Abstract
Many virus types are covered by a lipid bilayer. This structure called an envelope, is derived from the host cell and includes host- and virus-encoded proteins. Because envelope components first interact with the host, it is the trigger for infection, immunity and pathology. The roles of especially host-derived constituents are poorly understood. Focusing on herpes simplex type 1 (HSV1) as a model, we have shown that the envelope acquires the physiological initiators of coagulation from the host cell; tissue factor (TF) and procoagulant phospholipid (proPL). Unlike resting cells, where TF and proPL accessibility is carefully restricted, their expression is constitutive on the purified virus enabling factor VIIa (FVIIa)-dependant factor Xa (FXa) and thrombin generation. Interestingly, HSV1-encoded glycoprotein C (gC) on the virus enhances FXa production. In addition to coagulation proteases, HSV1 also facilitates fibrinolytic plasmin generation. HSV1 TF and gC combine to optimally enhance cultured cell infection when both FVIIa and FXa are available through protease activated receptor (PAR) 2. Plasmin also increases infection through PAR2, whereas thrombin provides an additive effect via PAR1. Thus, depending on the host cell, TF and proPL may be a general feature of enveloped viruses, enabling coagulation protease activation and PAR-mediated effects on infection.
The Virus Envelope
Many types of virus acquire a covering as they “bud” from the host cell. This structure, called an envelope, consists of a cell-derived lipid bilayer and the associated membrane-bound proteins, which are encoded by both viral and host genes. Since the host cell and the respective membrane are not constant, the envelope proteome is variable, and consequently the roles of host-encoded envelope proteins in the virus life-cycle and pathology are poorly understood. Numerous enveloped viruses affect hemostasis, such as herpes simplex virus types 1 (HSV1) [1] and 2 [2], cytomegalovirus [3], dengue virus [4], Ebola virus [5], hepatitis C virus [6], human immunodeficiency viruses 1 and 2 [7] and influenza virus [8]. As a model envelope virus, our focus has been primarily on HSV1, which uses host- and virus-encoded proteins to activate plasma coagulation proteases on its surface. Here we review our studies showing that HSV1 envelope receptors exploit these proteases to enhance cellular infection by triggering host signaling mechanisms.
Coagulation Proteases are activated on HSV1
Envelope procoagulant phospholipid
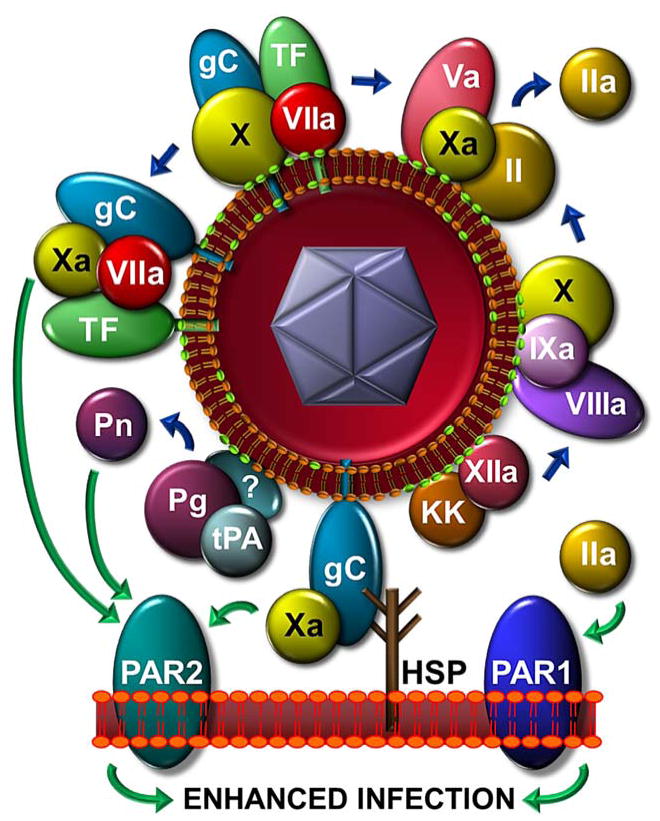
