Abstract
Options for the treatment of cartilage defects include chondral resurfacing with abrasion, debridement, autologous chondrocyte transplantation (ACT), matrix-induced chondrocyte transplantation (MACI), or osteochondral autologous transplantation (OATS). This article describes the new method of autologous matrix-induced chondrogenesis (AMIC), a 1-step procedure combining subchondral microfracture with the fixation of a collagen I/III membrane by a partially autologous fibrin glue. Indications and contraindications are provided; a technical note is given. This method is primarily applied in osteochondral lesions of the knee and ankle joints; other joints may qualify.
Keywords: autologous cartilage repair, AMIC, microfracturing
Introduction
Among the cartilage resurfacing procedures currently applied are abrasion arthroplasty and debridement. Britberg introduced the autologous chondrocyte transplantation (ACT), which involves a 2-step procedure transplanting chondrocytes previously taken from a donor site into a cartilage defect.1 Steadman developed the method of microfracturing in the 1990s. The calcified subchondral bone/cartilage border is perforated; consecutively fibrous cartilage is developed from mesenchymal stem cells.2-4
The matrix-associated chondrogenesis is a further development in cartilage resurfacing, which allows mesenchymal stem cells (MSCs) to develop into chondrocytes with the aid of a collagen scaffold.5,6 The matrix-induced autologous chondrocyte transplantation (MACI) combines this matrix technology with the ACT. A prospective randomized study by Bartlett et al. yielded similar results in both techniques.5 As the MACI method is even more expensive than the regular ACT, and both are not reimbursed by many health insurance companies in Europe, a more economic method was searched to perform cost-effective joint resurfacing.
The autologous matrix-induced chondrogenesis (AMIC) as first introduced by Behrens6-9 further develops the scaffold technique in combination with microfracturing. It is a 1-step procedure that involves microfracturing of the debrided cartilage lesion and a commercially available collagen I/III matrix for covering the blood clot and its MSCs. Fixation is with partial autologous fibrin glue in which the thrombin part is yielded from the patient’s serum. Fixation by sutures as performed with the MACI may lead to cartilage damage.6 This problem may be solved by gluing techniques.10 It should be noted that commercially available fibrin glue seems to act as an additional scaffold for chondrocytes and that its contents in TGF β are considerable, possibly helping in the differentiation of MSCs.8
The objective of AMIC is resurfacing of articular cartilage defects to restore a pain-free joint with physiological range of motion. The indications of AMIC are symptomatic full-thickness chondral and subchondral defects in the major joints, maximum size of 1.5 cm2, posttraumatic or osteochondrosis dissecans, and location in the main weightbearing area of the joint or maximum area of pain. The contraindications of AMIC are inflammatory diseases (e.g., rheumatoid arthritis), tumor, associated fracture, multiple lesions, noncompliant patient, extension deficit of more than 10°, flexion deficit of less than 100°, untreated meniscectomy without meniscal replacement, malalignment, and uncorrected ligament deficiency.
Preoperative Diagnostic Steps
After the patient’s history and physical examination are taken, standard weightbearing anteroposterior and lateral X-rays are performed. A posteroanterior weightbearing notch view of the knee may also be added to the preoperative work-up. If the knee is affected, a full-standing X-ray of both legs is taken to evaluate the axis, and an axial X-ray of the patella is taken. T1- and T2-weighted magnetic resonance imaging scans of the joint are taken to evaluate accompanying injuries and to gain a preoperative status of the cruciate ligaments and the cartilage.
Patient Information
Common surgical risks include postoperative infection, thrombosis, hemorrhage, delayed wound healing, partial weightbearing and limited range of motion for a minimum of 6 weeks, postoperative rehabilitation, alternative procedures, and self-limiting of cartilage lesions. The patient should be aware and agree that an arthrotomy will be performed.
Surgical Technique
The procedure may be performed in spinal or general anesthesia. The patient should be in a supine position. A tourniquet is advised for better views.
