Abstract
Precise regulation of the epigenome during perinatal development is critical to the formation of species-typical behavior later in life. Recent data suggests that Gadd45b facilitates active DNA demethylation by recruiting proteins involved in base excision repair (BER), which will catalyze substitution of 5-methyl-cytosine (5mC) for an unmodified cytosine. While a role for Gadd45b has been implicated in both hippocampal and amygdalar learning tasks, to the best of our knowledge, no study has been done investigating the involvement of Gadd45b in neurodevelopmental programming of social behavior. To address this, we used a targeted siRNA delivery approach to transiently knock down Gadd45b expression in the neonatal rat amygdala. We chose to examine social behavior in the juvenile period, as social deficits associated with neurodevelopmental disorders tend to emerge in humans at an equivalent age. We find that neonatal Gadd45b knock-down results in altered juvenile social behavior and reduced expression of several genes implicated in psychiatric disorders, including methyl-CpG-binding protein 2 (MeCP2), Reelin, and brain derived neurotrophic factor (BDNF). We furthermore report a novel role for Gadd45b in the programmed expression of α2-adrenoceptor (Adra2a). Consistent with Gadd45b’s role in the periphery, we also observed changes in the expression of pro-inflammatory cytokines interleukin-6 (Il-6) and interleukin- 1beta (Il-1beta) in the amygdala, which could potentially mediate or exacerbate effects of Gadd45b knockdown on the organization of social behavior. These data suggest a prominent role for Gadd45b in the epigenetic programming of complex juvenile social interactions, and may provide insight into the etiology of juvenile behavioral disorders such as ADHD, autism, and/or schizophrenia.
Keywords: Amygdala, Gadd45b, Methylation, Epigenetics, Social play, Reelin, MeCP2, Adra2a, Cytokines, Development
1. Introduction
While there is a growing body of literature investigating mechanisms involved in DNA methylation in the brain, less is known about the molecules involved in DNA demethylation. The growth arrest and DNA damage inducible factor 45 (Gadd45) family appears to be involved in active demethylation of the epigenome (Niehrs and Schäfer, 2012), and, in particular, Gadd45b appears necessary for rapid DNA demethylation in response to neuronal signaling (Ma et al., 2009). Interestingly, in patients with major psychosis, Gadd45b is overexpressed in regions of the cerebral cortex receiving projections from the amygdala (Gavin et al., 2011). Furthermore, aberrant epigenetic marks are found in the post-mortem neural tissue of adult psychiatric patients (Grayson et al., 2005; Nagarajan et al., 2006; Gavin et al., 2011; Dong et al., 2012; McGowan et al., 2009). Given the Gadd45 family’s involvement in demethylation (Niehrs and Schäfer, 2012) and localization to acetylated histones (Carrier et al., 1994), there is strong support for a role in the precise temporal and spatial regulation of DNA- and histone-modifying enzyme activity, which may in turn be critical for neurotypical development.
Gadd45b transcription occurs in response to environmental stressors (Takekawa and Saito, 1998), immune signaling (Liu et al., 2013), neuronal activity (Ma et al., 2009), and both hippocampal and amygdalar learning tasks (Keeley et al., 2006). While the relative importance of Gadd45b expression in the developmental programming of behavior remains unclear, two recent studies using adult Gadd45b knock-out mice demonstrated a role for Gadd45b in both hippocampal and striatal memory and learning tasks (Leach et al., 2012; Sultan et al., 2012). However, to our knowledge no studies have addressed a role for Gadd45b in the formation of social behaviors. Given the induction of Gadd45b in response to amygdala-related learning tasks (Keeley et al., 2006), we chose to investigate this brain region during development. We were specifically interested in the effect of Gadd45b on juvenile social behavior, a critical component of which is juvenile social play, that is thought to be both highly rewarding and a salient indicator of typical social development (Panksepp et al., 1984).
We have previously reported the sensitivity of this behavior to neonatal perturbations in epigenetic programming; specifically, a transient disruption to Mecp2 expression in the developing amygdala decreases juvenile social play behavior (Kurian et al., 2008). Though the role of Mecp2 in regulating gene expression is complex (Yasui et al., 2007; Chahrour et al., 2008), it is generally associated with gene repression. This raises the intriguing possibility that some genes must be repressed (e.g., those targeted by MeCP2) to activate play behavior; conversely, some genes may need to undergo transcriptional activation (e.g., through Gadd45b-assisted DNA demethylation) to inhibit play in order to achieve a species-typical level of the behavior. This led us to hypothesize that Gadd45b expression may be required to prevent an over-active play drive. We thus examined the consequence of siRNA-mediated, amygdalar Gadd45b knockdown on juvenile social development by assessing its impact on juvenile social play, sociability, and anxiety- like behavior.
We furthermore were interested in local expression of cytokines, as peripheral levels of Gadd45b are reported to regulate their availability (Lu et al., 2004). In particular, Gadd45b knockout mice produced less Il-6, a cytokine whose role in the central nervous system (CNS) may be to promote and stabilize excitatory synapse formation (Wei et al., 2012). To the best of our knowledge, Gadd45b has not been studied in the brain with respect to its potential effects on proinflammatory cytokine expression, despite a known role for these molecules in suppressing social behavior (Weil et al., 2006; Hennessy et al., 2014). We also examined genes known to influence play behavior, e.g., MeCP2, Adra2a (Kurian et al., 2008; Vanderschuren et al., 2008), and investigated a variety of genes previously associated with Gadd45b’s activity as an epigenetic regulator. This included BDNF, Reelin, and glutamate decarboxylase- 67 (Gad67) (Ma et al., 2009; Matrisciano et al., 2011).
2. Materials and method
2.1. Animals and weaning environment
Untimed-pregnant Sprague Dawley female rats (~15 d pregnant) were purchased from Charles River Laboratories (Wilmington, MA) and allowed to deliver normally. Cages were checked regularly to determine the day of birth (P0). On P0, litters were culled to 10 and were composed of 4 untreated females, 3 control siRNA males, and 3 Gadd45b siRNA males to maintain typical litter size and sex composition. Stimulus animals of the same strain that were uninfused, used for the sociability task, were also weaned into mixed sex litters. Animals raised to the juvenile period for behavioral testing were left with dams undisturbed following siRNA treatment until weaning. Juveniles were weaned at P21 into cages of 5 with mixed gender and treatment groups. Animals were housed under standard laboratory conditions (light/dark cycle of 12/12 h, food and water ad libitum). Standard 1200 cm2 cages were used throughout the duration of the experiment. All procedures were approved by the University of Wisconsin– Madison Animal Care and Use Committee.
