes were isolated from these newborn mice and subjected to migration assay by stimulation of EGF (ng/ml), insulin (10 μM) or vehicle. Results: A new hemophilic mouse model (FVIII-/-/Nu) was constructed successfully after genotyping verified by PCR. Compared to FVIII-/- mice, FVIII-/-/Nu and Nu mice showed greater degree of wound contraction and loss of the crust. Topical treatment with EGF exhibited faster wound healing than FVIII and ointment base. Insulin treatment showed more increased migration distance than treated with EGF or vehicle. FVIII-/-/Nu mice showed greater migration than FVIII-/- and Nu mice. Conclusions: A new mouse model (FVIII-/-/Nu) for wound healing in hemophilia A was constructed, and topical treatment of insulin may be a better therapy than EGF for healing wounds in hemophilia A.
Keywords: Wound healing, keratinocytes, recombinant human epidermal growth factor, insulin
Introduction
Hemophilia A in humans is a genetic disorder caused by a variety of mutations in clotting factor VIII (FVIII) gene located on the X chromosome, and it usually causes prolonged bleeding after injuries, tooth extractions, surgery and delayed or recurrent bleeding prior to complete wound healing [1]. It has been demonstrated that wound healing in hemophilic animals is slower than that in wild type animals, and many studies on wound healing have been focused on hemophilia B [2,3]. Generally, wound healing consists of five continuous, overlapping and precisely programmed phases in a precise and regulated manner, and these phases include hemostasis, inflammatory phase, proliferative phase, remodeling and scar formation, and scar maturation [4-6]. Wounds that exhibit impaired healing, including delayed acute wounds and chronic wounds, generally have failed to progress through the normal stage of healing [5].
In recent years animal models of hemophilia and other related diseases are significant for investigating novel treatments and to understand the pathophysiology of bleeding disorders in humans hemophilia [7]. Many models of hemophilia A have been established by animals (dogs, rats or sheep) with spontaneous mutations, or by targeted gene knock-out in mice [8-11], and among these models, rat model is most available for researching hemophilia A. In addition, even though impaired wound healing exists in hemophilic patients, animal models suitable for wound healing in hemophilic patients have been only reported on research of hemophilia B [12-14], and rare report on animal models of wounding healing in hemophilia A has been reported.
In the current study, to construct a new mice model (FVIII-/-/Nu), cross-mating was performed with wide-type nude mice and FVIII deficient (FVIII-/-) mice and genotypes of these mice were verified by PCR. Then mice were subjected a 5 mm-thickness wound on mice skin and treated with recombinant human epidermal growth factor (EGF) ointment, FVIII ointment or ointment base to heal the wounds. Additionally, primary keratinocytes were isolated and migration assay with stimulation by EGF, insulin or vehicle was analyzed.
Materials and methods
Mouse lines
Adult wild-type nude (BALB/cASlac-nu) mice, FVIII deficient (129S4-F8tm1Kaz/J, FVIII-/-) mice and heterozygous mice (FVIII-/-/Nu), all aged 6-10 weeks, weighted 25 ± 2 g were used in this study. Nude mice and FVIII-/- mice with bleeding disorders were purchased from Slac Laboratory Animal (Shanghai, China) and Jackson Laboratory (USA), respectively. FVIII-/-/Nu mice were generated in our laboratory by cross-mating with five female nude mice and five male FVIII-/- mice. Offspring obtained by mating were self-bred to produce mice with different genotypes, including wild-type nude mice, FVIII-/- mice and FVIII-/-/Nu mice. Total 54 male mice (6-10 weeks, 25 ± 2 g) were divided into three groups in average according to different genotypes in this study. Mice were housed in standard sterile cages and maintained under a temperature (22 ± 1°C) and light/dark (LD 12:12) environment with free access to food and water.
Animal care and experimental procedures were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University School of Pharmacy (SYXK-2007-0025).