The essential roles of thrombin in biology are highly regulated, requiring procoagulant phospholipid (proPL; e.g. phosphatidylserine) as a focal point for binding and assembly of the enzyme-cofactor-substrate complex that activates its precursor, prothrombin, and upstream proteases. ProPL serves to localize and concentrate clotting proteins to sites of vascular damage, where agonists induce its controlled accessibility. In contrast, we showed that purified HSV1, and other members of the herpes virus family constitutively express proPL on their envelope [2,9] and consequently circumvent the constraint of cell-localized thrombin production. The enzyme responsible for thrombin generation is a ternary complex between the protease factor Xa (FXa) and the cofactor Va (FVa) that associate with each other and proPL in the presence of Ca2+, termed prothrombinase. Direct binding and functional assays have demonstrated that purified HSV1 and other herpes viruses can assemble prothrombinase from purified proteins and in plasma [2,9]. Suggesting proPL may be a general virus envelope constituent, it has been defined on other viruses as essential to infection [10]. A model summarizing the procoagulant mechanisms we have identified on HSV1 is presented in Figure 1.
Figure 1. HSV1 initiates coagulation protease activation and enhances infection through PARs.
Host cell-derived TF and proPL (green polar-head), and virus-encoded gC on the HSV1 surface initiate coagulation protease activation (blue arrows). HSV1-cell attachment (e.g. via gC-heparan sulfate proteoglycan, HSP) positions the virus proximal to PAR1 for activation by thrombin, or to PAR2 for activation by FVIIa, FXa and plasmin (green arrows). The effects of these enzymes and PARs combine to enhance infection. Viral TF and gC, form an optimal quaternary PAR2-signaling complex with FXa and FVIIa. A novel gC/FXa PAR2 signaling combination is shown.
Host-derived tissue factor
Cells control prothrombinase assembly, not only by providing proPL, but also by producing the first FXa from its inactive precursor, factor X (FX). Our finding that purified HSV1 can initiate plasma coagulation [2], suggested a surface mechanism to generate FXa. The extrinsic tenase, the cellular FX-activating complex responsible for initiating coagulation, consists of the plasma-derived protease, factor VIIa (FVIIa), bound to its cofactor, tissue factor (TF). TF is an integral membrane protein present constitutively in epithelia and stromal cells. Exposure of the subendothelium or agonist-induced cellular expression localizes TF to sites of vascular damage. Of note, enveloped virus infection has been shown to enhance TF expression on at least endothelial cells, monocytes and macrophages [11,12]. Therefore host TF is a candidate receptor that could be incorporated into any virus envelope depending on the permissive cell-type during the course of infection. Indeed, we have identified TF on the surface of HSV1 and other herpes viruses propagated in various cell types by electron microscopy and functional analyses [2]. Our unpublished data also suggest that each of four purified dengue virus serotypes has TF activity. Thus, these viruses can bypass the cell-mediated regulation of clotting by not only providing their own proPL, but also TF to produce FXa and thrombin.