2.2. Gadd45b siRNA treatment
Gadd45b siRNA (Santa Cruz Biotechnology; catalog #sc-270211) and non-targeting control siRNA (Santa Cruz Biotechnology; catalog #sc-37007) were resuspended to 100 μM in ribonuclease-free water and Oligofectamine reagent (Invitrogen; catalog #12252-011) at a ratio of 2:1. Infusions directly into the neonatal rat amygdala were performed using a modified stereotaxic device (David Kopf Intruments; model #003445R), and coordinates from center and bregma suture lines were 1 mm lateral, 2 mm caudal, and 5.5 mm ventral as done previously in our lab (Forbes-Lorman et al., 2012, 2014; Jessen et al., 2010; Kurian et al., 2008; McCarthy et al., 2000). After cold anesthetization, rats were bilaterally infused with 1 μl (100 nmol) of either Gadd45b or control siRNA and allowed to recover under a warm lamp for approximately 20 min before being returned to the dam. Using this technique, we have reported that infusion of a dopamine D1 receptor agonist induces c-Fos expression localized to the injection site in the neonatal amygdala (Forbes-Lorman et al., 2012). In the current study, a separate set of 30 animals spread across P0–P2 was used for bilateral ink infusions to ensure targeting accuracy of the technique; the right and left amygdalae were successfully hit in all animals, as confirmed with rapid decapitation and microdissection.
To confirm knockdown in the first experiment, 12 animals (6 Gadd45b siRNA, 6 control siRNA) received two infusions 24 h apart from P0–P1 and were sacrificed 24 h later on P2. For behavioral analysis in the second experiment, 20 animals (9 Gadd45b siRNA, 11 control siRNA) received three infusions 24 h apart from P0–P2 and were then left to develop undisturbed with the dam until weaning. Animals were sacrificed at P33 after behavior testing.
2.3. Tissue collection
Neonatal rats were sacrificed via rapid decapitation. Juvenile rats were first anesthetized with isoflurane (Attane; catalog #NDC 66794-014-25) before rapid decapitation and tissue dissection. The amygdala and hypothalamus were dissected with razorblades and immediately snap frozen in isopentane (Fisher; catalog #O3551-4) on dry ice. Tissue samples were homogenized and total RNA and DNA were collected using an AllPrep DNA/RNA Mini Kit (Qiagen; catalog #80204).
2.4. Quantification of mRNA
RNA concentrations were determined using the Qubit Quantification Platform (Invitrogen; catalog #Q32857). RNA conversion to cDNA was performed in an Eppendorf MasterCycler Personal PCR machine via the ImPromII™ Reverse Transcription System (Promega; catalog #A3800). Real-time quantitative polymerase chain reaction (RT-PCR) was conducted using a Stratagene Mx3000P™ real time PCR system, and cDNA was amplified with GoTaq® Colorless Master Mix (Promega; catalog #M7132), SYBR green (Invitrogen; catalog #S33102) and ROX as a reference dye (Invitrogen; 12223-012). Following amplification, a dissociation melt curve and DNA gel analysis was performed to ensure the purity of PCR products. cDNA levels were normalized to a housekeeping gene, Ywhaz, using the ΔΔCT method (Table 1).
Table 1.
Primer sequences and Pubmed accession numbers for RT-qPCR.
| Primer | Accession | Forward | Reverse |
|---|---|---|---|
| Ywhaz | NM_013011 | TTGAGCAGAAGACGGAAGGT | GAAGCATTGGGGATCAAGAA |
| Gadd45b | NM_001008321 | GCTGGCCATAGACGAAGAAG | GCCTGATACCCTGACGATGT |
| Gadd45a | NM_024127 | GCTACTGGAGAACGACAAGAG | CCATTGTGATGAATGTGGGTTC |
| Gadd45g | NM_001077640 | CTGAATGTGGACCCTGACAAT | AACGCCTGGATCAACGTAAA |
| Reelin | NM_080394 | AGAGGACAATGCACTCGACATGGT | AAGCTGACTTCAGCACCACGGATA |
| Gad67 | M76177.1 | CTGCCATCCTGGTCAAGGAA | GAATCGCCTTGTCCCCTGTA |
| MeCP2 | NM_022673 | ACAGACTCACCAGTTCCTGCTTTG | ATGGAATCCTGTTGGAGCTGGTCT |
| BDNF | AY176065.1 | GCCCATGAAAGAAGCAAACGTCGA | TTTCTTCGTTGGGCCGAACCTTCT |
| Adra2a | NM_012739 | CTCGCTGAACCCTGTTGTCTA | TCACACGATGCGCTTTCT |
| Bcl2 | L14680.1 | GTGGATGACTGAGTACCTGAAC | GAGACAGCCAGGAGAAATCAA |
| Gfap | NM_017009.1 | GGTGTGGAGTGCCTTCGTAT | TACGATGTCCTGGGAAAAGG |
| Il-6 | NM_012589 | ATATGTTCTCAGGGAGATCTTGGAA | GTGCATCATCGCTGTTCATACA |
| Il-1beta | NM_031512 | CCCTGCAGCTGGAGAGTGTGG | TGTGCTCTGCTTGAGAGGTGCT |
| Tgf-beta | NM_021578 | ACCAACTACTGCTTCAGCTCCACA | TGTACTGTGTGTCCAGGCTCCAAA |
2.5. Behavioral testing
All behavioral tests were performed under dim red light and began 1–2 h after the dark phase of the light cycle began. Each animal was tested in all paradigms but was not repeat-tested in any task. Each behavior was video recorded with the exception of the open field test and the light/dark chamber, which were scored in real time. Videos were analyzed and scored using The Observer software (Noldus Information Technologies) by a trained observer who was blind to all treatments.
2.6. Juvenile social play behavior
The social play behavior paradigm was adapted from previously reported methods (Meaney and McEwen, 1986; Olesen et al., 2005). Animals were weaned on P21 and housed with littermates in groups of five, containing animals from each treatment condition (male control siRNA, male Gadd45b siRNA, and uninfused females to maintain normal sex ratios per litter). Animals were tail- and back-marked with a Sharpie and video recorded in their home cages twice per day (1 and 3 h after lights off) for 5 min trials over 5 d (P25–P29) for a total observation time of 50 min per animal. Play behavior was scored using the following criteria: (1) biting: one rat bites another; (2) chasing: one rat chases another; (3) pouncing: one rat pounces or lunges at another; (4) pinning: one rat stands over another, with its forepaws on the ventral surface of the opposing rat; and (5) boxing: both rats stand on hind legs and engage each other with forepaws.
2.7. Juvenile sociability
The sociability task was adapted from previous methods (Yang et al., 2011) using a three-chambered apparatus with removable dividers. On P30/31, test animals were placed in the chamber and allowed to freely explore for a 5 min acclimation period. The animal was then confined to the middle chamber and social and nonsocial stimuli were placed in opposing outside chambers. The dividers were removed and the test animal was allowed to explore the entire apparatus for 10 min. The social stimulus was an age- and sex-matched novel juvenile rat of the same strain that had not received infusions, held in a perforated plastic container (7 × 7 × 16 cm) that permitted sight, sound, scent, and minimal tactile contact. The nonsocial stimulus was an empty, identically perforated plastic container. Location in any chamber was scored as having all 4 paws inside the chamber.
2.8. Juvenile anxiety-like behavior
Under red light, 2 h after the dark phase of the light cycle began, animals were tested on three separate 5 min tasks – the open field arena, the elevated plus maze, and the light/dark box. Each animal (aged 31–32 d) was tested in this order, with 5 min inter-test intervals. Lux meter analysis revealed that there were approximately 15 (dim), 125 (low/moderate), and 7000 (bright/aversive) lumens for the EPM, open field, and light dark box, respectively.