DNA extraction and polymerase chain reaction (PCR)
Mouse DNA was extracted from tail biopsies as briefly outlined below. The last 1 to 3 mm of the mice tail was obtained and placed directly into an eppendorf tube. Total 50 μl digestion buffer (1X GB, 1% b-mercaptoethanol, 0.5% Triton X-100) was added to the tube and incubated at 95°C for 5 min. Proteinase K was added at a final concentration of 1 mg/ml. After incubation at 55°C for 1 hr, vortex vigorously for about 20 s, and incubation at 55°C for 1 hr, the mixture was heat-denatured at 95°C for 5 min. The tubes were then spun to pellet the undigested stuff, and 1 μl supernatant was taken for PCR reaction to confirm the successful genotype.
The primers used for PCR were designed according to JAX Mice Database Genotyping Protocols (JAX Mice Database, The Jackson Laboratory, Bar Harbor, ME). The primer sequences were as follows: oIMR1752 (5’-TGT GTC CCG CCC CTT CCT TT-3’), oIMR1753 (5’-TGC AAG GCC TGG GCT TAT TT-3’), and oIMR1754 (5’-GAG CAA ATT CCT GTA CTG AC-3’). PCR was performed with initial denaturation at 94°C for 3 min, then 30 cycles of amplification (98°C for 30 s, 61.2°C for 30 s, 68°C for 1 min), and a final extension at 68°C for 2 min.
Different treatment in full thickness wound model
Mice in each group were anaesthetized with 0.4% sodium pentobarbital (1 ml/100 g body weight; i.p.). The dorsal hair of mice was shaved and removed. A full-thickness skin was excised using a 5-mm biopsy punch. The FVIII ointment (Shanghai RAAS Blood products Co., Ltd, Shanghai, China), the EGF ointment (Hao Hai Healthcare, Shanghai, China) or the ointment base (control) was administered topically to animals daily for 9 days starting on the day of the incision. The FVIII ointment dose (30 IU) and EGF dose (10 μg/g) was according to the instructions of the manufacturer and a previous report [15]. The ointment base was comprised of 80% vaseline and 20% liquid paraffin. Mice were housed individually during the whole experimental period. The wound size was recorded daily in 2 dimensions, and the percentage of wound closure was also calculated from wound surface areas obtained from wound measurements.
Primary keratinocyte isolation
Primary keratinocytes were isolated from the skin of newborn mice as described previously [16]. Briefly, the whole skin of the mice was dissected, treated with cold, sterile 0.25% trypsin solution and incubated at 4°C for 24 hr. The epidermis was separated from the dermis with sterile forceps, minced and suspended in 10 ml of growth medium. The suspension was shaken firmly to release keratinocytes, and then passed through a sterile 70 μm nylon filter to remove cornified sheets. The entire 10 ml of keratinocyte suspension obtained from each skin was plated onto 10 cm tissue culture dish coated with 30 μg/ml denatured rat tail collagen. Mouse keratinocytes were cultured until reaching > 95% confluence. The keratinocytes were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C, 5% CO2, and sub-cultured twice every 4-6 days. The keratinocytes obtained were further subjected to migration assay in vitro. In this study, keratinocytes were isolated from 3 neonates in each group, and cells which exhibited better growth were used for the experiment. The isolation experiment was performed in triplicates.
Migration assay
Primary keratinocytes were plated onto culture dishes, and scratch wounds were made reaching certain confluence. Keratinocytes were starved for 24 hr before the migration assay. Total 1 ml pipette tip was used to scratch the cells on a 6-well plate and then the edges of the scratches were marked. Cell migration was measured at a given times by calculating the distance from the initial cell edge to the edge of the migrating cells.
Effect of insulin and EGF on keratinocyte migration
To study the effects of insulin and EGF on keratinocytes migration, cells were treated with 10 μM insulin, 40 ng/ml EGF or vehicle after marking positions of scratch wounds. Besides, to remove the dependent influence of cell proliferation on keratinocyte migration, cells were treated with 10 μg/ml mitomycin C, cell proliferation inhibitor, for 2 hr, followed by treatment with the same dose of insulin or EGF. After that, cell migration was monitored by microscopy at 0, 20 and 40 hr after treatment from the initial edge to the new edge of the cells, and migration distance at 20 and 40 hr was quantified.
