Abstract
We have shown previously that the bleomycin (BLM) carbohydrate moiety can recapitulate the tumor cell targeting effects of the entire BLM molecule, that BLM itself is modular in nature consisting of a DNA-cleaving aglycone which is delivered selectively to the interior of tumor cells by its carbohydrate moiety, and that there are disaccharides structurally related to the BLM disaccharide which are more efficient than the natural disaccharide at tumor cell targeting/uptake. Because BLM sugars can deliver molecular cargoes selectively to tumor cells, and thus potentially form the basis for a novel antitumor strategy, it seemed important to consider additional structural features capable of affecting the efficiency of tumor cell recognition and delivery. These included the effects of sugar polyvalency and net charge (at physiological pH) on tumor cell recognition, internalization, and trafficking. Since these parameters have been shown to affect cell surface recognition, internalization, and distribution in other contexts, this study has sought to define the effects of these structural features on tumor cell recognition by bleomycin and its disaccharide. We demonstrate that both can have a significant effect on tumor cell binding/internalization, and present data which suggests that the metal ions normally bound by bleomycin following clinical administration may significantly contribute to the efficiency of tumor cell uptake, in addition to their characterized function in DNA cleavage. A BLM disaccharide-Cy5** conjugate incorporating the positively charged dipeptide d-Lys-d-Lys was found to associate with both the mitochondria and the nuclear envelope of DU145 cells, suggesting possible cellular targets for BLM disaccharide–cytotoxin conjugates.
The bleomycins (Figure 1) are glycopeptide-derived natural products,1 which were first identified as secondary metabolites from culture broths of Streptomyces verticillus following their isolation as Cu(II) chelates.1,2 The bleomycins were shown to possess significant anticancer activity,3,4 and some of the BLMs are now employed clinically as antitumor agents, both as single agents and in combination chemotherapy.5,6 Interestingly, it has also been found that radionuclide complexes of the bleomycins bind selectively to a variety of tumor cells,7−14 which likely contributes to their selectivity as antitumor agents. Redox-active metal complexes of the BLMs mediate oxidative degradation of DNA producing both single- and double-strand breaks,15−22 and the double-strand DNA breaks are believed to form the basis for the antitumor activity of BLM.23
Figure 1.
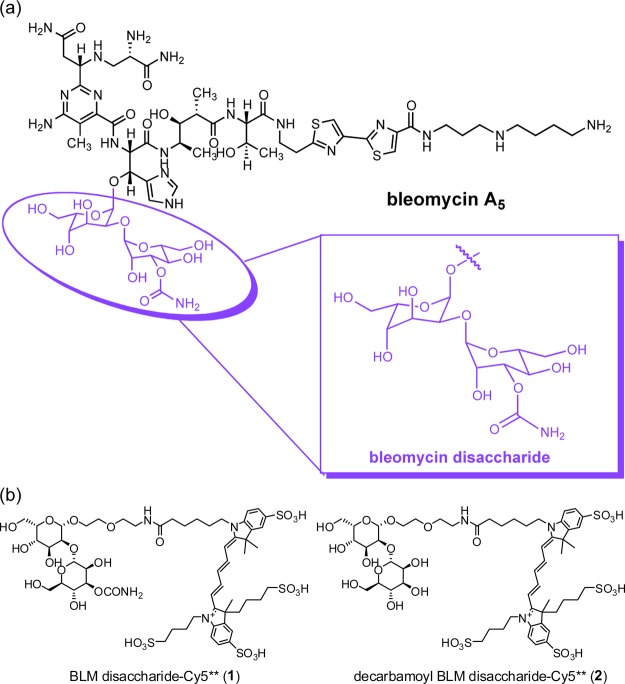
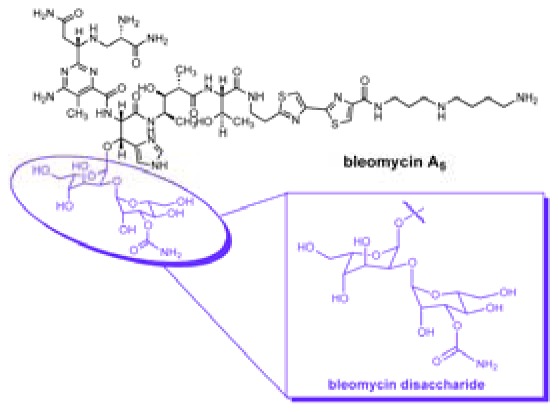
(a) Structure of BLM A5, the highlighted domain shows the BLM disaccharide. (b) Structures of BLM disaccharide-Cy5** (1) and decarbamoyl BLM disaccharide-Cy5** (2).
In recent studies, it has been shown that the tumor cell selectivity of the bleomycins resides in the disaccharide moiety, which consists of l-gulose and 3-O-carbamoyl-d-mannose subunits,24−26 and that systematic variation of the position and substitution of the carbamoyl moiety afforded disaccharide analogues with significantly improved cancer cell binding/uptake.27 The BLM disaccharide has been shown to be capable of delivering molecular cargoes to cancer cells, including the cytotoxic BLM aglycone28 and dyes such as the cyanine dye Cy5**.25,26 As part of an ongoing effort to develop a practical strategy to use a BLM saccharide as a delivery vehicle for other therapeutic agents, it seemed of interest to explore other aspects of this carrier molecule that might increase its efficiency for selective cargo delivery to tumor cells. In addition, we were also interested in defining the intracellular distribution of BLM–disaccharide conjugates. This information might be useful for designing conjugates that can deliver cargoes to specific intracellular sites.
Presently, we report that a cluster of three BLM disaccharides exhibited enhanced tumor cell targeting and uptake; such effects of polyvalency are typical of the involvement of one or more carbohydrate receptors on the surface of tumor cells. Additionally, it was found that the addition of Cu(II) to bleomycin A5-Cy5** significantly increased its efficiency of tumor cell binding/uptake, and that Fe(III)•BLM-Cy5**, Cu(II)•BLM-Cy5**, Mn(III)•BLM-Cy5**, and Zn(II)•BLM-Cy5** exhibited binding and uptake in four cultured cancer cell lines, but not in matched noncancer cell lines. It seemed likely that the enhanced binding/uptake was due to the positive charge associated with the chelated metal ions. While the BLM disaccharide lacks the intrinsic metal ion chelating properties of BLM itself, the introduction of two lysine residues (the side chains of which are positively charged at physiological pH) between the disaccharide and Cy5** moieties of the conjugate also resulted in a significant enhancement in tumor cell targeting and uptake. These findings have important implications for the design of tumor selective agents capable of delivering cytotoxic cargoes to tumor cells, and may also indicate a role for the metal constituent in tumor cell binding/uptake of metalloBLMs, as well as the long recognized role of redox-active metals in the activation of oxygenated metalloBLMs for DNA degradation by this class of drug.