The procedure starts with an arthroscopy of the affected joint to verify the size and location of the defect and also the amount of accompanying disorder. If the size is located and an AMIC procedure is recommended, a minimally invasive arthrotomy is performed,11 and the defect is visualized openly (Fig. 1). The defective cartilage tissue and subchondral bone are removed with a curette. Microfracturing is performed with the corresponding microfracturing probes (Fig. 2). The size of the defect is evaluated, and a collagen membrane is measured. It should be slightly undersized to avoid dislocation after movement. An aluminum template may be used to determine the size of the lesion. For preparation of the fibrin glue, several options exist: a partially autologous fibrin glue may be manufactured by centrifuging a blood sample from the patient and mixing the yielded thrombin with allogenic fibrinogen. Completely allogenic fibrin glue as commercially available or suturing for fixation may also be performed. The partially autologous fibrin glue is the authors’ fixation method of choice. Suturing may also be performed but may lead to cartilage damage.6 Knecht et al. have confirmed that gluing with fibrin glue leads to sufficient stability.10 It should be kept in mind that according to the original method, the collagen membrane should be placed slightly below the cartilage level to avoid displacement. If the joint is gently moved intraoperatively, inspection of the site usually yields that the membrane remains in place. The membrane is attached to cover the defect (Figs. 3 and 4).
Figure 1.
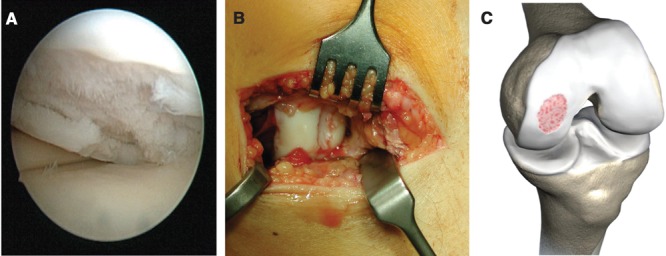
The defect is clearly visible on the medial left femoral condyle. The arthroscopic view (A) demonstrates a grade 4 lesion according to the Outerbridge classification. This is confirmed by a mini open arthrotomy (B). (C) Reproduced with kind permission by Geistlich (Wolhusen, Switzerland).
Figure 2.
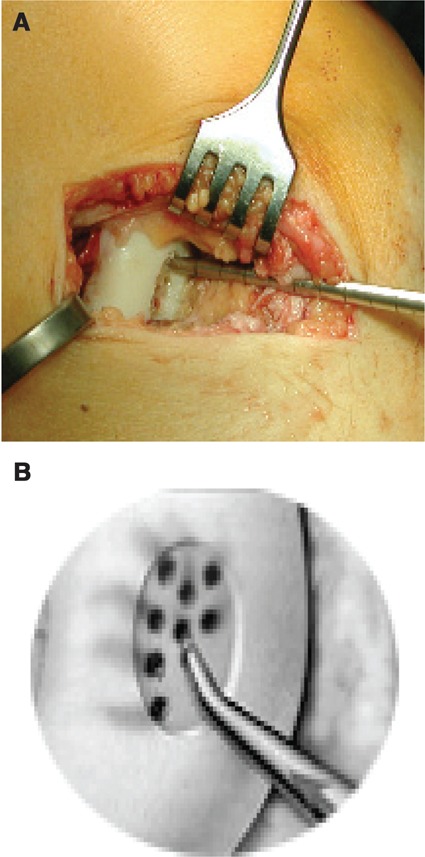
(A) After curettage of the lesion, microfracturing may be performed. A blood clot should form. (B) Reproduced with kind permission by Geistlich (Wolhusen, Switzerland).
Figure 3.
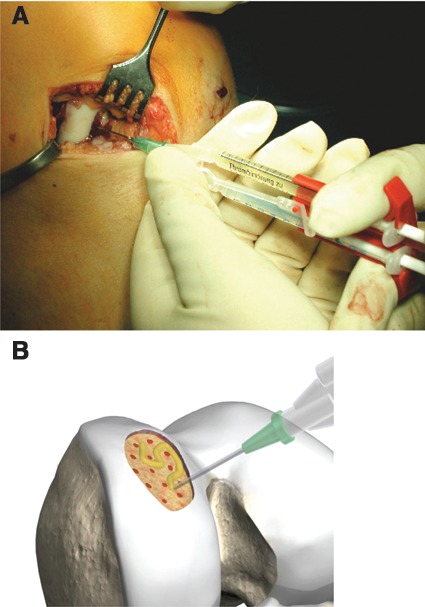
(A) The collagen membrane is fixed with partially autologous fibrin glue, which is the original AMIC method. Allogenic fibrin glue may also be applied for fixation. (B) Reproduced with kind permission by Geistlich (Wolhusen, Switzerland).