Statistical analysis
Statistical analysis was performed using the GraphPad 5.0 Prism Pocket Programme (GraphPad Software lnc., San Diego, CA, USA). Data were converted to percentages of scatter plot.
Results
Mouse genotyping identification
Hemophilic mice (FVIII-/-) were produced originally by disruption of FVIII gene through replacing 7 bp at the 3’ end of exon 16 and 286 bp at the 5’ end of intron 16 with a neomycin resistance cassette (Figure 1A). RNA and protein assays indicated that the mutated allele produced protein with levels of activity less than 1% of wild-type levels. PCR products of FVIII-/- mice, wild-type nude mice and FVIII-/-/Nu mice DNA were 420 bp, 620 bp and 420 bp & 620 bp, respectively (Figure 1B).
Figure 1.
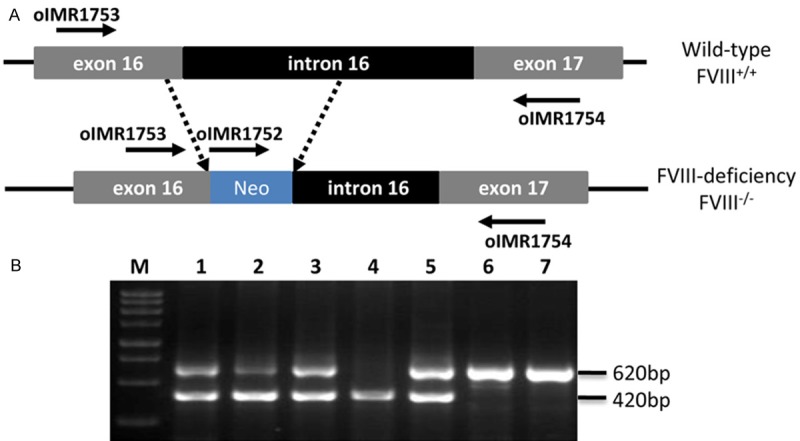
PCR results of mice genotyping. A. Scheme for replacing of exon 16 and intron 16 by neo cassette. B. Gel analysis of PCR products. Lane M: DNA marker; Lane 1, 2, 3, and 5: FVIII-/-/Nu mice; Lane 4: FVIII-/- mice; Lane 6 and 7: Nude mice.
Wound healing in hemophilia A mice
There was no difference of the wound size among all the mice under the same treatment at the beginning of post injury, and the differences became obvious on the subsequent 9 days (Figure 2A-C). Compared with FVIII-/- mice, FVIII-/-/Nu and Nu mice showed greater degree of wound contraction and loss of the crust, and the wound was completely healed on day 9.
Figure 2.
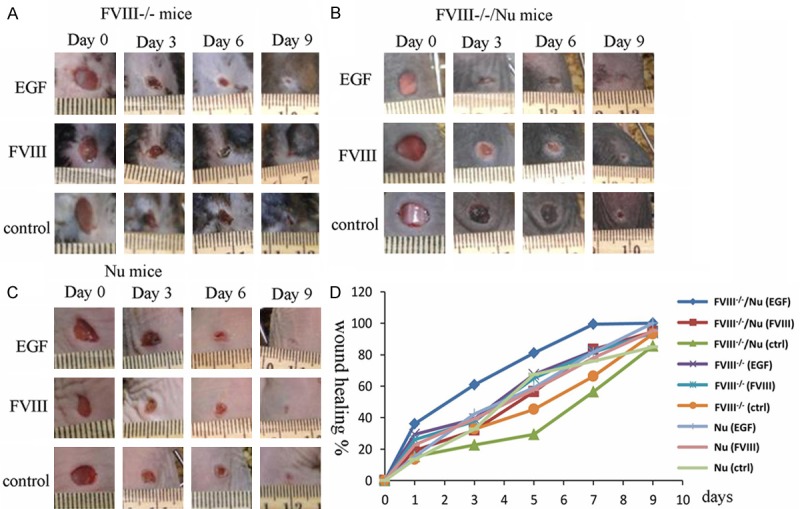
The results of wound healing with EGF (10 μg/g), FVIII (30 IU) or control (ointment base) treated in FVIII-/- mice (A), FVIII-/-/Nu mice (B and C) Nu mice group. (D) Representative statistics showed comparisons among EGF, FVIII and ointment base treatment. Wound healing was expressed as the percentage of the original wound.