Materials and Methods
Metallobleomycins
Fe(III)•BLM-Cy5**, Mn(III)•BLM-Cy5**, and Zn(II)•BLM-Cy5** were prepared by admixture of equimolar amounts of the respective metal ions to metal free BLM-Cy5**, the latter of which was synthesized starting from BLM A5 as described previously.25 Cu(II)•BLM-Cy5** is an intermediate in the synthesis of metal free BLM-Cy5**.25
Cell Growth Conditions
C6 (ATCC CCL-107) rat glioma cells, CTX TNA2 (ATCC CRL-2006) rat astrocytes, SW 1088 (ATCC HTB-12) human brain cancer cells, SVG p12 (ATCC CRL-8621) normal human brain cells, SW480 (ATCC CCL-228) human colon cancer cells, and CCD-112CoN (ATCC CRL-1541) normal human fetal colon cells were grown in MEM medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (HyClone) and 1% penicillin–streptomycin mix antibiotic supplement. A549 (ATCC CCL-185) human lung carcinoma cells, A498 (ATCC HTB-44) human kidney carcinoma, BxPC-3 (ATCC CRL-1687) human pancreatic adenocarcinoma, BT474 (ATCC HTB-20) human breast ductal carcinoma cells, and MCF-7 (ATCC HBT-22) human breast adenocarcinoma were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (HyClone, South Logan, UT) and 1% penicillin–streptomycin antibiotic mixture (Cellgro, Manassas, VA). MCF-10A (ATCC CRL-10317) human fibrocystic breast basal epithelial cells were grown in MEGM (Invitrogen, Grand Island, NY) medium supplemented with 100 ng/mL cholera toxin (Sigma-Aldrich) and 1% penicillin–streptomycin mix antibiotic supplement. DU145 (ATCC HTB-81) human prostate carcinoma, WI38 (ATCC CCL-75) human fetal lung fibroblasts, SW1783 (ATCC HTB-13) human grade III astrocytoma, PZ-HPV-7 (ATCC CRL-2221) human HPV-18 transformed prostate epithelium cells, and RWPE-1 (ATCC CRL-11609) human HPV-18 transfected prostate epithelium cells were grown in MEM medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (HyClone) and 1% penicillin–streptomycin mix antibiotic supplement. The cells were incubated at 37 °C under a humidified atmosphere of 5% CO2 and 95% air.
Fluorescence Microscopy
Fluorescence images were acquired using a Zeiss Axiovert 200M inverted microscope fitted with an AxioCam MRm camera equipped with a 300 W xenon lamp (Sutter, Novato, CA), and Cy5 cyanine filter (Chroma, Bellows Falls, VT). The cells were grown on 16-well Lab-Tek glass chamber slides at a cell density of 5000 cells/well (Thermo Scientific, Waltham, MA) at 37 °C for 48 h. Cells were rinsed twice with phosphate buffered saline (PBS) and the medium was replaced with RPMI 1640 (no phenol red) when the cell density reached 70% confluence. The cells were treated with the dye-labeled conjugates to the final desired concentration. Cell incubation was carried out at 37 °C for 1 h, then the cells were washed with PBS and fixed with 4% paraformaldehyde at 37 °C for 5 min. Finally, the slide was mounted with Prolong Antifade Gold reagent with DAPI (Invitrogen). All images were recorded and the target cells counted using a 40× oil objective. To ensure accurate intensity measurements, the exposure time and laser time were kept the same. Pixel intensity was quantified using AxioVision release 4.7 version software, and the mean pixel intensity was generated as gray level.
Results
Synthesis of Disaccharide–Dye Conjugates
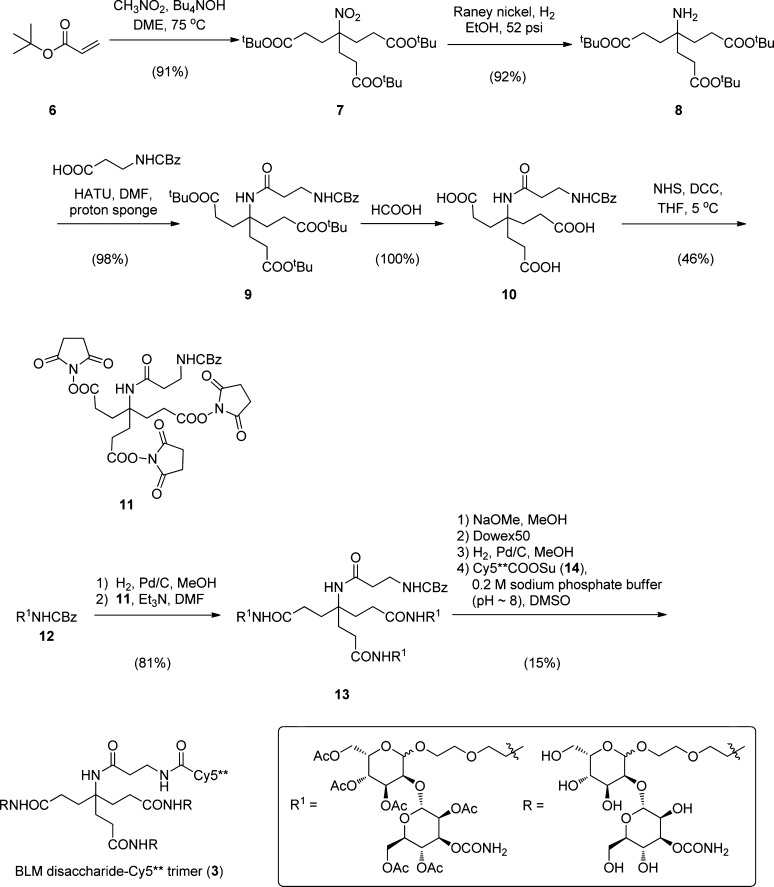
The method used for the preparation of the BLM disaccharide-Cy5** trimer (3) is outlined in Scheme 1. Key intermediate 4-(2-tert-butoxycarbonylethyl)-4-nitro-heptanedioic acid di-tert-butyl ester (7) was obtained in 91% yield by the condensation of nitromethane with 3 equiv of tert-butyl acrylate (6). Following reduction of the nitro functional group to an amine over Raney Ni in 92% yield, the amine was acylated with CBz-β-alanine (98% yield), and the tert-butyl esters were removed in quantitative yield by treatment with formic acid at room temperature, affording triacid 10 as a colorless oil. The triacid was activated as the tris-N-hydroxysuccinimide ester (11) and then condensed with the free amine resulting from removal of the CBz protecting group from 12. Peracetylated BLM-disaccharide trimer (13) was obtained as a colorless oil in 81% overall yield from 11. Following removal of the O-acetyl protecting groups (NaOMe in MeOH) and the CBz protecting group (H2, Pd/C), condensation with the activated ester of Cy5** (Cy5**COOSu (14)) afforded BLM disaccharide-Cy5** trimer (3), which was isolated in an overall yield of 15% from 13 after purification by C18 reversed phase HPLC.
Scheme 1. Synthetic Route Utilized for the Preparation of BLM disaccharide-Cy5** trimer.