Virus-encoded glycoprotein C
HSV1-encoded glycoprotein C (gC) expression on the infected endothelial cell surface has been attributed to enhanced FX activation [13]. Since gC is a transmembrane component of the HSV1 envelope, we became interested in its role as a possible contributor to envelope-mediated coagulation. With a gC-null virus mutant, TF inhibitory antibodies and solubilized recombinant gC (sgC), we demonstrated that gC on the surface of HSV1 enhances FVIIa-dependent FX activation [14]. Viral gC contributes to FX binding and sgC enhances FVIIa-dependent FX activation by approximately 1000-fold in the presence of purified HSV1. A Ca2+-dependent association of FX with virus particles was facilitated when gC was present in the envelope of HSV1 [14]. Interestingly, sgC has an as yet unidentified binding partner(s) on HSV1 with ~100 sites/virus particle. sgC bound through this mechanism provides an equivalent number of additional FX binding sites. To unambiguously investigate the role of virus envelope TF, we produced novel host protein-restricted HSV1 that is either positive or negative for TF. These were further made either positive or negative for virus-encoded gC [15]. The initial data suggest that gC functions to enhance the cofactor activity of TF on the virus surface toward FX activation with only limited activity in the absence of TF (unpublished). Our observations add to the repertoire of gC functions, which is also involved in complement evasion [16] and virus-host cell interactions [17].
Intrinsic tenase
Once physiological FXa generation is started by TF/FVIIa, its production is amplified by a complex between the protease factor IXa, its specific cofactor VIIIa (FVIIIa) and proPL, the intrinsic tenase. We have recently reported FVIII-dependent plasma clot formation initiated by low levels of virus [18]. Utilizing FVII-deficient plasma and various inhibitors to dissect the clotting pathway, we have also shown that HSV1 can initiate plasma coagulation through the contact phase of coagulation, which amplifies FXa/thrombin generation through the intrinsic tenase by upstream factor XIIa and kallikrein activation [18]. Combined with TF and gC, HSV1 has at least three mechanisms on its envelope contributing to FXa function. Other procoagulant enveloped viruses may similarly deliver and localize activated coagulation proteases to the surface of host cells as one of the first events in the infection mechanism.
Envelope Coagulation Cofactors Enhance HSV1 Cell Infection through Protease Activated Receptors
Coagulation proteases increase HSV1 infection
Thrombin, FVIIa, FXa and other proteases such as fibrinolytic plasmin, stimulate many cell types by exposing a “tethered-ligand” at the N-terminus of G-protein-linked protease activated receptors (PARs) [19]. Due to the procoagulant enzymes generated on the HSV1 envelope, we consequently investigated a role for host cellular PAR1 and PAR2 in infection (Figure 1). Using purified proteases, synthetic PAR-activating peptides and inhibitory antibodies to PAR-mediated signaling, we showed that thrombin enhanced cultured endothelial cell and fibroblast infection exclusively through PAR1 [20]. Independent of PAR1, a PAR2-mediated infection pathway was triggered by FXa, FVIIa [15] or plasmin [21]. Since the effects of PAR1 and PAR2 on infection were additive [15], different G-protein-coupled intracellular pathways may contribute to HSV1 replication. Like other viruses [22], we found that plasmin generation from its purified precursor plasminogen by tissue plasminogen activator was increased in the presence of purified HSV1 [21]. This simultaneous generation of clot-dissolving and -forming proteases may explain why HSV1 or other enveloped viruses have not been correlated more strongly to thrombosis. Interestingly, outside of the herpes virus family, the plasminogen pathway has been shown to promote early-stage influenza A replication with PAR1 playing a key role and correlating strongly to pathogenesis in mice [23,24].
TF and gC on HSV1 promote infection mediated by FVIIa and FXa
In addition to its pivotal role in coagulation, TF has been identified as a signaling cofactor that presents FVIIa and FXa/FVIIa to PARs, thereby dropping the concentration of these proteases to a relevant physiological range [25]. Using our TF+/−, gC+/− HSV1 panel, TF+/gC+ HSV1 in the presence of both FVIIa and FXa optimally enhanced infection in vitro [15]. Other cofactor/protease combinations also increased HSV1 replication, including a newly identified signaling cofactor-protease pair, gC/FXa. These results demonstrated a cofactor signaling function when TF is derived from a different surface than the cellular PAR2. Thus the virus has evolved to activate coagulation proteases to trans-modulate cell function, analogous to tumor-derived TF+ microparticles during hypoxia [26]. While it is currently unclear at which stages of viral infection PARs are activated, cell membrane-HSV1 envelope fusion may influence PAR cleavage by virus envelope cofactor complexes.