2.8.1. Open field test
Rats were placed into a corner of an 80 cm square arena with opaque walls 40 cm high and allowed to move freely for 5 min. The arena floor had markings that divided it into 25 equal square (16 × 16 cm) divisions. A light was shone onto the middle of the test while the perimeter remained dark. The rat’s movement in the arena was scored as the number of crossings between squares during the 5 min testing period and perimeter crosses, middle crosses, groomings, defecations, and stretching from one square to another were scored.
2.8.2. Elevated plus maze
The elevated plus maze is a Plexiglas structure standing 50 cm off the floor and consisting of two opposing 100 cm runways that cross at the center. One runway is open, whereas the other is closed with 39-cm-high Plexiglas walls with an opening in the center to allow crosses. Rats were placed in the center of the maze facing an open arm and maze exploration was recorded for 5 min. Parameters quantified were entries into as well as time spent in the open and closed arms and the center of the maze arms. An entry was counted when all four paws crossed into a certain portion of the maze.
2.8.3. Light/dark chamber
The light/dark chamber is a large, Plexiglas chamber split by an opaque Plexiglas insert into two compartments: a light side where a white incandescent lamp was shining (35 × 38 × 39 cm), and smaller opaque, dark side (25 × 38 × 39 cm). An opening in the lower corner of the Plexiglas insert (6 × 10 cm) allows the animal to move freely between the light and dark chambers. The animal was placed in the corner of the light side of the box facing the opening and was observed for 5 min. Latency to enter the dark chamber and total time in light were recorded.
2.9. Quantification of DNA methylation
DNA methylation was assessed using an adapted version of the methylation sensitive restriction enzyme (MSRE) assay (Auger et al., 2011; Hashimoto et al., 2007). Briefly, 240 ng of DNA from each rat was divided equally into two tubes and digested with either HpaII (New England Biolabs; catalog #R0171) or HhaI (New England Biolabs; catalog #R0139) in the same buffer conditions at 37 °C for 90 min. Enzymes were subsequently inactivated by heating to 65 °C for 20 min. A no-DNA control was added to ensure purity of the restriction enzyme reaction. Primers were designed such that either HhaI or HpaII cut sites were contained within the region of interest, but not both. To assess relative amounts of DNA methylation, RT-qPCR was performed as described above.
2.10. Statistical analysis
Behavioral data and PCR data were analyzed using either a two-tailed Student’s t test or a one-way ANOVA with repeated measures as necessary in the sociability task and EPM (chamber duration as a within-subjects variable) using Prism 5 (GraphPad Software, Inc.). Potential outliers were screened for using the Grubbs test for outliers (http://graphpad.com/quickcalcs/Grubbs1.cfm). All reported measures are listed as mean ± SEM. Significance was defined as a p value of <0.05.
3. Results
3.1. Confirmation of Gadd45b siRNA targeting and knockdown
To confirm specificity of Gadd45b siRNA in the amygdala, animals receiving two rounds of infusions 24hrs apart and sacrificed at PN2 were examined for Gadd45b expression, as well as for the expression of Gadd45 family members Gadd45a and Gadd45g. Gadd45b siRNA-infused males showed an approximate 20% reduction in Gadd45b mRNA levels (Fig. 1). In an adjacent brain region, the hypothalamus, no knockdown was observed, suggesting the amygdala was successfully targeted and diffusion of siRNA to surrounding areas did not occur. Additionally, no significant knockdown of either Gadd45a or Gadd45g mRNA levels was observed, suggesting specificity of Gadd45b siRNA in the amygdala.
Fig. 1.
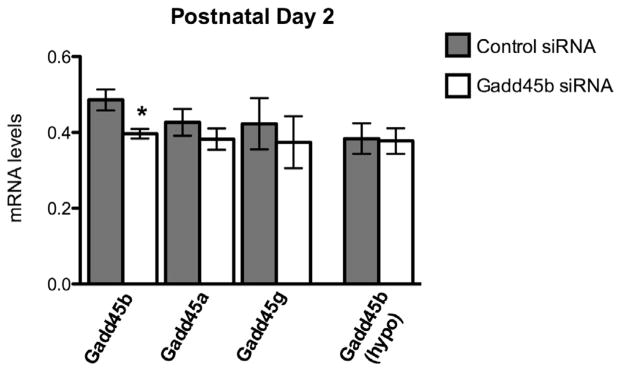
Gadd45b siRNA specificity in the amygdala. Two days of Gadd45b siRNA gives approximately 20% knockdown of mRNA (control: 0.4861 ± 0.0276, N = 5; Gadd45b: 0.3967 ± 0.0130, N = 5); *p = 0.0191. Gadd45b siRNA does not significantly decrease mRNA levels of closely related family members Gadd45a (control: 0.4267 ± 0.03536, N = 6; Gadd45b: 0.3825 ± 0.02830, N = 6; p = 0.3525) or Gadd45g (control: 0.423 ± 0.06764, N = 6, Gadd45b: 0.374 ± 0.06849, N = 6; p = 0.6229). Gadd45b siRNA does not spread to the adjacent hypothalamus (control: 0.3838 ± 0.04025, N = 4; Gadd45b: 0.3775 ± 0.03378, N = 6; p = 0.9079).
3.2. Neonatal Gadd45b siRNA increases frequency of juvenile social play behavior
Normal levels of neonatal Gadd45b mRNA in the amygdala appear to be important for the organization of juvenile social play, as a transient disruption in its expression altered this behavior later in development. Animals given siRNA treatment from PN0–PN2 and allowed to develop to the juvenile period were recorded in the home cage with mixed group littermates two times daily from PN25–PN29. Measures are reported as the average frequency across all observations. One control male outlier was removed from the analysis. t-Test analysis revealed that Gadd45b siRNA treatment more than doubled (~2.5×) levels of juvenile social play (Fig. 2A). Individual components of play including boxing, chasing, and pouncing were also increased in Gadd45b siRNA treated animals (Fig. 2B–D), though frequency of pinning was not significantly different between treatment groups (Fig. 2E). Biting was infrequently observed and no statistically significant differences were found (data not shown).
Fig. 2.
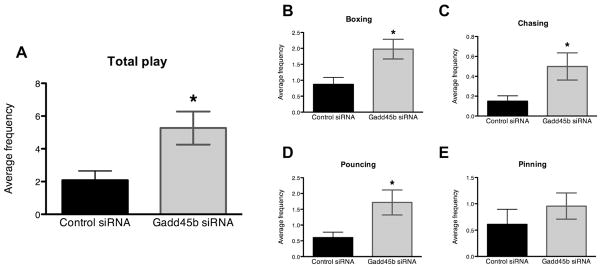
Neonatal Gadd45b siRNA infusion increases the initiation of play fighting. (A) Transient neonatal disruption of Gadd45b expression in the amygdala more than doubles the frequency of juvenile social play (control siRNA males: 2.064 ± 0.5007, N = 9. Gadd45b siRNA males: 4.664 ± 1.060, N = 8, p = 0.0361). (B) Average frequency of boxing increased 2.27-fold in Gadd45b siRNA treated males (1.976 ± 0.3082, N = 7) compared to control siRNA treated males (0.8708 ± 0.2199, N = 8); p = 0.0107. (C) Chasing increased 3.32-fold in Gadd45b siRNA treated males (0.4984 ± 0.1364, N = 7) compared to controls (0.1500 ± 0.05345, N = 8); p = 0.0265. (D) Pouncing increased 2.85-fold in Gadd45b siRNA males (1.717 ± 0.3971, N = 7) relative to control males (0.6028 ± 0.1723, N = 8); p = 0.0183. (E) Pinning was not statistically different between Gadd45b siRNA males (0.9571 ± 0.2494, N = 7) and control males (0.6111 ± 0.2840, N = 9); p = 0.3909.