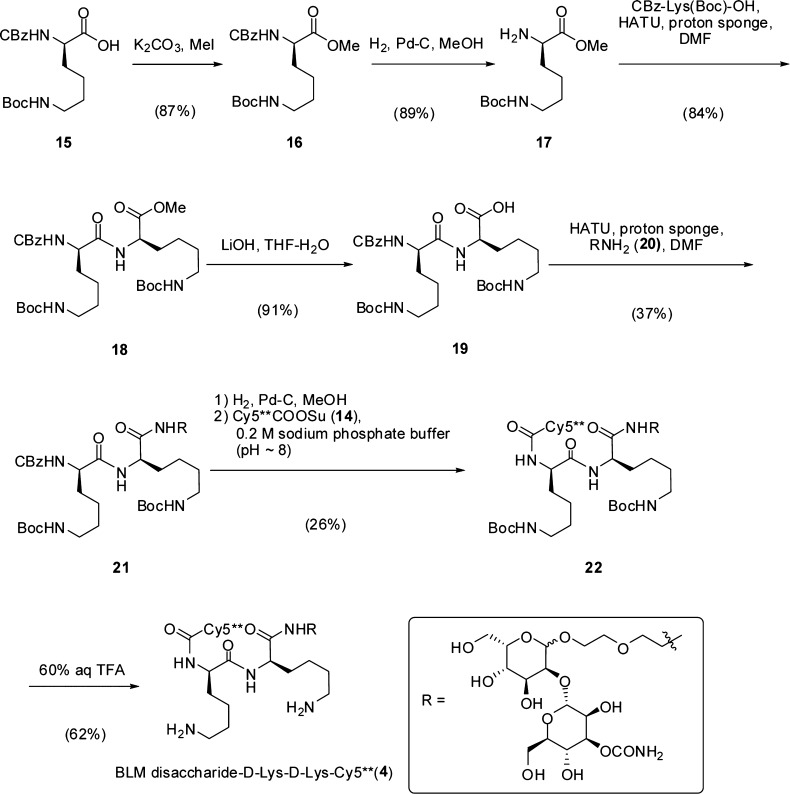
The synthetic route used for preparation of the BLM disaccharide conjugate incorporating d-Lys-d-Lys (4) is outlined in Scheme 2. Nε-Boc-protected CBz-d-Lys (15) was converted to the respective methyl ester (16) by treatment with methyl iodide (87% yield) and then the CBz group was removed by hydrogenolysis over palladium-on-carbon, affording free amine 17 in 89% yield. Condensation of 15 and 17 then afforded fully protected d-Lys-d-Lys derivative 18 as a colorless oil. Demethylation (LiOH, aq tetrahydrofuran) provided free acid 19 which was conjugated to disaccharide 20 via a linker having an amine functional group. The d-Lys-d-Lys-disaccharide intermediate 21 was obtained in modest (37%) yield as a colorless solid. Hydrogenolysis of 21 over palladium-on-carbon, followed by treatment with Cy5**COOSu (14) in 0.2 M aq sodium phosphate buffer (pH ∼8) gave the bis-Boc-protected conjugate 22 in low (26%) yield following purification by C18 reversed phase HPLC. Deprotection using 60% aqueous CF3COOH, followed by purification using reversed phase HPLC, then afforded Nα-Cy5**-d-lysyl-d-lysyl-BLM-disaccharide (4) as a blue solid. The respective conjugate containing l-lysyl-l-lysine (5) was prepared analogously starting from l-lysine (Scheme S1).
Scheme 2. Synthetic Route Utilized for the Preparation of BLM Disaccharide-d-Lys-d-Lys-Cy5**.
Cell Binding/Uptake of Disaccharide–Dye Conjugates
The Cy5** conjugate of the disaccharide moiety of bleomycin has previously been shown to bind selectively to a number of cultured cancer cells, but not to normal cell lines of the same general cell type.25 The generality of this observation is critical to a strategy which envisions using the BLM disaccharide to direct cytotoxic agents selectively to tumor cells. Accordingly, in the present study additional cancer and matched normal cell lines were studied. As shown in Figure 2, significant uptake was observed for monolayer cultures of C6 rat glioma cells (but not for CTX TNA2 rat astrocyte cells), SW 1088 human brain cancer cells (but not for SVG p12 normal human brain cells), and SW480 colon cancer cells (but not CCD-112CoN human fetal colon cells). Also tested were BT474 human breast ductal carcinoma and SW1783 human astrocytoma cell lines, both of which exhibited significant binding and uptake of BLM disaccharide–Cy5** (not shown).
Figure 2.
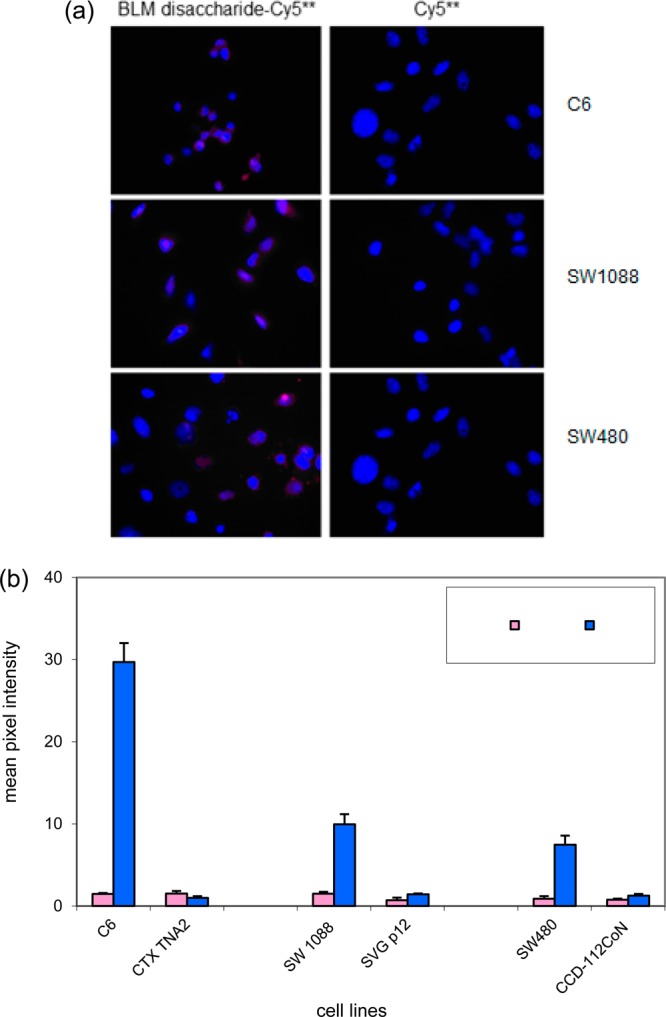
(a) Comparison of binding/uptake of Cy5** and BLM disaccharide–Cy5** conjugate (1) in three cancer cell lines. The cells were treated with 25 μM BLM disaccharide–Cy5** (1) or Cy5** at 37 °C for 1 h, washed with PBS, and fixed with 4% paraformaldehyde. The cell nuclei were stained with DAPI. Fluorescence imaging was carried out after a 2 s exposure. (b) Quantification of the binding/uptake of BLM disaccharide–Cy5** and Cy5** in three sets of matched cancer and normal cell lines. The cells were treated with 25 μM dye (conjugate) and irradiated for 2 s prior to imaging.
As noted previously,27 the presence of a carbamoyl moiety has been found to be essential for binding/uptake of the BLM disaccharide–Cy5** conjugate by tumor cells, and has been documented for several tumor cell lines. The importance of this characteristic to our proposed therapeutic strategy prompted the testing of additional tumor cell lines for this property. As illustrated in Figure 3, the C6 rat glioma and SW1783 human astrocytoma cancer cell lines were shown to share this property with cancer cell lines tested previously, two of which (DU145 human prostate carcinoma and A549 human lung carcinoma epithelial cells) were again tested in parallel for verification of earlier findings. As in earlier experiments, the presence of the carbamoyl moiety on mannose was essential for binding/uptake.
Figure 3.
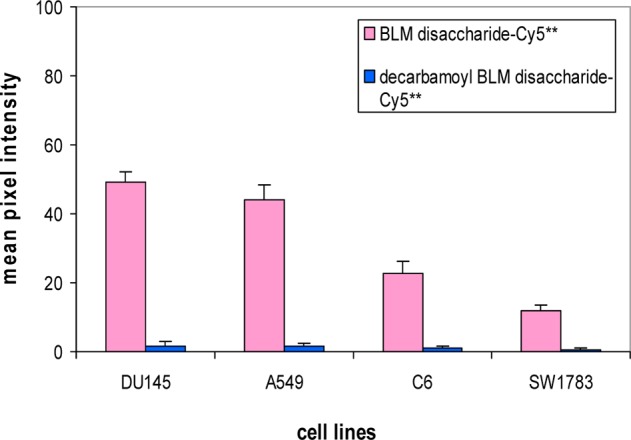
Quantification of the binding/uptake of BLM disaccharide–Cy5** (1) and decarbamoyl BLM disaccharide–Cy5** (2) in four cancer cells. The cells were treated with 25 μM dye conjugates and irradiated for 2 s prior to imaging.
