Abstract
Gender-moderated gene–environment interactions are rarely explored, raising concerns about inaccurate specification of etiological models and inferential errors. The current study examined the influence of gender, negative and positive daily life events, and GABRA2 genotype (SNP rs279871) on alcohol dependence, testing two- and three-way interactions between these variables using multilevel regression models fit to data from 2,281 White participants in the Collaborative Study on the Genetics of Alcoholism. Significant direct effects of variables of interest were identified, as well as gender-specific moderation of genetic risk on this SNP by social experiences. Higher levels of positive life events were protective for men with the high-risk genotype, but not among men with the low-risk genotype or women, regardless of genotype. Our findings support the disinhibition theory of alcohol dependence, suggesting that gender differences in social norms, constraints and opportunities, and behavioral undercontrol may explain men and women’s distinct patterns of association.
Keywords: Gender, Genetics, Gene–environment interaction, Daily hassles and uplifts, Alcohol dependence
Introduction
In the past decade, there has been significant interest in identifying the complex and interacting pathways through which genetic predisposition and environmental factors are associated with complex behavioral disorders (Moffitt et al. 2006; Rutter et al. 2006). Alcohol dependence and drinking behaviors have been identified as a particularly rich case for examining gene–environment interactions (Bearman and Brückner 2002; Guo 2006). Problem drinking is linked to gene expression through multiple pathophysiological systems, but is also driven by social influences like socioeconomic status, family structure, stressful conditions, and socialization (e.g., Singer and Ryff 2001). As a result, the effects of genetic factors are likely to vary substantially depending on numerous environmental contingencies. For example, a genetic effect may be strong and robust in the absence of social controls (e.g., low parental monitoring), but weak or nonexistent when controls are present (Dick et al. 2009). A lack of attention to GxE in this case is problematic since it would likely lead to underestimation of the strength of both genetic and environmental influences, potentially impeding the researcher’s ability to detect statistically significant effects (Heath and Nelson 2002).
In alcohol research, features of the social environment have been shown to mediate or moderate the effect of hereditary predispositions towards alcohol use that involve disinhibition and behavioral undercontrol. For instance, studies using a sample of Finnish adolescent twins found that genetic risk factors have relatively little influence on drinking behaviors in rural areas, attributing this interaction to limited access to alcohol and lower exposure to adolescent drinking in these environments (Dick et al. 2001; Rose et al. 2001). Likewise, research indicated that the influence of high-risk genotypes on alcohol misuse was diminished in the presence of positive family relations and high parental monitoring (Dick et al. 2009; Nilsson et al. 2005; Pescosolido et al. 2008). Religious upbringing also exhibited protective effects, reducing the influence of genetic risk on alcohol outcomes (Koopmans et al. 1999). These GxE effects likely relate to the behavioral under-control-disinhibition theory of alcohol dependence, in which individuals are genetically predisposed to impulsivity and externalizing behavior through neurological pathways (Dick et al. 2010; Villafuerte et al. 2012). These heritable traits may manifest as conduct disorder or other externalizing problems in childhood, and as alcohol or drug dependence in adulthood when these substances are more accessible (Dick et al. 2006a). In addition, conditions such as marriage and employment can serve as external social control functions in adulthood, particularly when they are rewarding, reducing the effects of behavioral undercontrol-disinhibition and related genotypes on alcohol use (Eitle et al. 2010; Heath and Nelson 2002; Umberson et al. 2010). Consequently, in combination with ineffective social control, permissive social norms, and exposure to problem drinking through deviant peers, genetic risk for behavioral disinhibition may lead to alcohol misuse and dependence (Dick et al. 2009; Zucker et al. 2011); conversely, when opportunity to drink is constrained and/or social norms are strongly present, genetic risk for disinhibition may find more limited expression.
However, because research has pointed to important gender differences in the social pressures, opportunities, and expectations that promote or constrain drinking behavior, the disinhibition pathway may lead to distinct patterns of alcohol consumption and dependence in men and women. Broadly, research has consistently documented higher rates of alcohol use and alcohol-related problems among men (Gomberg 1997). Estimates based on nationally-representative samples suggested that the male to female ratio for the prevalence of alcohol dependence was about 2.5:1 (Grant 1997; Kessler et al. 1994). Though the gender gap in alcohol consumption has been narrowing over time, recent estimates indicate that men in the youngest cohorts are still nearly twice as likely to develop dependence compared to similar-aged women (Keyes et al. 2008).
Research on gender norms and social control processes indicates that disinhibition pathways in alcohol misuse may operate differently among men and women. Studies have reported that the American public perceives harsher social sanctions in response to alcohol and drug use by women and girls relative to men and boys (for reviews, see Nolen-Hoeksema and Hilt 2006; Schmidt et al. 1990). This stigma may derive from the conflict between alcohol misuse and feminine social norms and obligations relating to virtue, emotionality, and nurturing and caregiving for children and families (Bancroft 2009; Schulte et al. 2009). In contrast, alcohol consumption and its effects are consistent with aspects of the masculine gender role, including aggression and instrumentality. In fact, women who more strongly adhere to feminine gender norms have been shown to report lower frequency and quantity of alcohol use, while endorsement of masculine norms was associated with increased drinking in men (Horwitz and White 1987; Huselid and Cooper 1992; Landrine et al. 1988; Ricciardelli et al. 2001). Moreover, both women and men perceive that the ‘‘typical woman’’ consumes less alcohol than the ‘‘typical man,’’ and this expectation has a protective effect on drinking behaviors for women, but not for men (Lewis and Neighbors 2004).