3.3. Gadd45b siRNA treatment alters juvenile sociability
As seen in Fig. 3, control males spent significantly more time in the social chamber of a 3-chambered sociability apparatus during a 10 min test when compared to Gadd45b siRNA males (control: 62.31 ± 5.85%, N = 9 vs. Gadd45b: 39.25 ± 9.01%, N = 7; p = 0.0423). No significant differences in the percent time spent in the non-social (control: 26.42 ± 4.41%, N = 9 vs. Gadd45b: 36.64 ± 9.45%; p = 0.308) or middle (control: 11.26 ± 2.10%, N = 9 vs. Gadd45b: 11.45 ± 2.03%, N = 7; p = 0.952) chambers were observed. There were no differences in the number of exits and entries into the social chamber (Supplementary Fig. 1), indicating that overall locomotion was not affected in this task.
Fig. 3.
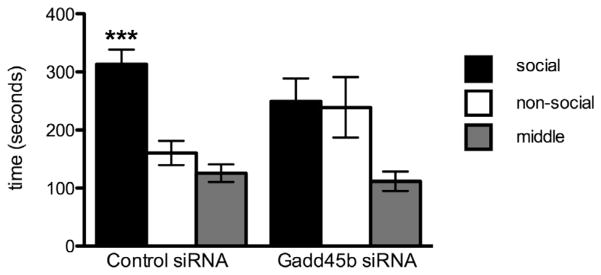
Gadd45b siRNA infused males exhibit abnormal social behavior, with no preference for the social chamber. Repeated measures ANOVA analysis reveals a strong preference for the social chamber in control siRNA males (F = 15.23, p < 0.0005), which Gadd45b siRNA males appear to lack (F = 2.599, p = 0.1233).
3.4. Gadd45b siRNA treatment does not alter juvenile anxiety-like behavior
Gadd45b siRNA infusion had no significant effects on anxiety, as tested by the elevated plus maze (EPM), open field maze, and the light/dark chamber (Supplementary Figs. 2–5). Student’s t-tests for percent time spent in the closed arm (control: 72.2 ± 6.09%, N = 8; Gadd45b: 67.5 ± 7.94%, N = 7, p = 0.640) (control: 12.1 ± 3.39%, N = 8; Gadd45b: 13.9 ± 4.26%, N = 7, p = 0.742), open arm (control: 12.1 ± 3.39%, N = 8; Gadd45b: 13.9 ± 4.26%, N = 7, p = 0.742), and the middle (control: 15.7 ± 4.30%, N = 8; Gadd45b: 18.6 ± 4.12%, N = 7, p = 0.636) reveal no significant differences.
3.5. siRNA knockdown of Gadd45b expression is transient
Juvenile amygdala samples were analyzed for Gadd45b mRNA levels to examine whether siRNA treatment was transient or lasting (Fig. 4). As expected, Gadd45b mRNA levels show no significant differences between control and Gadd45b siRNA treated groups in the amygdala on P33. Gadd45a mRNA levels were also unchanged, but intriguingly Gadd45g expression was significantly reduced by 33% at this later time point. Gadd45b mRNA expression was not altered in the juvenile hypothalamus.
Fig. 4.
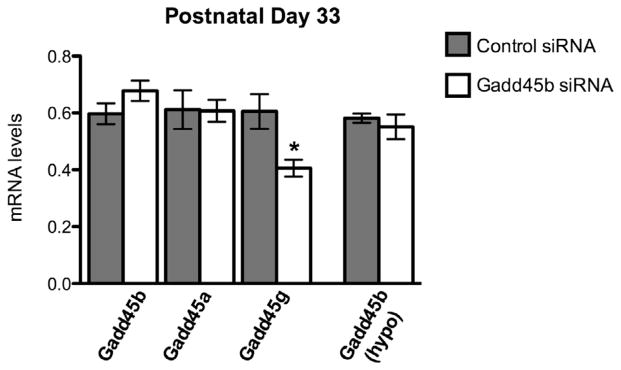
The decrease in Gadd45b expression is transient (control: 0.5970 ± 0.03689, N = 8 vs. Gadd45b: 0.6779 ± 0.03609, N = 7; p = 0.1433). Gadd45a expression remains unchanged at the juvenile time point (control: 0.612 ± 0.0677, N = 9; Gadd45b: 0.607 ± 0.0390, N = 7; p = 0.958). Gadd45g expression was decreased by 33% (control: 0.605 ± 0.0610, N = 9; Gadd45b: 0.406 ± 0.0296, N = 6; p = 0.0255). No change in Gadd45b expression is seen in the hypothalamus at P33 (control: 0.5818 ± 0.01635, N = 7; Gadd45b: 0.5507 ± 0.04331, N = 7; p = 0.5151).
3.6. Analysis of gene expression in neonatal and juvenile samples
Reelin exhibited a transient but dramatic 75% reduction in expression of mRNA at P2 (Fig. 5A). We saw no change in expression of Gad67 at either time point. Levels of BDNF were unchanged at P2; however, MeCP2 was decreased by approximately 30% neonatally. This effect was transient, as levels of MeCP2 had returned to control levels in the juvenile samples. Though no difference was seen at P2, BDNF mRNA was decreased at P33 by 25%. Additionally, Adra2a showed a 25% decrease in expression at the juvenile time point (Fig. 5B), but not at the neonatal time point (Fig. 5A), suggesting a lasting organizational effect of Gadd45b siRNA on gene expression. B-cell CLL/Lymphoma 2 (Bcl2) was not significantly different at either time point. Furthermore, expression of glial fibrillary acidic protein (GFAP) was highly variable at the neonatal time point but not significantly different in either sample set.
Fig. 5.