Additionally, there was no difference between the EGF treatment and control during the first 3 postoperative days (Figure 2D). However, the EGF treated group showed significantly faster wound healing from the 4th day than of the other two groups. The group treated with FVIII healed faster than control group but slower than treated with EGF group. Moreover, FVIII-/-/Nu mice exhibited greater wound healing speed during healing period than FVIII-/- mice.
Effects of insulin and EGF on the keratinocytes migration
Keratinocyte migration distance was time-dependent in three treatment groups (Figure 3A). Besides, keratinocytes migration distance increased in insulin treated group than that in EGF treated and vehicle treated group. Even though proliferation was obviously halted, migration was still taking place in the same manner. Among the three treatment groups, keratinocytes migration distance in FVIII-/-/Nu mice was higher than that in FVIII-/- and Nu mice after (Figure 3B).
Figure 3.
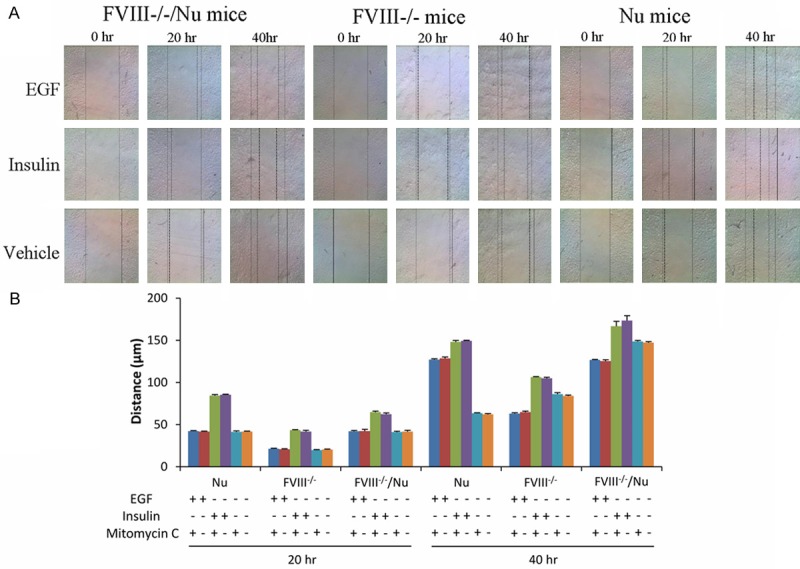
Effects of insulin (10 μM), EGF (40 ng/ml) or vehicle on keratinocytes migration in different mice after scratch wounding of 0 hr, 20 hr and 40 hr (A). The dotted line in the scratched area represents the limit of cell migration. The continuous line indicates the border of the scratched area. (B) Quantitative expression of the effects of insulin, EGF and mitomycin C on the keratinocytes migration. “+” treated in the groups, “-” without treatment.
Discussion
In the current study, a new mouse model (FVIII-/-/Nu) for wound healing in hemophilia A has been established. Besides, we found that EGF could accelerate wound healing in hemophilic mice than treatment by FVIII. Keratinocytes migration was time-dependent and insulin stimulation could increase migration distance over those treated with EGF and vehicle. Furthermore, compared with FVIII-/- mice, FVIII-/-/Nu mice exhibited greater healing speed of wounds during healing period and keratinocyte migration with stimulation of insulin or EGF.
Animal models of wound healing in hemophilia are in large part responsible for understanding of wound healing in hemophilia and calculating its treatment [10]. However, due to the differences between rat skin and human skin, contraction is the predominant form of wound healing in mice [17]. In addition, researchers have reported decrease in the overall healing time of rat wounds due to more rapid wound contraction than epithelialization [18-20]. In our study, compared with FVIII-/- mice, FVIII-/-/Nu mice showed greater degree of wound contraction and loss of the crust, and faster speed of wound healing during healing period. Thus, FVIII-/-/Nu mice model may be more suitable for evaluating therapeutics for wound healing in hemophilia A.