In a murine model, we have recently presented data in abstract form showing that purified TF+ HSV1 is much more infectious than TF- HSV1. This enhanced infectivity is furthermore inhibited by virus-specific TF antibodies, which strongly suggests that the host cell membrane plays a key role in virology by transferring a cofactor advantage to the virus envelope. Highlighting the multi-functional and divergent roles of coagulation proteases within virology, PARs have not only been shown to enhance infection [15,24,27] but also to up-regulate the opposing innate immune response to influenza A and the non-enveloped coxsackie virus [28]. Interestingly, non-enveloped adenovirus, associates with the zymogen FX to facilitate infection, and to directly stimulate innate immune defenses [29]. While further studies must be conducted in vivo, our results for HSV1 suggest that constituents obtained from the host cell membrane aid infection, possibly tipping the balance toward evading immune surveillance.
References
- 1.Siscovick DS, Schwartz SM, Corey L, Grayston JT, Ashley R, Wang SP, Patsy BM, Tracy RP, Kuller LH, Kronmal RA. Chlamydia pneumoniae, herpes simplex virus type 1, and cytomegalovirus and incident myocardial infarction and coronary heart disease death in older adults - The Cardiovascular Health Study. Circulation. 2000;102:2335–2340. doi: 10.1161/01.cir.102.19.2335. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland MR, Raynor CM, Leenknegt H, Wright JF, Pryzdial ELG. Coagulation initiated on herpesviruses. Proc Natl Acad Sci USA. 1997;94:13510–13514. doi: 10.1073/pnas.94.25.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie PD, Nieto FJ, Adam E, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1 and coronary heart disease. Archives Int Med. 2000;160:2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 4.van Gorp EC, Minnema MC, Suharti C, Mairuhu AT, Brandjes DPM, ten Cate H, Hack CE, Meijers JCM. Activation of coagulation factor XI, without detectable cntact activation in dengue haemorrhagic fever. Brit J Haematol. 2001;113:94–99. doi: 10.1046/j.1365-2141.2001.02710.x. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishizaka N, Ishizaka Y, Takahashi E, Tooda E-I, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 7.Witz M, Lehman J, Korzets Z. Acute brachial artery thrombosis as the initial manifestation of human immunodeficiency virus infection. Am J Hematol. 2000;64:137–139. doi: 10.1002/(sici)1096-8652(200006)64:2<137::aid-ajh13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Muamoto Y, Ozaki H, Takada A, Park C-H, Sunden Y, Umemura T, Kawaoka Y, Matsuda H, Kida H. Highly pathogenic H5N1 influenza virus causes coagulopathy in chickens. Microbiol Immunol. 2006;50:73–81. doi: 10.1111/j.1348-0421.2006.tb03764.x. [DOI] [PubMed] [Google Scholar]
- 9.Pryzdial ELG, Wright JF. Prothrombinase assembly on an enveloped virus: evidence that the cytomegalovirus surface contains procoagulant phospholipid. Blood. 1994;84:3749–3757. [PubMed] [Google Scholar]
- 10.Morizono K, Chen ISY. Receptors and tropisms of envelope viruses. Curr Opin Virol. 2011;1:13–18. doi: 10.1016/j.coviro.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Key NS, Bach RR, Vercellotti GM, Moldow CF. Herpes simplex virus type 1 does not require productive infection to induce tissue factor in human umbilical vein endothelial cells. Lab Invest. 1993;68:645–651. [PubMed] [Google Scholar]