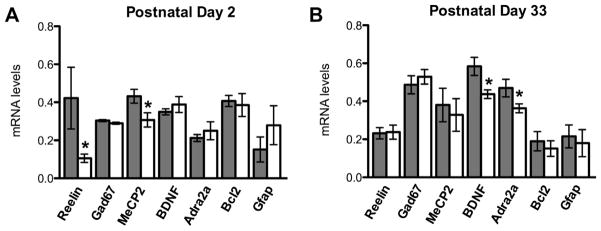
Gadd45b mRNA reduction alters the expression of several genes implicated in psychiatric disorders. (A) Reelin mRNA levels at P2 show a marked decrease compared to controls (control: 0.422 ± 0.162, N = 6; Gadd45b: 0.106 ± 0.0219, N = 6, p = 0.041). There was no change in Gad67 (control: 0.304 ± 0.00529, N = 4; Gadd45b: 0.290 ± 0.00524, N = 5, p = 0.107), but MeCP2 mRNA levels are also reduced at P2 (control: 0.432 ± 0.0366, N = 6; Gadd45b: 0.307 ± 0.0373, N = 6, p = 0.0384). BDNF and Adrenoceptor α2A (Adra2a) mRNA levels are unchanged at P2 (control: 0.350 ± 0.0161, N = 5; Gadd45b: 0.388 ± 0.0422, N = 5, p = 0.414; control: 0.212 ± 0.0184, N = 6; Gadd45b: 0.250 ± 0.0474, N = 6, p = 0.472, respectively). Anti-apoptotic protein Bcl2 mRNA is unchanged (control: 0.407 ± 0.0295, N = 5; Gadd45b: 0.385 ± 0.0601, N = 6, p = 0.769), and glial cell marker GFAP levels are not significantly different (control: 0.152 ± 0.0658, N = 6; Gadd45b: 0.279 ± 0.102, N = 5, p = 0.306). (B) The reduction in Reelin is transient (control: 0.232 ± 0.0298, N = 8; Gadd45b: 0.238 ± 0.0375, N = 7, p = 0.901) and no change in Gad67 levels is seen at P33 (control: 0.486 ± 0.0475, N = 8; Gadd45b: 0.529 ± 0.0380, N = 7, p = 0.504). The effect of Gad45b siRNA on MeCP2 is also transient (control: 0.381 ± 0.0879, N = 9; Gadd45b: 0.328 ± 0.0856, N = 7, p = 0.683) BDNF is significantly reduced by P33 (control: 0.584 ± 0.0476, N = 9; Gadd45b: 0.437 ± 0.0227, N = 7, p = 0.0239), as is Adra2a (control: 0.469 ± 0.0460, N = 8; Gadd45b: 0.350 ± 0.0227, N = 7, p = 0.0450). There was no change in Bcl2 (control: 0.190 ± 0.0507, N = 9; Gadd45b: 0.151 ± 0.0409, N = 6, p = 0.599) or GFAP (control: 0.215 ± 0.0606, N = 9; Gadd45b: 0.180 ± 0.0713, N = 7, p = 0.706). Control siRNA shown in dark gray; Gadd45b siRNA shown in white.
Gadd45 family members were originally described in white blood cells of the periphery; we thus examined the expression of several cytokines in the amygdala and observed a transient decrease in pro-inflammatory cytokines Il-6 and Il-1beta but not in transforming growth factor beta (Tgf-beta) at postnatal day 2 (Fig. 6A).
Fig. 6.
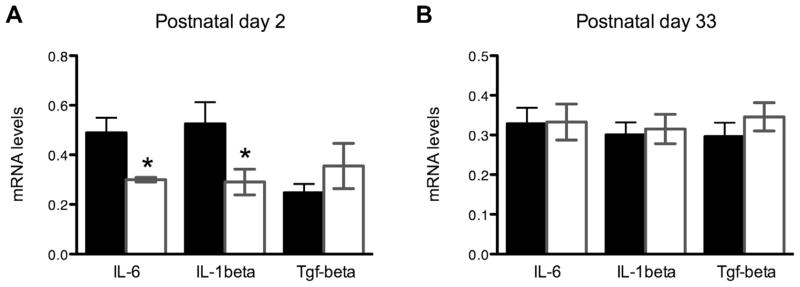
(A) Pro-inflammatory cytokines Il-6 (control: 0.489 ± 0.135, N = 5; Gadd45b: 0.300 ± 0.0184, N = 4, p = 0.029) and Il-1beta (control: 0.525 ± 0.213, N = 6; Gadd45b: 0.290 ± 0.0127, N = 6, p = 0.043), but not Tgf-beta (control: 0.247 ± 0.0353, N = 6; Gadd45b: 0.355 ± 0.0914, N = 6, p = 0.294) show less expression at PN2. (B) This effect was transient; no changes in these cytokines was observed in the juvenile period (Il-6: control: 0.329 ± 0.0398, N = 8; Gadd45b: 0.333 ± 0.0455, N = 7, p = 0.947. Il-1beta: control: 0.300 ± 0.0314, N = 9; Gadd45b: 0.315 ± 0.0370, N = 6, p = 0.766. Tgf-beta: control: 0.296 ± 0.0348, N = 8; Gadd45b: 0.346 ± 0.0355, N = 7, p = 0.337).
3.7. Gadd45b siRNA epigenetically alters methylation of the Adra2a promoter in the amygdala
We chose to further examine the promoter region of Adra2a, shown in Fig. 7, using a methylation-sensitive restriction enzyme method. Site 1 primers were designed around a cAMP responsive element-binding protein (CREB) transcription-factor binding site distal to the transcriptional start site (TSS), and Site 2 primers were designed around an early growth response protein 1 (Egr-1) site more proximal to the TSS.
Fig. 7.
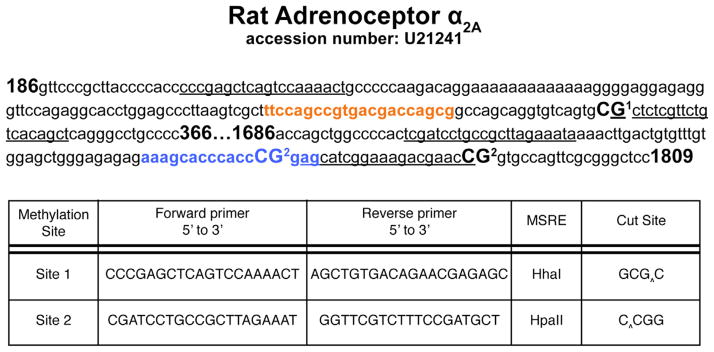
Methodological details for methylation sensitive restriction enzyme (MSRE) analysis of CpG sites within the rat Adra2a promoter (GenBank Accession No. U21241). Primer annealing sequences used for examining methylation at sites 1 and 2 are underlined. Each methylation site examined is bolded and numbered. MatInspector predicted transcription factor binding sites are indicated (CREB site shown in box A; Egr-1 is shown in box B). Primers used to investigate the methylation status at two distinct promoter sites are indicated. Cut sites for each MSRE are indicated by an arrow. Methylation at these CpG sites protects against enzyme digestion and allows for PCR amplification.
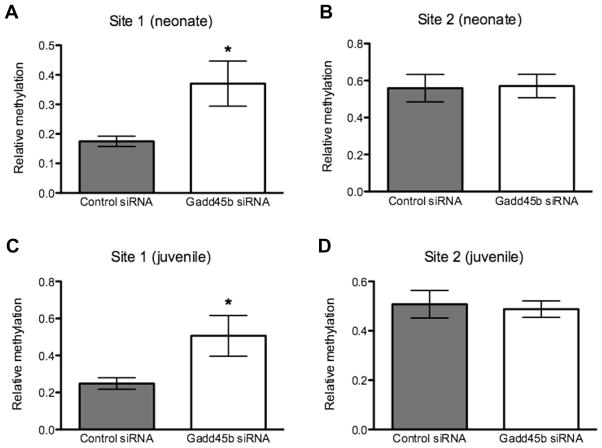
In the neonatal period, the Gadd45b siRNA infused animals showed a significant 52% increase in Adra2a promoter methylation at Site 1 (Fig. 8A), with no effect on methylation at Site 2 (Fig. 8B). We found a significant, 51% increase in methylation of the Adra2a promoter in the juvenile amygdala at Site 1 (Fig. 8C), but again no significant change at Site 2 (Fig. 8D).
Fig. 8.