It has been reported that the EGF treatments in wound healing have the efficacy of accelerating wound healing in normal and diabetic rats [21,22]. In particular, the topical application of EGF (10 μg/g) could accelerate the healing of open wounds in a full thickness wound mouse model by boosting myofibroblast proliferation and collagen synthesis [15]. Besides, the epidermis and dermis-mediated events have been proved particular sensitivity to a single EGF dose, which revealed a dose-dependent manner of EGF in treatment wound healing in rats [23]. In our study, we also demonstrated that a topical treatment with EGF ointment (10 μg/g) exhibited faster wound healing than topical FVIII treatment and ointment base.
As an essential feature of wound healing, re-epithelialization is depended on two basic functions of keratinocytes: migration and proliferation [24]. Keratinocytes migration is an important step in skin re-epithelialization which is an essential feature of wounds healing [25]. It has been reported that when topically applied to skin excision wounds, insulin could accelerate re-epithelialization, stimulate keratinocyte migration and promote the maturation of the healing tissue in a time-dependent and dose-dependent manner [26]. Additionally, topical application of small dose of insulin (1 U) could accelerate burn wound healing and insulin may stimulate EGF expression in wound marginal keratinocytes in scalded rats [27]. Here, our study verified the stronger role of insulin (10 μM) stimulation in the longer keratinocytes migration distance than EGF (40 ng/ml). Thereby, a topical treatment of insulin appeared to be a better therapy than EGF for healing wounds in hemophilia A.
Our study still has some limitations. First, the dose-dependent effect of EGF, FVIII ointment and insulin which are important parameters for determination of their role on wound healing was not performed. Second, topical application of FVIII ointment was only applied on, and intravenous administration of FVIII was not made.
In conclusion, a new mouse model (FVIII-/-/Nu) for wound healing in hemophilia A was constructed, and topical treatment of insulin may be a better therapy than EGF for healing wounds in hemophilia A. Findings of this study may provide new insights for wound healing studies in hemophilia.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81201769 and 81373319) and Shanghai Government Science and Technology Funding (10431903900).
Disclosure of conflict of interest
None.
References
- 1.Booth CJ, Brooks MB, Rockwell S, Murphy JW, Rinder HM, Zelterman D, Paidas MJ, Compton SR, Marks PW. WAG-F8(m1Ycb) rats harboring a factor VIII gene mutation provide a new animal model for hemophilia A. J Thromb Haemost. 2010;8:2472–2477. doi: 10.1111/j.1538-7836.2010.03978.x. [DOI] [PubMed] [Google Scholar]
- 2.Monroe DM, Mackman N, Hoffman M. Wound healing in hemophilia B mice and low tissue factor mice. Thromb Res. 2010;125:S74–S77. doi: 10.1016/j.thromres.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman M, Harger A, Lenkowski A, Hedner U, Roberts HR, Monroe DM. Cutaneous wound healing is impaired in hemophilia B. Blood. 2006;108:3053–3060. doi: 10.1182/blood-2006-05-020495. [DOI] [PubMed] [Google Scholar]
- 4.Enoch S, Leaper DJ. Basic science of wound healing. Surgery (Oxford) 2007;26:31–37. [Google Scholar]
- 5.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Lozier JN, Nichols TC. Animal Models of Hemophilia and Related Bleeding Disorders. Semin Hematol. 2013;50:175–184. doi: 10.1053/j.seminhematol.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingdon HS, Hassell TM. Hemophilic dog model for evaluating therapeutic effectiveness of plasma protein fractions. Blood. 1981;58:868–872. [PubMed] [Google Scholar]