- 12.Geibert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Inf Dis. 2003;188:1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 13.Etingin OR, Silverstein RL, Friedman HM, Hajjar DP. Viral activation of the coagulation cascade: Molecular interaction at the surface of infected endothelial cells. Cell. 1990;61:657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland MR, Friedman HM, Pryzdial ELG. Herpes simplex virus type 1-encoded glycoprotein C enhances coagulation factor VIIa activity on the virus. Thromb Haemost. 2004;92:947–955. doi: 10.1160/TH04-04-0242. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland MR, Ruf W, Pryzdial ELG. Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection. Blood. 2012;119:3638–3645. doi: 10.1182/blood-2011-08-376814. [DOI] [PubMed] [Google Scholar]
- 16.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris JD, Friedman HM. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein C. J Exp Med. 1999;190:1637–1646. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tal-Singer R, Peng C, Ponce de Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershom ES, Sutherland MR, Lollar P, Pryzdial ELG. Involvement of the contact phase and intrinsic pathway in herpes simplex virus-initiated plasma coagulation. J Thromb Haemost. 2010;8(5):1037–1043. doi: 10.1111/j.1538-7836.2010.03789.x. [DOI] [PubMed] [Google Scholar]
- 19.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland MR, Friedman HM, Pryzdial ELG. Thrombin enhances herpes simplex virus infection of cells involving protease-activated receptor 1. J Thromb Haemost. 2007;5:1055–1061. doi: 10.1111/j.1538-7836.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- 21.Gershom ES, Vanden Hoek AL, Meixner SC, Sutherland MR, Pryzdial ELG. Herpesviruses enhance fibrin clot lysis. Thromb Haemost. 2012;107:760–768. doi: 10.1160/TH11-08-0601. [DOI] [PubMed] [Google Scholar]
- 22.LeBouder F, Morello E, Rimmelzwaan GF, Bosse F, Pechoux C, Delmas B, Riteau B. Annexin II incorporated into influenza virus particles supports virus replication by converting plasminogen into plasmin. J Virol. 2008;82:6820–6828. doi: 10.1128/JVI.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berri F, Rimmelzwaan GF, Hanss M, Albina E, Foucault-Grunenwald M-L, Le VB, Vogelzang-van Trierum SE, Gil P, Camerer E, Martinez D, Lina B, Lijnen R, Carmeliet P, Riteau B. Plasminogen controls inflammation and pathogenesis of influenza virus infections via fibrinolysis. PLoS Pathogens. 2013;9:1–12. doi: 10.1371/journal.ppat.1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoufache K, Berri F, Nacken W, Vogel AB, Delenne M, Camerer E, Coughlin SR, Carmeliet P, Lina B, Rimmelzwaan GF, Planz O, Ludwig S, Riteau B. PAR1 contributes to influenza A virus pathogenicity in mice. J Clin Inv. 2013;123:206–214. doi: 10.1172/JCI61667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothmeier A, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133–149. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 26.Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a pro-angiogenic pathway involving cancer cell microvesicles and PAR-2 mediated HB-EGF signaling in endothelial cells. Proc Natl Acad Sci U S A. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aerts L, Hamelin M-E, Rheaume C, Lavigne S, Couture C, Kim W, Susan-Resiga D, Prat A, Seidah NG, Vergnolle N, Riteau B, Boivin G. Modulation of protease activated receptor 1 influences human metapneumovirus disease severity in a mouse model. PloS One. 2013;8:1–13. doi: 10.1371/journal.pone.0072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoniak S, Owens AP, Baunacke M, Williams JC, Lee RD, Weithauser A, Sheridan PA, Malz R, Luyendyk JP, Esserman DA, Trejo J, Kirchhofer D, Blaxall BC, Pawlinski R, Beck MA, Rauch U, Mackman N. PAR-1 contributes to the innate immune response during viral infection. J Clin Inv. 2013;123:1310–1322. doi: 10.1172/JCI66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, Miyake K, MacDonald JW, Bammler TK, Beyer RP, Ferin FM, Stewart PL, Shayakhmetov DM. Coagulation factor X activates innate immunity to human species C adenovirus. Science. 2012;338:795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]