Another important experiment employed a Cy5** conjugate tethered to a trivalent cluster of BLM disaccharides (Figure 4a). As shown in the Figure 4a,c, when treated with a 25 μM concentration of the trimeric cluster (3, Scheme 1), the binding/uptake of BLM disaccharide–Cy5** trimer by DU145 cells was almost 3-fold greater than that of BLM disaccharide–Cy5** and twice that observed for BLM–Cy5** itself, suggesting the importance of multivalency in the recognition and internalization of the BLM disaccharide by cultured tumor cells.
Figure 4.
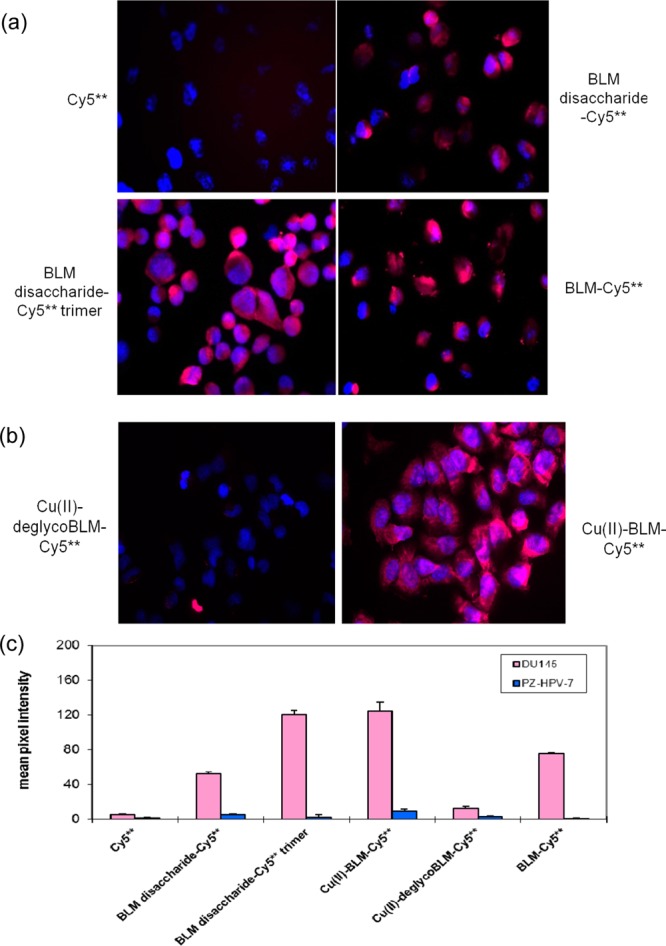
(a) Comparison of the binding/uptake of BLM disaccharide–Cy5**, Cy5**, BLM disaccharide–Cy5** trimer (3), and BLM–Cy5** in DU145 prostate carcinoma cells. (b) Comparison of the binding/uptake of Cu(II)•BLM-Cy5** and Cu(II)•deglycoBLM-Cy5** in DU145 prostate carcinoma cells. (c) Quantification of the binding/uptake of 25 μM dye conjugates in DU145 and PZ-HPV-7 cells. The cells were treated with 25 μM dye conjugates and irradiated for 2 s prior to imaging.
Bleomycin is often administered clinically by injection in metal free form.29 However, the drug acts to cleave DNA as a metal complex with Fe2+,15,16 or possibly Cu+,30 and has long been thought to sequester metal ions soon after i.v. administration.2 Accordingly, the cellular binding and uptake of metalloBLMs by DU145 cells was evaluated employing Cu(II)•BLM-Cy5** (Figure 4b). As shown in the figure, Cu(II)•BLM-Cy5** associated very efficiently with DU145 prostate carcinoma cells. Quantification of binding/uptake revealed that Cu(II)•BLM-Cy5** was about twice as efficient as the metal free BLM-Cy5** conjugate, and at least as efficient as any other conjugate studied (Figure 4b,c). While the analogous experiment using the BLM disaccharide moiety bound directly to Cu2+ could not be carried out due to the lack of efficient multidentate ligands for Cu2+ within the disaccharide moiety, the importance of the disaccharide to DU145 prostate cacinoma cell binding/uptake was evaluated by repeating the same experiment using Cu(II)•deglycoBLM-Cy5**. As shown in Figure 4b,c, in the absence of the disaccharide moiety, no significant cell association/uptake was noted.
The effect of bound Fe(III) and Cu(II) on tumor cell binding and internalization was studied further using four additional cancer cell lines and matched normal cell lines; the results are summarized in Figure 5. As shown, Fe(III)•BLM-Cy5** and Cu(II)•BLM-Cy5** were bound/internalized to the same extent by MCF-7 human breast adenocarcinoma, and to a greater extent than metal-free BLM-Cy5**. None of the conjugates associated to a significant extent with human breast basal epithelial cells (MCF-10A). Similar results were noted for A549 lung carcinoma cells and C6 rat glioma cells, although the addition of Cu(II) or Fe(III) afforded only a small increase in uptake; again, their matched normal cell lines displayed little affinity for any of the BLM-Cy5** conjugates. Interestingly, in an experiment carried out in triplicate, SW1783 human astrocytoma cells displayed a much greater affinity for Fe(III)•BLM-Cy5** than for Cu(II)•BLM-Cy5**, although both metalloBLMs still exhibited a preference for this cancer cell line relative to SVGp12 human fetal SV40 transformed astrocytes. Enhancement of tumor cell binding/uptake was also documented using Zn(II)31 and Mn(III)32 chelates of BLM-Cy5** (Figure 5), suggesting that the effect is due primarily to the positive charge common to each of the metal cations studied. Further, the differences observed between metal free BLM and individual metalloBLMs, among the various metalloBLMs, and from one cell line to another with the same species argues that the observed results cannot be explained via metal ion exchange to afford a common metalloBLM different from that introduced to the culture medium.
Figure 5.
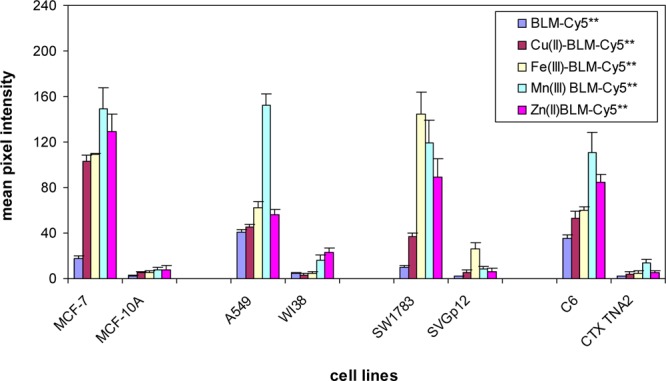
Effect of metal ions on the internalization of BLM-Cy5** conjugates in four sets of matched cancer and normal cell lines. The cells were treated with 25 μM Cu(II)•BLM-Cy5**, Fe(III)•BLM-Cy5**, Mn(III)•BLM-Cy5**, or Zn(II)•BLM-Cy5** at 37 °C for 1 h, washed with PBS, and fixed with 4% paraformaldehyde. Fluorescence imaging was carried out with a 2 s exposure time.