Figure 4.
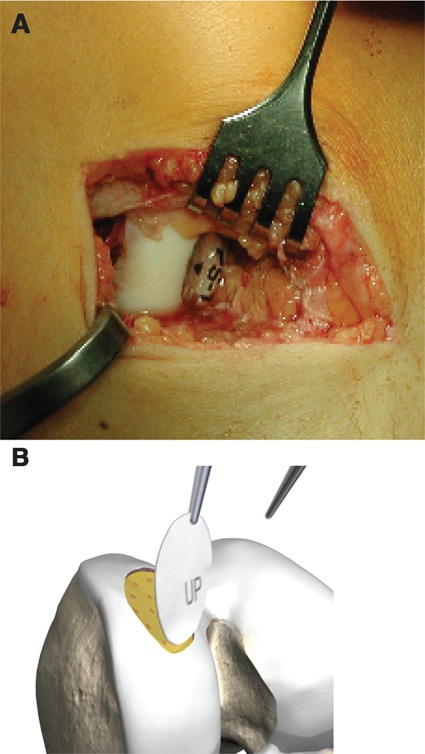
(A) The collagen membrane is definitely fixed with partially autologous fibrin glue. (B) Reproduced with kind permission by Geistlich (Wolhusen, Switzerland).
The postoperative management is as follows: maximum weightbearing of 15 to 20 kg for 6 weeks, and common thrombose prophylaxis with a low molecular heparine. Removal of a possible drain 24 hours after surgery is recommended. Care should be exercised while the suction is removed, as membrane dislocation could result.
Maximum flexion of the knee should not exceed 30° for 2 weeks following the operation. Assisted active physical therapy may be applied, minding the weight and range of motion limits. Isometric quadriceps training, straight length raising, and hamstring isometrics are possible.
Flexion is raised to 60° and 90° for another 2 weeks, respectively. The patient is seen in the outpatient clinic 6 weeks after the appointment. Postoperative physical therapy should focus on muscle strengthening exercises as well as progressively increased joint mobilization. After 6 weeks, progressive exercising is possible with light biking in a slight resistance setting.
Combining microfracturing with a collagen I/III scaffold may be a cost-effective option to resurface cartilage defects. Prospective studies should compare the results of the AMIC procedure as compared to microfracturing alone and MACI.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Funding: The authors received no financial support for the research and/or authorship of this article.
References
- 1. Britberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39 Suppl:S40-9. [DOI] [PubMed] [Google Scholar]
- 2. Mithoefer K, Wiliams RJ, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-20. [DOI] [PubMed] [Google Scholar]
- 3. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MM, Gill TJ, Rodhen WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;15(5):477-84. [DOI] [PubMed] [Google Scholar]
- 4. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391 Suppl:S362-9. [DOI] [PubMed] [Google Scholar]
- 5. Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective randomized study. J Bone Joint Surg Br. 2005;87(B):640-5. [DOI] [PubMed] [Google Scholar]
- 6. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation: a 5-year follow-up. Knee. 2006;13(3):194-202. [DOI] [PubMed] [Google Scholar]
- 7. Gille J, Ehlers EM, Okroi M, Ruslies M, Behrens P. Apoptotic chondrocyte death in cell matrix biocomposites used in autologous chondrocyte transplantation. Annals Anat. 2002;184(4):317-23. [DOI] [PubMed] [Google Scholar]
- 8. Gille J, Meisner U, Ehlers EM, Mueller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005;37:339-48. [DOI] [PubMed] [Google Scholar]
- 9. Kramer J, Boehrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix guided human mesenchymal stem cells. Cell Mol Life Sci. 2006;6(5):616-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stuessi E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res B Appl Biomater. 2007;83(1):50-7. [DOI] [PubMed] [Google Scholar]
- 11. Anders S, Schaumburger J, Schubert T, Grifka J, Behrens P. Matrix-associated autologous chondrocyte transplantation: minimally invasive technique in the knee. Oper Orthop Traumatol. 2008;20(3):208-19. [DOI] [PubMed] [Google Scholar]