- 9.Porada C, Sanada C, Long C, Wood J, Desai J, Frederick N, Millsap L, Bormann C, Menges S, Hanna C. Clinical and molecular characterization of a re-established line of sheep exhibiting hemophilia A. J Thromb Haemost. 2010;8:276–285. doi: 10.1111/j.1538-7836.2009.03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth C, Brooks M, Rockwell S, Murphy J, Rinder H, Zelterman D, Paidas M, Compton S, Marks P. WAG-F8m1Ycb rats harboring a factor VIII gene mutation provide a new animal model for hemophilia A. J Thromb Haemost. 2010;8:2472–2477. doi: 10.1111/j.1538-7836.2010.03978.x. [DOI] [PubMed] [Google Scholar]
- 11.Monahan P. The expanding menagerie: animal models of hemophilia A. J Thromb Haemost. 2010;8:2469–2471. doi: 10.1111/j.1538-7836.2010.04053.x. [DOI] [PubMed] [Google Scholar]
- 12.Monroe D, Hoffman M, Roberts H, Hedner U. Progressive improvement in wound healing with increased therapy in haemophilia B mice. Haemophilia. 2013;19:926–932. doi: 10.1111/hae.12220. [DOI] [PubMed] [Google Scholar]
- 13.McDonald AG, Yang K, Roberts HR, Monroe DM, Hoffman M. Perivascular tissue factor is down-regulated following cutaneous wounding: implications for bleeding in hemophilia. Blood. 2008;111:2046–2048. doi: 10.1182/blood-2007-05-092916. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman M, Monroe DM. Low intensity laser therapy speeds wound healing in hemophilia by enhancing platelet procoagulant activity. Wound Repair Regen. 2012;20:770–777. doi: 10.1111/j.1524-475X.2012.00828.x. [DOI] [PubMed] [Google Scholar]
- 15.Kwon YB, Kim HW, Roh DH, Yoon SY, Baek RM, Kim JY, Kweon H, Lee KG, Park YH, Lee JH. Topical application of epidermal growth factor accelerates wound healing by myofibroblast proliferation and collagen synthesis in rat. J Vet Sci. 2006;7:105–109. doi: 10.4142/jvs.2006.7.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodivala-Dilke K. Primary Mouse Keratinocyte Culture. In: Wise C, editor. Epithelial Cell Culture Protocols. Humana Press; 2002. pp. 139–144. [DOI] [PubMed] [Google Scholar]
- 17.Greenhalgh DG. Models of wound healing. J Burn Care Res. 2005;26:293–305. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]
- 18.Dorsett-Martin WA. Rat models of skin wound healing: a review. Wound Repair Regen. 2004;12:591–599. doi: 10.1111/j.1067-1927.2004.12601.x. [DOI] [PubMed] [Google Scholar]
- 19.Truong AT, Kowal-Vern A, Latenser BA, Wiley DE, Walter RJ. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J Burns Wounds. 2005;4:e4. [PMC free article] [PubMed] [Google Scholar]
- 20.Moulin V, Auger FA, Garrel D, Germain L. Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns. 2000;26:3–12. doi: 10.1016/s0305-4179(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 21.Dogan S, Demirer S, Kepenekci I, Erkek B, Kiziltay A, Hasirci N, Muftuoglu S, Nazikoglu A, Renda N, Dincer UD, Elhan A, Kuterdem E. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int Wound J. 2009;6:107–115. doi: 10.1111/j.1742-481X.2009.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong JP, Park SW. The combined effect of recombinant human epidermal growth factor and erythropoietin on full-thickness wound healing in diabetic rat model. Int Wound J. 2014;11:373–378. doi: 10.1111/j.1742-481X.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlanga J, Moreira E, Perez L, Boix E, Gonzalez T, Lopez-Saura P. Wound healing promotion in rats treated with EGF is dose dependent. Biotecnología Aplicada. 1996;13:181–185. [Google Scholar]
- 24.Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 25.Kirfel G, Herzog V. Migration of epidermal keratinocytes: mechanisms, regulation, and biological significance. Protoplasma. 2004;223:67–78. doi: 10.1007/s00709-003-0031-5. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Petreaca M, Yao M, Martins-Green M. Cell and molecular mechanisms of keratinocyte function stimulated by insulin during wound healing. BMC Cell Biol. 2009;10:1–15. doi: 10.1186/1471-2121-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhang X, Zhang Z, Fang P, Xu W. [Effects of topical application of insulin on the wound healing in scalded rats] . Zhonghua Shao Shang Za Zhi. 2004;20:98–101. [PubMed] [Google Scholar]