To validate the assumption concerning the effects of the positive charge of the metal ions on tumor cell targeting/uptake, and provide a tool more readily applicable to drug design, two BLM disaccharide conjugates were prepared in which a lysine dipeptide was introduced between the disaccharide and Cy5** dye moieties. Conjugate 4 (Scheme 2) incorporated d-lysyl-d-lysine, while conjugate 5 (Scheme S1) contained l-lysyl-l-lysine. As anticipated, both of these conjugates were bound/internalized by DU145 cells more efficiently than BLM disaccharide–Cy5** (1) itself (Figure 6) and conjugate 4 was more effective than 5, no doubt reflecting the greater susceptibility of the embedded l-lysyl-l-lysine moiety to proteolytic degradation.
Figure 6.
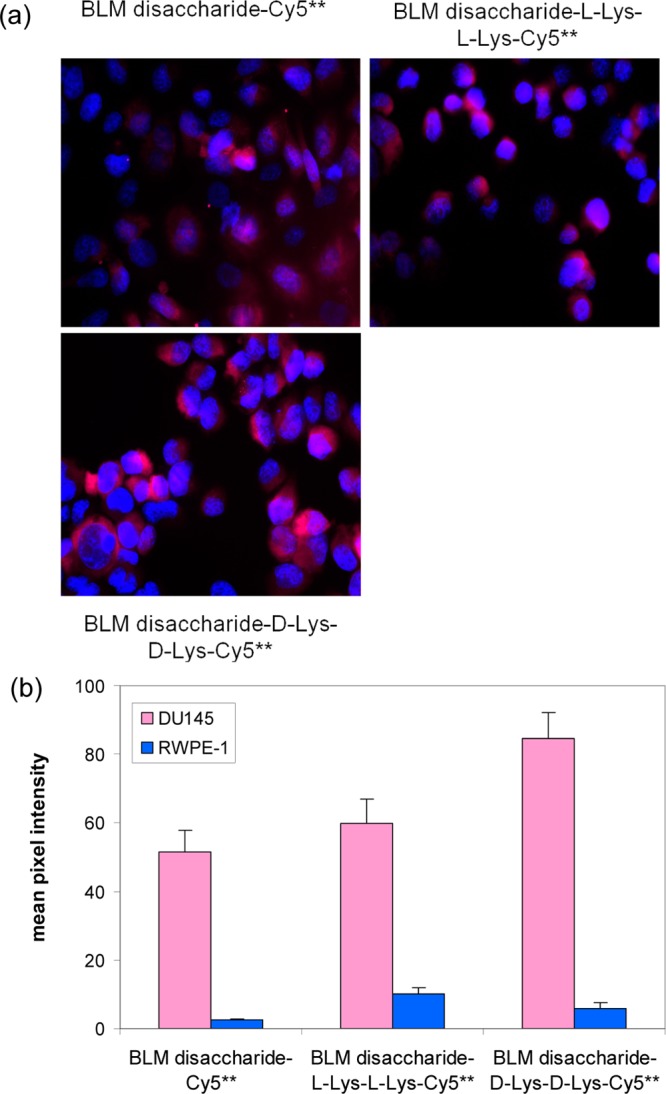
(a) Effect of lysine linkers on the internalization of BLM disaccharide–Cy5** conjugates (4 and 5) on binding/uptake in four cell lines. The cells were treated with 25 μM BLM disaccharide–Cy5** analogues at 37 °C for 1 h, washed with PBS, and fixed with 4% paraformaldehyde. Fluorescence imaging was carried out with a 2 s exposure time. (b) Quantification of the binding/uptake of 25 μM dye conjugates in different cancer cells and irradiated for 2 s prior to imaging.
The foregoing experiments were carried out using fixed cell imaging, which was entirely sufficient to enable the conclusions enumerated above. In order to address the issue of intracellular trafficking of the internalized BLM (disaccharide)–Cy5** conjugates, initial experiments were also carried out employing live cell imaging. These were performed using cultured DU145 cells and BLM disaccharide conjugate 1; the images shown in Figure 7a were taken after a 4 h incubation period. As is clear from the images, the dye-labeled conjugate was associated primarily with the mitochondria and with the nuclear envelope of the DU145 cells, two organelles of potential interest for targeting in tumor cells.
Figure 7.

(a) Uptake of BLM disaccharide–Cy5** and (b) BLM disaccharide-d-Lys-d-Lys-Cy5** in DU145 cells was studied by live cell imaging. The cells were grown as a monolayer culture for 48 h, then treated with 25 μM BLM disaccharide–Cy5** (1) or BLM disaccharide-d-Lys-d-Lys-Cy5** (4) for 0.5, 1.0, or 4.0 h. Prior to each time point, the cells were treated with 100 nM mito-tracker green (mitochondrial staining) and 2.5 μg/mL Hoechst 33342 (nuclear staining) for 20 min and then irradiated for 3.5 s prior to imaging. The results after 4 h are shown. (c) Quantification of Cy5** in the mitochondria is shown.
An additional experiment involving live cell imaging was carried out under the same conditions noted for Figure 7a, but using BLM disaccharide–Cy5** conjugate 4, containing an embedded d-lysyl-d-lysine peptide. As is clear from Figure 7b and c, both the amount and the distribution were altered relative to the observation in Figure 7a, with a significant accumulation of conjugate 4 within the mitochondria.
Discussion
In previous studies, novel conjugates of BLM disaccharides25,27 and monosaccharides26 have been used to define the structural requirements for interaction of these BLM sugars selectively with tumor cells. It has been demonstrated that the carbamoyl functional group is required for selective binding/uptake of both the BLM mono- and disaccharides,26,27 but that other BLM disaccharides in which the position or nature of the carbamoyl moiety had been altered could actually afford 2–4-fold more efficient targeting in some cases.27 Interestingly, while the BLM monosaccharide was entirely sufficient to support tumor cell targeting and uptake when employed in isolation,26 two BLM congeners in which the l-gulose moiety of the BLM disaccharide was replaced with glucose clearly did not support tumor cell targeting/uptake,28 establishing the importance of the molecular context in which the carbamoylmannose moiety is presented to the cell.
The discovery of uncomplicated carbohydrate derivatives capable of selectively targeting tumor cells,25−27 and delivering molecular cargoes to those cells,25−28 in principle may enable novel strategies for the delivery of potential therapeutic agents to cells in support of antitumor therapy. The applicability of the strategy will necessarily be in direct proportion to the generality of tumor-selective targeting, arguing for the need to study a substantial variety of tumor cell types, and to ensure that the targeting mechanisms are fundamentally similar among these tumor cell types. Additionally, since not all antitumor agents work by the same molecular mechanism, the intracellular trafficking of the sugar–drug conjugates is of obvious importance. The present study was designed to begin to address these issues, thereby supporting efforts to select appropriate antitumor drugs for conjugation and design carbohydrate–drug conjugates likely to exhibit selective antitumor properties.
In previous studies using BLM saccharide–dye conjugates, it was found that both the BLM mono- and disaccharide dye conjugates selectively bound to a monolayer culture of a cancer cell line, but not to a matched normal cell line, for five sets of cell lines.25,26 In the present study, this observation of selective binding was extended to C6 rat glioma cells, SW1088 human brain cancer cells, and SW480 human colon adenocarcinoma cancer cells, but not to any of three matched normal cell lines (Figure 2). Also tested, albeit in the absence of matched normal control cells, were BT474 human breast carcinoma cells and SW1783 human astrocytoma cell lines, both of which exhibited significant binding/uptake of BLM disaccharide–Cy5**. Thus, a total of 10 cancer cell lines have now been documented as substrates for at least one BLM saccharide–dye conjugate, and eight of these have been shown to lack comparable interactions with a matched normal cell line.
An important hallmark of BLM saccharide–dye interaction with cultured cancer cells is the need for a carbamoyl functional group in an appropriate position of mannose.26,27 This characteristic has been demonstrated for BLM mono- and disaccharide–Cy5** conjugates using four cancer cell lines. In the present study, an additional two cancer cell lines (C6 rat glioma cells and SW1783 human astrocytoma cells) were shown to share this property.