Neonatal Gadd45b siRNA treatment epigenetically alters Adra2a promoter DNA methylation near a CREB binding site at P2 (control: 0.175 ± 0.0175, N = 5 vs. Gadd45b: 0.371 ± 0.0764, N = 6; p = 0.0489) and P33 (control: 0.249 ± 0.0310, N = 8 vs. Gadd45b: 0.506 ± 0.110, N = 7; p = 0.0328) (A and C) but a downstream Egr-1 site shows no methylation difference at either P2 (control: 0.558 ± 0.0744, N = 6; Gadd45b: 0.571 ± 0.0634, N = 6; p = 0.902) or P33 (control: 0.508 ± 0.0557, N = 9; Gadd45b: 0.488 ± 0.0333, N = 7; p = 0.781) (B and D).
4. Discussion
Our findings indicate that a transient knockdown of Gadd45b expression in the neonatal amygdala can cause lasting changes in juvenile social behaviors. Specifically, we report that Gadd45b siRNA treatment results in a dramatic increase in the overall frequency of juvenile social play behavior, recorded twice a day for 5 min over the course of 5 days for a total of 50 min of play per animal and reported as an average frequency (Fig. 2A). ‘Play’ involves a complex suite of behaviors, including pouncing, chasing, boxing and pinning; all of these components were increased in the Gadd45b siRNA-treated animals relative to controls, with the exception of pinning (Fig. 2B–E). This may be indicative of a generally increased drive to engage in play. We have previously reported that MeCP2 knockdown within the developing amygdala results in decreased juvenile social play (Kurian et al., 2008), suggesting that epigenetic processes associated with gene repression are important for programming, and possibly activating, typical levels of juvenile social play. In the current study, disruption to a molecule involved in DNA demethylation increased play, which may indicate Gadd45b is involved in the formation of inhibitory pathways governing this behavior. Together, these data indicate that the developmental programming of juvenile social play behavior is perhaps under repressive control by epigenetic modifications within the newborn amygdala.
To further examine Gadd45b’s role in juvenile social interactions, we used the three-chambered test for sociability to investigate anti-social behavior in rodents (Moretti et al., 2005; Yang et al., 2011). We find that neonatal Gadd45b disruption results in lower levels of juvenile sociability; specifically, Gadd45b siRNA-treated animals spent 37% less time in the social chamber than control siRNA-treated animals (Fig. 3). While these behavioral results may appear contradictory when compared to the play behavior data, an increased drive to play could impede performance in the sociability task. Specifically, by depriving Gadd45b-siRNA treated rats of the ability to engage in play with the stimulus rat, higher impulsivity behavior and thus greater exploration of the non-social chamber may occur. Alternatively, the molecular pathways regulating play and sociability could be regulated differently and therefore be dissociable. The seemingly disparate behavioral phenotype observed with Gadd45b siRNA treatment has been previously observed in spontaneously hypertensive rats (SHRs), which are a commonly used animal model of ADHD. Specifically, SHRs show increased play behavior towards conspecifics (Ferguson and Cada, 2004) and decreased social interaction (Calzavara et al., 2011) when compared to the parental Wistar-Kyoto strain.
The similar behavioral phenotype of Gadd45b-siRNA treated rats and SHRs with regard to play and sociality also appears to be supported by parallel disruptions in Adra2a. That is, SHRs show reduced levels of the norepinephrine-binding autoreceptor Adra2a in the brain (Olmos et al., 1991), and Gadd45b siRNA treatment also results in significantly decreased expression of Adra2a in the juvenile amygdala (Fig. 5B). The reduced levels of Adra2a may be a result of reduced Gadd45b expression allowing for increased methylation near a CREB-binding site in the promoter (Fig. 8A and C). The effect of Gadd45b siRNA on Adra2a promoter methylation at Site 1 was observed during the neonatal period and lasts into the juvenile period; however, the change in mRNA did not occur until P33. This suggests that the organization of Adra2a expression occurs early in development and that epigenetic programming of CpG sites near the CREB-binding site may be important for driving Adra2a expression later in life. That is, while reducing Gadd45b resulted in increased methylation of the Adra2a promoter, it did not result in a functional consequence on Adra2a mRNA levels until the juvenile period when social play emerges. Importantly, previous data have implicated Adra2a as an important molecule in regulating juvenile social play behavior. Pharmacological Adra2a antagonism increases juvenile social play in rats (Siviy and Baliko, 2000). Furthermore, pretreatment with an Adra2a antagonist can significantly reverse deficits in play behavior incurred by the administration of either amphetamine or methylphenidate (Vanderschuren et al., 2008). These data suggest that Adra2a acts to suppress juvenile social play. The directionality of Gadd45b regulated Adra2a expression is consistent with its suppressive role on juvenile social play behavior; thus the Gadd45b-mediated programming of the Adra2a promoter observed in this study may provide some mechanistic insight into a potent regulator of complex social behavior.
We furthermore report a 30% decrease in Mecp2 mRNA expression in the neonatal amygdala that was no longer detected at P33 (Fig. 5). A behavioral study using Mecp2308/Y mice, in which the Mecp2 protein is truncated, revealed significantly decreased social interactions with an unfamiliar stimulus animal in the three-chambered sociability task, whereas social behavior towards familiar animals did not differ from wild type controls (Moretti et al., 2005), which appears to be consistent with our current behavioral data set. However, a previous study from our lab in which we targeted the amygdala with Mecp2 siRNA resulted in decreased juvenile social play behavior (Kurian et al., 2008). It is possible that the decrease in Adra2a expression due to Gadd45b siRNA treatment outcompetes the deficits in social play behavior induced by Mecp2 knockdown, as Adra2a antagonists profoundly affect the frequency of juvenile social play (Vanderschuren et al., 2008). It is also possible that genetic manipulation of the Mecp2 protein and the effect of Gadd45b siRNA alter as yet unidentified binding partners of Mecp2 that would change its effect on gene transcription (Yasui et al., 2007; Chahrour et al., 2008). Nevertheless, Mecp2 deficiency is implicated in both Rett syndrome and autism spectrum disorders (ASDs), both of which are characterized by abnormal social interactions. Future work is necessary to determine if pharmacological α2-adrenoceptor manipulation can overcome some of the social deficits observed in Mecp2 deficient rodent models.
It is important to note that there were no alterations in anxiety-like behavior (Supplementary Figs. 2–5), suggesting that the behavioral disruptions were not universal, but may be limited to juvenile social interactions. Furthermore, genes involved in cell death or cellular differentiation were unaltered by Gadd45b siRNA treatment within the amygdala. Specifically, expression of either the anti-apoptotic protein Bcl2 (Garcia et al., 1992) or GFAP – an intermediate filament found in glia, astrocytes and neural stem cells (Imura et al., 2003), which is typically altered in response to damage (Bignami and Dahl, 1974) – was not different between treatment groups at either time point (Fig. 5), suggesting that we have not globally altered cell death or response to damage within the amygdala. Finally, lesioning the amygdala decreases social play behavior (Meaney et al., 1981), whereas Gadd45b siRNA resulted in the opposite effect. Combined, these data indicate that it is unlikely that Gadd45b siRNA infusions resulted in global amygdala disruption.