In an earlier study, it was found that a Cy5** conjugate containing a trimeric cluster of 3-O-carbamoylmannose exhibited enhanced binding/uptake to each of six different cancer cell lines, relative to the binding observed for BLM monosaccharide–Cy5**.26 The enhancements observed ranged from 1.6- to 2.3-fold, and were greatest for A549 cells and least pronounced for DU145 cells. The apparent effect of multivalency in the interaction suggested the possible involvement of a carbohydrate receptor, some of which have been shown to exhibit multivalent effects.33,34 The possible multivalent effects of the BLM disaccharide in its interaction with cultured cancer cells has not been addressed to date due to the greater complexity of the synthetic chemistry required to elaborate a trimeric cluster of the BLM disaccharide. Nonetheless, the successful synthesis of BLM disaccharide–Cy5** trimer (3) is outlined in Scheme 1, and enabled this critical experiment to be carried out. As shown in Figure 4a,c, conjugate 3 was almost 3-fold more efficient than BLM disaccharide–Cy5** (1) in binding/uptake by DU145 human prostate carcinoma cells, which had exhibited the least enhancement in the presence of BLM monosaccharide–Cy5** trimer.26 An experiment run in parallel using PZ-HPV-7 human HPV-18 transformed prostate epithelium cells verified the selectivity for the tumor cell line (Figure 4c).
Preliminary evidence has now been provided for the possible involvement of one or more facilitative glucose transporters in the binding/uptake of BLM by cancer cells.28 Such transporters are known to be up-regulated in cancer cells to support the greater use of glycolysis for ATP production as compared with mitochondrial oxidative phosphorylation.35 The evidence presented included the pretreatment of normal lung WI-38 cells and normal kidney cells with a sublethal dose of the mitochondrial complex I inhibitor rotenone36 to induce the enhanced use of glycolysis in these noncancer cell lines. In both cases, this resulted in significantly enhanced uptake of BLM-Cy5**.28 Further, when treated with the known GLUT1 inhibitors cytochalasin B37 and phloretin,38 BLM-Cy5** binding/uptake by SW-480 colon cancer cells was reduced substantially, and in a dose-dependent fashion.28
One of the most striking findings in the current study involved the metalloBLMs Cu(II)•BLM and Fe(III)•BLM, both of which have been studied extensively over a period of years.39,40 As shown in Figure 4b and c, Cu(II)•BLM-Cy5** was bound/internalized about twice as efficiently as the metal-free species (BLM–Cy5**) or the BLM disaccharide–Cy5** conjugate (1). The observed binding was clearly dependent on the BLM carbohydrate moiety, since Cu(II)•deglycoBLM–Cy5** showed minimal binding/uptake (Figure 4b,c). The observation was extended by testing both Cu(II)•BLM-Cy5** and Fe(III)•BLM-Cy5** in four sets of cancer cell lines and their matched normal control cell lines. As shown in Figure 5, the two metalloBLM–Cy5** conjugates exhibited enhanced binding in all four cancer cells lines relative to metal free BLM–Cy5**, and relative to the results obtained for Cu(II)•BLM–Cy5** and Fe(III)•BLM–Cy5** in the normal cell lines. The two metalloBLM–Cy5** conjugates gave comparable results in three of the cancer cell lines studied, but not in SW1783 human astrocytoma cells, for which Fe(III)•BLM–Cy5** proved to be the more efficient ligand.
The nature of the enhancement occasioned by the presence of the Cu2+ and Fe3+ cations was further defined by studying the effects of the Zn(II) and Mn(III) chelates of BLM–Cy5**. As shown in Figure 5, both of these cations, which have been shown previously to form stable chelates with bleomycin,31,32 also potentiated the selective binding of BLM–Cy5** to all four cancer cells lines. The observation of enhanced binding/uptake of positively charged species by mammalian cells is well documented,41,42 and not entirely surprising given the negatively charged phospholipids common to the external membranes of eukaryotic cells.43,44 However, in the present case, each of the conjugates also contains Cy5**, which has four negatively charged sulfonate functional groups such that the metalloBLM–Cy5** conjugates likely retain a net negative charge under the experimental conditions employed. Further, both normal and cancer cells have phospholipids in their membranes, but the selectivity for tumor cells is retained.
The foregoing findings for metalloBLMs may have important implications for the mechanism of action of BLM as an antitumor agent. It has long been recognized that DNA cleavage by bleomycin requires the presence of a redox-active metal ion for oxygen activation. Fe(II)•BLM is generally considered to be the metalloBLM species responsible for oxygen activation in a therapeutic setting, although there is also some evidence compatible with the possible participation of Cu(I)•BLM.30 It has been posited that, following i.v. administration as a metal-free species, bleomycin sequesters Cu2+ in the serum, and this metal ion is later exchanged for Fe2+ intracellularly.2 The cancer cell binding/uptake data presented in Figures 4 and 5 suggests that either of these metalloBLMs would gain access to tumor cells much more readily than metal-free BLM, especially since the BLM family antitumor agents employed clinically also have positively charged C-termini and lack the negatively charged sulfonate groups present in the conjugates studied here.
One further conclusion evident in the present data should also be noted. The results shown in Figures 4 and 5 document that tumor cell binding and uptake are facilitated by four different metal ions, each of which has been shown to form a stable chelate with BLM, and three of which are redox-active and can effect DNA cleavage.15,16,30,32 The observed effects are clearly different than those obtained in the absence of added metal ions, arguing that each of the metal chelates must survive at least the initial binding event (facilitated by the disaccharide) to the cancer cell surface in order to produce the more efficient tumor cell binding and uptake actually observed. Persistence of the metal complexes following cell uptake might well alter the cellular distribution of the internalized bleomycins as well, potentially leading to enhanced association with the cell mitochondria (cf., Figure 7a,b).
In order to realize the enhanced efficiency of tumor cell binding/uptake occasioned by the presence of a chelated metal cation, it would be useful from the perspective of medicinal chemistry to be able to achieve the same effect using a positively charged organic functional group. Accordingly, two conjugates (4 and 5) were prepared in which d or l-lysyl-lysine was embedded between the BLM disaccharide and Cy5** reporter group. As shown in Figure 6, both of these positively charged functionalities enhanced binding/uptake to cultured DU145 cells. As in the case of BLM, the conjugated Cy5** contained four negatively charged groups, such that enhanced uptake was achieved with a molecule lacking a net positive charge.