To further characterize the molecular effects of Gadd45b knockdown, we examined the mRNA levels of several candidate genes linked to Gadd45b expression. A recent study using Gadd45b ChIP revealed binding to the promoter regions of Reelin, Gad67, and BDNF in the hippocampus (Matrisciano et al., 2011) – genes previously implicated in the etiology of psychiatric disorders. Interestingly, expression of these genes was differentially affected by Gadd45b siRNA between the neonatal and juvenile amygdala data sets. While Gad67 failed to reach significance at either time point, there was a dramatic 75% neonatal reduction in Reelin expression that was no longer present at P33. Conversely, the expression of BDNF was not different from controls at P2, but was significantly decreased during the juvenile period (Fig. 5). The temporally specific effect of Gadd45b manipulation warrants future study, and may depend on the availability of as yet unidentified co-factors to localize it to each promoter region.
Reduced expression of BDNF was suggested as a mechanism to explain GABAergic interneurons’ vulnerability to environmental stressors in the amygdala (Guilloux et al., 2012), though the ontology of this reduction in BDNF remains elusive. Our data suggests that Gadd45b-mediated expression of Reelin early in development may belie this change; Reelin, a large glycoprotein preferentially excreted from GABAergic neurons (Pesold et al., 1998), colocalizes with integrins (Rodriguez et al., 2000) and is thought to stabilize dendritic spines and connections with interneurons (Guidotti et al., 2000). The large reduction in the expression of Reelin seen in our experiment could result in abnormal neurite outgrowth and synapse stabilization of GABAergic interneurons from or within the amygdala. Furthermore, while little is known about Gadd45g, it was previously demonstrated that BDNF expression in the ventral tegmental area (VTA) is necessary to maintain typical Gadd45g levels in the nucleus accumbens (Koo et al., 2012). Thus, a cascade of gene expression changes beginning with early, perinatal programming of synapse-stabilizing genes like Reelin by Gadd45b may have lasting consequences on the integrity of GABAergic interneurons, which could in turn, through changes in BDNF expression, influence the expression profile of their postsynaptic targets, as with the reduced levels of Gadd45g seen in this study (Fig. 4). Consequently, Gadd45b manipulation may disproportionally affect the formation of GABAergic signaling pathways in and from the amygdala to other brain regions, which may underlie the behavioral disinhibition of social play observed here.
Finally, we investigated the expression of several pro-inflammatory cytokines, as Gadd45b has been studied extensively with respect to innate immunity and cytokine production in the periphery (for excellent reviews see Hoffman and Liebermann, 2007; Schmitz, 2013). Importantly, a functional role for Gadd45b in the production of Il-6 was demonstrated in activated white blood cells of a Gadd45b knockout mouse (Lu et al., 2004). To our knowledge, no study has examined whether Gadd45b alters cytokine production in the brain, despite both a neuroprotective role for neuronally-expressed Il-6 (Gadient and Otten, 1997) and emerging data to suggest proinflammatory cytokines – including Il-6 and Il-1beta – can affect social behavior (Weil et al., 2006; Hennessy et al., 2014). We observed a 40% reduction in the expression of Il-6 in the neonatal amygdala of our Gadd45b siRNA animals (Fig. 6A). We also observed a 45% reduction in the expression of a potent inducer of Il-6 expression, Il-1beta (Ringheim et al., 1995). Tgf-beta, previously shown to induce expression of Gadd45b in retinal ganglion cells (Liu et al., 2013), is linked to Gadd45b signaling in the dentate gyrus (Haditsch et al., 2013), and may be upstream of Gadd45b signaling whereas Il-6 and Il-1beta may be downstream effectors. Consistent with this idea, we observed no change in Tgf-beta expression at either time point (Fig. 6).
At present, we are unable to discern whether reduced expression of Il-6 and Il-1beta is a consequence of less Gadd45b activity, as seen previously in white blood cells, or if the significantly reduced availability of Reelin causes an indirect change through altered synapse formation. It is of interest that Il-6 has been shown to specifically promote the formation and stability of tyrosine hydroxylase (TH)-positive neurons during postnatal rat brain development (Kushima et al., 1992) – TH being the rate-limiting step in production of catecholamines, including norepinephrine. Although a social defeat paradigm did establish a role for β-adrenergic receptor signaling in Il-1beta-dependent anxiety behavior (Wohleb et al., 2011), it remains to be determined whether there are links between the availability of Il-6 and expression of Adra2a, which could help integrate the signaling pathways altered in our current data set.
While the functional consequences of cytokine regulation in programming juvenile social interactions is unclear, reduced levels of cytokines, including Il-6 and Il-1beta, are associated with aggressive behavior in adult humans (Provençal et al., 2013) and mice (Alleva et al., 1998). Juvenile rough and tumble play behavior as described herein is not thought to be an aggressive behavior – rather, it is reported to be a joyful and rewarding activity (Burgdorf et al., 2008). Generally speaking, the aggressive behaviors linked to cytokine levels may be ascribed to alterations in sociability, consistent with previous observations (Weil et al., 2006; Hennessy et al., 2014); mechanisms to understand these links are of considerable interest, as altered levels of cytokines have been linked to a variety of environmental stressors and neuropsychiatric disorders (for review, see Bilbo and Schwarz, 2012).
5. Conclusions
Herein we have demonstrated a novel role for Gadd45b-mediated programming of social behaviors, altered epigenetic programming of Adra2a, and raised the intriguing idea of reduced cytokine expression as a mediating pathway for altered juvenile social development. These effects are rather complex in that they increase juvenile social play but disrupt sociability; however, the behavioral and molecular changes seen here appear to be consistent with social alterations found in several animal models of human neurodevelopmental disorders. Interestingly, Gadd45b knockdown resulted in both changes specific to social behaviors and to an epigenetically programmed decrease in the expression of Adra2a. It will be important for future work to determine if pharmacologically targeting this receptor would be therapeutic in the treatment of neurodevelopmental disorders presenting with social deficits. A parsimonious explanation of the data presented here is that increased gene expression in the amygdala is required to inhibit certain aspects of juvenile social play behavior; reducing the cell’s ability to demethylate genes, such as Adra2a, may disinhibit the system and cause atypical levels of play due to abnormal epigenetic programming.
Supplementary Material
Acknowledgments
This work supported by NIH #T32 GM008688 to S.L.K. and NSF IOS-112207 to A.P.A.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2015.02.018.
Footnotes
Author contributions
S.L.K. and A.P.A. designed research, S.L.K. and L.C. performed research, S.L.K. and L.C. analyzed data, S.L.K. and A.P.A. wrote the paper.
Financial interests
The authors declare no competing financial interests.