If the BLM saccharides are employed to deliver conjugated cytotoxins or other agents selectively to tumor cells, the ability to realize effective therapeutic intervention will also depend on the ability of the delivered agent to gain access to the relevant subcellular compartment(s). At present no information is available concerning the trafficking of BLM or its carbohydrates following internalization. As shown in Figure 7a, which involved the live cell imaging of cultured DU145 cells 4 h after treatment with BLM disaccharide 1, the conjugate was substantially associated both with the nuclear membrane and with the mitochondria. In comparison, conjugate 4 was more substantially associated with the cell mitochondria. Association of the conjugate (having an embedded d-Lys-d-Lys dipeptide) with the nuclear membrane is unsurprising, and completely consistent with the putative mechanism of action of bleomycin, involving degradation of chromatin. The mitochondrial association may also reflect the presence of the embedded positively charged dipeptide, as such species are known to accumulate in mitochondria due to the high membrane potential associated with the inner mitochondrial membrane.45−48 It may also be noted that damage to mitochondrial DNA is repaired primarily by base excision repair, such that the repair of BLM-mediated damage to mitochondrial DNA may be less facile than chromatin repair. In fact, it has been reported that mitochondrial DNA is more sensitive to BLM than nuclear DNA,49 that mitochondrial DNA repair occurs only in part following BLM treatment,50 and that BLM-induced apoptosis requires the presence of DNA within the mitochondria.51
Conclusions
The binding of the disaccharide moiety of bleomycin has now been demonstrated for a total of 10 cultured cancer cell lines using a conjugate of the disaccharide and cyanine dye Cy5**. This reinforces the observations made initially using microbubbles containing conjugated BLM or BLM disaccharide. Unexpectedly, chelation of BLM–Cy5** to any of four different metal ions resulted in significantly enhanced cellular uptake, and this still proved to be selective for cancer cells. That the enhanced uptake resulted from the positive charge associated with the metal ions in the formed metalloBLMs was supported by the observation that a disaccharide–Cy5** conjugate containing an embedded bis-lysine moiety also exhibited enhanced uptake. For the BLM disaccharide conjugate containing bis-lysine, live cell imaging revealed that the intracellular distribution of the conjugate was also altered, favoring accumulation in the cell mitochondria. This is consistent with the potential across the inner mitochondrial membrane.45−48 To the extent that metalloBLMs retain their chelated metal ion following cancer cell uptake, it would also be logical to expect that they might also exhibit a similar change in intracellular distribution.
Glossary
Abbreviations
- APCI
atmospheric pressure chemical ionization
- BLM
bleomycin
- CBz
carboxybenzyl
- Cy5**
cyanine 5**
- DAPI
4′,6-diamino-2-phenylindole
- DMAP
N,N-dimethylaminopyridine
- DME
dimethoxyethane
- DMF
dimethylformamide
- FAB
fast atom bombardment
- Hz
Hertz
- HPLC
high performance liquid chromatography
- MALDI-TOF
matrix-assisted laser-desorption-ionization
- PBS
phosphate buffered saline
- Rf
ratio of fronts
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TMSOTf
trimethylsilyl triflate
Supporting Information Available
Procedures for the synthesis and characterization of BLM disaccharide analogues prepared for study. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.5b00277.
This work was supported by National Institutes of Health research grant CA140471, awarded by the National Cancer Institute.
The authors declare the following competing financial interest(s): Sidney Hecht is a consultant to Isis Pharmaceuticals.
Supplementary Material
References
- Umezawa H.; Suhara Y.; Takita T.; Maeda K. (1966) Purification of bleomycins. J. Antibiot. 19, 210–215. [PubMed] [Google Scholar]
- Umezawa H. (1979) Advances in bleomycin studies. In Bleomycin: Chemical, Biochemical and Biological Aspects (Hecht S. M., Ed.) pp 24–36, Springer-Verlag, New York. [Google Scholar]
- Carter S. K., Crooke S. T., and Umezawa H., Eds. (1978) Bleomycin: Current Status and New Developments, pp 9–14, Academic Press, Orlando, FL. [Google Scholar]
- Sikic B. I., Rozencweig M., and Carter S. K., Eds. (1985) Bleomycin Chemotherapy, pp 3–35, Academic Press, Orlando, FL. [Google Scholar]
- Levi J. A.; Raghavan D.; Harvey V.; Thompson D.; Sandeman T.; Gill G.; Stuart-Harris R.; Snyder R.; Byrne M.; Kerestes Z. (1993) The importance of bleomycin in combination chemotherapy for good-prognosis germ cell carcinoma. Australasian germ cell trial group. J. Clin. Oncol. 11, 1300–1305. [DOI] [PubMed] [Google Scholar]
- Hecht S. M. (2012) Bleomycin Group Antitumor Agents. In Anticancer Agents from Natural Products, 2nd ed. (Cragg G. M., Kingston D. G. I., and Newman D. J., Eds.) pp 451–478, CRC Press, FL. [Google Scholar]
- Silberstein E. B.; Kornblut A.; Shumrick D. A.; Saenger E. L. (1974) 67Ga as a diagnostic agent for the detection of head and neck tumors and lymphoma. Radiology 110, 605–608. [DOI] [PubMed] [Google Scholar]
- Jones S. E.; Lilien D. L.; O’Mara R. E.; Durie B. G. M.; Salmon S. E. (1975) Indium-111 bleomycin tumor scanning in lymphoma. Med. Pediatr. Oncol. 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Silverstein M. J.; Verma R. C.; Greenfield L.; Morton D. L. (1976) 111Indium-bleomycin breast and axilla imaging. Cancer 37, 36–42. [DOI] [PubMed] [Google Scholar]
- Rasker J. J.; Beekhuis H.; van de Wal A. M.; Homan van der Heide J. N.; Woldring M. G. (1976) Cobalt-57-bleomycin scanning of hila and mediastinum in patients with bronchial carcinoma: a prospective study. Thorax 31, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton I. E.; Todd J. H.; Turner R. L. (1977) Static and dynamic imaging with indium-111 labelled bleomycin in the localization of squamous cell neoplasia. Br. J. Radiol. 50, 508–512. [DOI] [PubMed] [Google Scholar]
- Bekerman C.; Moran E. M.; Hoffer P. B.; Hendrix R. W.; Gottschalk A. (1977) Scintigraphic evaluation of lymphoma: a comparative study of 67Ga-citrate and 111In-bleomycin. Radiology 123, 687–694. [DOI] [PubMed] [Google Scholar]
- DeRiemer L. H.; Meares C. F.; Goodwin D. A.; Diamanti C. I. (1979) BLEDTA: tumor localization by a bleomycin analog containing a metal-chelating group. J. Med. Chem. 22, 1019–1023. [DOI] [PubMed] [Google Scholar]
- Goodwin D. A.; Meares C. F.; DeRiemer L. H.; Diamanti C. I.; Goode R. L.; Baumert J. E.; Sartoris D. J.; Lantieri R. L.; Fawcett H. D. (1981) Clinical studies with In-111 BLEDTA, a tumor-imaging conjugate of bleomycin with a bifunctional chelating agent. J. Nucl. Med. 22, 787–792. [PubMed] [Google Scholar]
- Sausville E. A.; Peisach J.; Horwitz S. B. (1978) Effect of chelating agents and metal ions on the degradation of DNA by bleomycin. Biochemistry 17, 2740–2746. [DOI] [PubMed] [Google Scholar]
- Sausville E. A.; Stein R. W.; Peisach J.; Horwitz S. B. (1978) Properties and products of the degradation of DNA by bleomycin and iron(II). Biochemistry 17, 2746–2754. [DOI] [PubMed] [Google Scholar]
- Povirk L. F. (1983) In Molecular Aspects of Anti-cancer Drug Action (Neidle S., and Waring M., Eds.) pp 157–181, Vol. 3, Macmillan, London. [Google Scholar]
- Keller T. J.; Oppenheimer N. J. (1987) Enhanced bleomycin-mediated damage of DNA opposite charged nicks. A model for bleomycin-directed double strand scission of DNA. J. Biol. Chem. 262, 15144–15150. [PubMed] [Google Scholar]
- Povirk L. F.; Han Y. H.; Steighner R. J. (1989) Structure of bleomycin-induced DNA double-strand breaks: predominance of blunt ends and single-base 5′ extensions. Biochemistry 28, 5808–5814. [DOI] [PubMed] [Google Scholar]
- Absalon M. J.; Kozarich J. W.; Stubbe J. (1995) Sequence-specific double-strand cleavage of DNA by Fe-bleomycin. 1. The detection of sequence-specific double-strand breaks using hairpin oligonucleotides. Biochemistry 34, 2065–2075. [DOI] [PubMed] [Google Scholar]
- Absalon M. J.; Wu W.; Kozarich J. W.; Stubbe J. (1995) Sequence-specific double-strand cleavage of DNA by Fe-bleomycin. 2. mechanism and dynamics. Biochemistry 34, 2076–2086. [DOI] [PubMed] [Google Scholar]
- Keck M. V.; Manderville R. A.; Hecht S. M. (2001) Chemical and structural characterization of the interaction of bleomycin A2 with d(CGCGAATTCGCG)2. Efficient, double-strand DNA cleavage accessible without structural reorganization. J. Am. Chem. Soc. 123, 8690–8700. [DOI] [PubMed] [Google Scholar]
- Roy B.; Hecht S. M. (2014) Hairpin DNA sequences bound strongly by bleomycin exhibit enhanced double-strand cleavage. J. Am. Chem. Soc. 136, 4382–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J.-C.; Schmaltz R. M.; Tsosie K. S.; Belohlavek M.; Hecht S. M. (2009) Carbohydrate dependent targeting of cancer cells by bleomycin–microbubble conjugates. J. Am. Chem. Soc. 131, 2438–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Schmaltz R. M.; Bozeman T. C.; Paul R.; Rishel M. J.; Tsosie K. S.; Hecht S. M. (2013) Selective tumor cell targeting by the disaccharide moiety of bleomycin. J. Am. Chem. Soc. 135, 2883–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya C.; Yu Z.; Rishel M. J.; Hecht S. M. (2014) The carbamoylmannose moiety of bleomycin mediates selective tumor cell targeting. Biochemistry 53, 3264–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madathil M. M.; Bhattacharya C.; Yu Z.; Paul R.; Rishel M. J.; Hecht S. M. (2014) Modified bleomycin disaccharides exhibiting improved tumor cell targeting. Biochemistry 53, 6800–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B. R.; Ghare M. I.; Bhattacharya C.; Paul R.; Yu Z.; Zaleski P. A.; Bozeman T. C.; Rishel M. J.; Hecht S. M. (2014) The disaccharide moiety of bleomycin facilitates uptake by cancer cells. J. Am. Chem. Soc. 136, 13641–13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke S. T.; Comis R. L.; Einhorn L. H.; Strong J. E.; Broughton A.; Prestayko A. W. (1977) Effects of variations in renal function on the clinical pharmacology of bleomycin administered as an iv bolus. Cancer Treat. Rep. 61, 1631–1636. [PubMed] [Google Scholar]
- Ehrenfeld G. M.; Shipley J. B.; Heimbrook D. C.; Sugiyama H.; Long E. C.; van Boom J. H.; van der Marel G. A.; Oppenheimer N. J.; Hecht S. M. (1987) Copper dependent cleavage of DNA by bleomycin. Biochemistry 26, 931–942. [DOI] [PubMed] [Google Scholar]
- Manderville R. A.; Ellena J. F.; Hecht S. M. (1995) Interaction of Zn(II)•Bleomycin with d(CGCTAGCG)2. A binding model based on NMR experiments and restrained molecular dynamics calculations. J. Am. Chem. Soc. 117, 7891–7903. [Google Scholar]
- Ehrenfeld G. M.; Murugesan N.; Hecht S. M. (1984) Activation of oxygen and mediation of DNA degradation by manganese-bleomycin. Inorg. Chem. 23, 1496–1498. [Google Scholar]
- Spiess M. (1990) The asialoglycoprotein receptor: A model for endocytotic transport receptors. Biochemistry 29, 10009–10018. [DOI] [PubMed] [Google Scholar]
- Choudhury A. K.; Tao Z.-F.; Hecht S. M. (2001) Synthesis and DNA cleavage activity of a novel bleomycin A5 glycoconjugate. Org. Lett. 3, 1291–1294. [DOI] [PubMed] [Google Scholar]
- Kroemer G.; Pouyssegur J. (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 6, 472–482. [DOI] [PubMed] [Google Scholar]
- Li N.; Ragheb K.; Lawler G.; Sturgis J.; Rajwa B.; Melendez J. A.; Robinson J. P. (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278, 8516–8525. [DOI] [PubMed] [Google Scholar]
- Pinkofsky H. B.; Dwyer D. S.; Bradley R. J. (2000) The inhibition of GLUT1 glucose transport and cytochalasin B binding activity by tricyclic antidepressants. Life Sci. 66, 271–278. [DOI] [PubMed] [Google Scholar]
- Afzal I.; Cunningham P.; Naftalin R. J. (2002) Interactions of ATP, oestradiol, genistein and the anti-oestrogens, faslodex (ICI 182780), and tamoxifen, with the human erythrocyte glucose transporter, GLUT1. Biochem. J. 365, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeman T. C.; Nanjunda R.; Tang C.; Liu Y.; Segerman Z. J.; Zaleski P. A.; Wilson W. D.; Hecht S. M. (2012) Dynamics of bleomycin interaction with a strongly bound hairpin DNA substrate, and implications for cleavage of the bound DNA. J. Am. Chem. Soc. 134, 17842–17845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.; Paul A.; Alam M. P.; Roy B.; Wilson W. D.; Hecht S. M. (2014) A short DNA sequence confers strong bleomycin binding to hairpin DNAs. J. Am. Chem. Soc. 136, 13715–13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender P. A.; Rothbard J. B.; Jessop T. C.; Kreider E. L.; Wylie B. L. (2002) Oligocarbamate molecular transporters: design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J. Am. Chem. Soc. 124, 13382–13383. [DOI] [PubMed] [Google Scholar]
- Cooley C. B.; Trantow B. M.; Nederberg F.; Kiesewetter M. K.; Hedrick J. L.; Waymouth R. M.; Wender P. A. (2009) Oligocarbonate molecular transporters: oligomerization-based syntheses and cell-penetrating studies. J. Am. Chem. Soc. 131, 16401–16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S.; Murray D. (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–610. [DOI] [PubMed] [Google Scholar]
- de Kroon A. I. P. M.; Rijken P. J.; De Smet C. H. (2013) Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog. Lipid Res. 52, 374–394. [DOI] [PubMed] [Google Scholar]
- Szewczyk A.; Wojtczak L. (2002) Mitochondria as a pharmacological target. Pharmacol. Rev. 54, 101–127. [DOI] [PubMed] [Google Scholar]
- Murphy M. P.; Smith R. A. (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 47, 629–656. [DOI] [PubMed] [Google Scholar]
- Hoye A. T.; Davoren J. E.; Wipf P.; Fink M. P.; Kagan V. E. (2008) Targeting mitochondria. Acc. Chem. Res. 41, 87–97. [DOI] [PubMed] [Google Scholar]
- Odeh A. M.; Craik J. D.; Ezzeddine R.; Tovmasyan A.; Batinic-Haberle I.; Benov L. T. (2014) Targeting mitochondria by Zn(II) N-Alkylpyridylporphyrins: The impact of compound sub-mitochondrial partition on cell respiration and overall photodynamic efficacy. PLoS One 9, e108238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. O.; Neims A. H. (1987) Mitochondrial DNA damage by bleomycin. Biochem. Pharmacol. 36, 2769–2774. [DOI] [PubMed] [Google Scholar]
- Shen C. C.; Wertelecki W.; Driggers W. J.; LeDoux S. P.; Wilson G. L. (1995) Repair of mitochondrial DNA damage induced by bleomycin in human cells. Mutat. Res. 337, 19–23. [DOI] [PubMed] [Google Scholar]
- Brar S. S.; Meyer J. N.; Bortner C. D.; Van Houten B.; Martin W. J. II (2012) Mitochondrial DNA-depleted A549 cells are resistant to bleomycin. Am. J. Physiol. 303, L413–L424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.