References
- Alleva E, Cirulli F, Bianchi M, Bondiolotti GP, Chiarotti F, De Acetis L, Panerai AE. Behavioral characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur J Neurosci. 1998;10 (12):3664–3672. doi: 10.1046/j.1460-9568.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Coss D, Auger AP, Forbes-Lorman RM. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male. Proc Natl Acad Sci. 2011;108 (10):4242–4247. doi: 10.1073/pnas.1100314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Dahl D. Astrocyte-specific protein and radial glia in the cerebral cortex of newborn rat. Nature. 1974;252 (5478):55–56. doi: 10.1038/252055a0. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and the developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33 (3):267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122 (4):357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Calzavara MB, Levin R, Medrano WA, Almeida V, Sampaio APF, Barone LC, Frussa-Filho R, Abílio VC. Effects of antipsychotics and amphetamine on social behaviors in spontaneously hypertensive rats. Behav Brain Res. 2011;225 (1):15–22. doi: 10.1016/j.bbr.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Carrier F, Smith ML, Bae I, Kilpatrick KE, Lansing TJ, Chen C, Engelstein M, Friend SH, Henner WD, Gilmer TM, Kastan MB, Fornace AJ. Characterization of human Gadd45, a p53-regulated protein. J Biol Chem. 1994;269 (51):32672–32677. [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Gavin DP, Chen Y, Davis J. Upregulation of TET1 and downregulation of APOBEC3A and APOBEC3C in the parietal cortex of psychotic patients. Trans Psychiatry. 2012;2 (9):e157–e159. doi: 10.1038/tp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Cada AM. Spatial learning/memory and social and nonsocial behaviors in the Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacol Biochem Behav. 2004;77 (3):583–594. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7 (3):230–238. doi: 10.4161/epi.7.3.19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Kurian JR, Auger AP. MeCP2 regulates GFAP expression within the developing brain. Brain Res. 2014;1543:151–158. doi: 10.1016/j.brainres.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6) – a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52 (5):379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Garcia I, Martinou I, Tsujimoto Y, Martinou JC. Prevention of programmed cell death of sympathetic neurons by bcl-2 proto-oncogene. Science. 1992;258:302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- Gavin DP, Sharma RP, Chase KA, Matrisciano F, Dong E, Guidotti A. Growth arrest and DNA-damage-inducible, beta (GADD45b)-mediated DNA demethylation in major psychosis. Neuropsychopharmacology. 2011;37:531–542. doi: 10.1038/npp.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, DiGiorgi Gerevini V, Dwivedi Y, Grayson D, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in Reelin and glutamic acid decarboylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57 (11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haditsch U, Anderson MP, Freewoman J, Cord B, Babu H, Brakebusch C, Palmer TD. Neuronal Rac1 is required for leaning-evoked neurogenesis. J Neurosci. 2013;33 (30):12229–12241. doi: 10.1523/JNEUROSCI.2939-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kokubun S, Itoi E, Roach HI. Improved quantification of DNA methylation using methylation-sensitive restriction enzymes and real-time PCR. Epigenetics. 2007;2 (2):86–95. doi: 10.4161/epi.2.2.4203. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml PA. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav Immun. 2014;37:15–20. doi: 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B, Liebermann DA. Role of gadd45 in myeloid cells in response to hematopoietic stress. Blood Cells Mol Dis. 2007;39 (3):344–347. doi: 10.1016/j.bcmd.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23 (7):2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen HM, Kolodkin MH, Bychowski ME, Auger CJ, Auger AP. The nuclear receptor corepressor has organizational effects within the developing amygdala on juvenile social play and anxiety-like behavior. Endocrinology. 2010;151 (3):1212–1220. doi: 10.1210/en.2009-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley MB, Wood MA, Isiegas C, Stein J, Hellman K, Hannenhalli S, Abel T. Differential transcriptional response to nonassociative and associative components of classical fear conditioning in the amygdala and hippocampus. Learn Mem. 2006;13 (2):135–142. doi: 10.1101/lm.86906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robinson MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28 (28):7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima Y, Hama T, Hatanaka H. Interleukin-6 as a neurotrophic factor for promoting the survival of cultured catecholaminergic neurons in a chemically defined medium from fetal and postnatal rat midbrains. Neurosci Res. 1992;13:267–280. doi: 10.1016/0168-0102(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Leach PT, Poplawski SG, Kenney JW, Hoffman B, Liebermann DA, Abel T, Gould TJ. Gadd45b knockout mice exhibit selective deficits in hippocampus-dependent long-term memory. Learn Mem. 2012;19 (8):319–324. doi: 10.1101/lm.024984.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Sun X, Suyeoka G, Garcia JGN, Leiderman YI. TGFβ signaling induces expression of Gadd45b in retinal ganglion cells. Invest Opthalmol Vis Sci. 2013;54 (2):1061–1069. doi: 10.1167/iovs.12-10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Ferrandino AF, Flavell Gadd45β is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol. 2004;5 (1):38–44. doi: 10.1038/ni1020. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang MI, Pow-anpongkul N, Flavell RA, Lu B, Ming GI, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323 (5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Dong E, Gavin DP, Nicoletti F, Guidotti A. Activation of group II metabotropic glutamate receptors promotes DNA demethylation in the mouse brain. Mol Pharm. 2011;80 (1):174–182. doi: 10.1124/mol.110.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Mong JA, Sickel MJ, Davis AM. Antisense oligodeoxynucleotides as a tool in developmental neuroendocrinology. Methods. 2000;22 (3):239–248. doi: 10.1006/meth.2000.1075. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with child abuse. Nat Neurosci. 2009;12 (3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Dodge AM, Beatty WW. Sex-dependent effects of amygdaloid lesions on the social play of prepubertal rats. Physiol Behav. 1981;26 (3):467–472. doi: 10.1016/0031-9384(81)90175-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, McEwen BS. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398 (2):324–328. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14 (2):205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1 (4):e1–e11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Schäfer A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol. 2012;22 (4):220–227. doi: 10.1016/j.tcb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in the neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146 (9):3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Olmos G, Miralles A, Barturen F, García-Sevilla JA. Decreased density and sensitivity of alpha 2-adrenoceptors in the brain of spontaneously hypertensive rats. Eur J Pharm. 1991;205 (1):93–96. doi: 10.1016/0014-2999(91)90776-m. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play; theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8 (4):465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing γ-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provençal N, Suderman MJ, Vitaro F, Szyf M, Tremblay RE. Childhood chronic physical aggression associates with adult cytokine levels in plasma. PLoS One. 2013;8 (7):e69481. doi: 10.1371/journal.pone.0069481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringheim GR, Burgher KL, Heroux JA. Interleukin-6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin-6 production in the brain. J Neuroimmunol. 1995;63:113–123. doi: 10.1016/0165-5728(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Pesold C, Liu WS, Kriho V, Guidotti A, Pappas GD, Costa E. Colocalization of integrin receptors and Reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc Natl Acad Sci. 2000;97 (7):3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz I. Gadd45 proteins in immunity. Adv Exp Med Biol. 2013;793:51–68. doi: 10.1007/978-1-4614-8289-5_4. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Baliko CN. A further characterization of alpha-2 adrenoceptor involvement in the rough-and-tumble play of juvenile rats. Dev Psychobiol. 2000;37 (1):25–34. doi: 10.1002/1098-2302(200007)37:1<25::aid-dev4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sultan FA, Wang J, Tront J, Liebermann DA, Sweatt JD. Genetic deletion of gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci. 2012;32 (48):17059–17066. doi: 10.1523/JNEUROSCI.1747-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95 (4):521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, van Leeuwen N, de Vries TJ, Schoffelmeer ANM. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012;1822 (6):831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Bowers SL, Pyter LM, Nelson RJ. Social interactions alter proinflammatory cytokine gene expression and behavior following endotoxin administration. Brain Behav Immun. 2006;20:72–79. doi: 10.1016/j.bbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31 (17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;56 (8.26):1–16. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci. 2007;104 (49):19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.