At the same time, gender differences in behavioral undercontrol also disadvantage men with respect to alcohol outcomes (Nolen-Hoeksema and Hilt 2006). Research has consistently reported higher scores on measures of impulsivity, sensation-seeking, and disinhibition among men (Cross et al. 2011; Zuckerman and Kuhlman 2000), and these traits have been strongly associated with heavy alcohol use and alcohol-related problems (Caspi et al. 1997; Sher et al. 2005). Moreover, some studies indicated that behavioral undercontrol was exclusively or more strongly related to heavy drinking and alcohol-related problems among men compared to women (Caspi et al. 1996; Rutledge and Sher 2001b). In sum, women appear to be less likely to engage in drinking as a result of internal behavioral undercontrol and related traits, and, moreover, a strong set of external social norms is in place to discourage women and girls from alcohol misuse. Taken together, these strands of research suggest that women may be less susceptible to developing alcohol dependence through genetic and environmental mechanisms in the disinhibition pathway.
These gender differences in mechanisms of disinhibition in alcohol dependence merit attention in so far as they might lead to gender-specific GxE effects. We refer to this phenomenon as a GxExE effect, conceptualizing gender as an environmental variable—an approach that has garnered growing support in the genetics literature (Ober et al. 2008). ‘‘Sex’’ refers to biological and physical characteristics distinguishing men and women, and clearly has a genetic basis in sex chromosomes and epigenetic regulation. ‘‘Gender,’’ however, is a social construction encompassing cultural conventions, roles, and behaviors adopted by men and women that shape their experiences and activities. Sex and gender often overlap in meaningful ways, such that differences between men and women are attributable to a combination of social and biological forces that often cannot be extricated from one another. However, in the case of the current study, the literature reviewed above points to gender differences in mechanisms that we feel are attributable in larger part to social forces (e.g., gender norms). Thus, gender as environment captures the cumulative effects on men and women of living in a social world where such categories matter—a process that undoubtedly has some roots in biological sex differences.
When men and women experience shared environments differently (Bird and Rieker 2008), whether due to biological or social factors, gender is likely to moderate the influence of genetics and/or environments in etiological pathways, leading to GxExE effects. A GxExE effect is one in which gene–environment interactions are observable in only one gender group or the magnitude or pattern of gene–environment interaction (e.g., direction) differs between the two genders (Shanahan et al. 2008). For example, because women’s drinking behavior is subjected to greater external social control by gender norms, having friends who engage in alcohol misuse might have a stronger adverse effect in men compared to women (an ExE effect). Hypothetically, then, exposure to deviant peer groups might trigger a candidate gene associated with alcohol dependence (a GxE effect), but only for men (a GxExE effect).
If ignored, gender-differentiated GxE effects can increase the likelihood of null findings and obscure complex interactions between genetic predisposition and gendered experiences, motivations, and expectations. Consequently, Shanahan and Hofer (2005) argue for more complex models that go beyond the interaction of genotype and only one indicator of the social environment. Despite evidence of gender differences in mechanisms of affect regulation and disinhibition in alcohol dependence, existing studies have rarely examined gender-specific gene–environment interactions involved in these etiological pathways. Frequently, studies have been statistically underpowered to support complex interaction models (Patsopoulos et al. 2007). However, even when gender differences in genetic effects were examined, inappropriate methods were often used (Brookes et al. 2004).
The aim of the present study is to explore whether gene–environment interaction in disinhibition pathways is moderated by gender. To accomplish this, two- and three-way interactions between gender, positive and negative daily life events, and genotype at GABRA2 SNP rs279871 are examined. GABRA2 is an ideal candidate gene for examining genetic influences in disinhibition pathways because it plays a major role in impulsivity (Villafuerte et al. 2012). GABRA2 encodes the production of the alpha 2 subunit of the GABA-A receptor protein and influences hyperexcit-ability and the effectiveness of inhibitory processors in the brain (Begleiter and Porjesz 1999; Edenberg et al. 2004). Single nucleotide polymorphisms at GABRA2 are associated with increased risk for alcohol and drug dependence in adulthood, as well as conduct disorder and externalizing behaviors in childhood (Agrawal et al. 2006; Dick et al. 2006a, 2009; Edenberg et al. 2004; Enoch et al. 2010; Philibert et al. 2009). GABRA2 is thought to influence emotional reactivity and the inhibition or facilitation of rash or ill-considered behaviors (Kreek et al. 2005).
Findings on gender differences in the effects of GABRA2 have been mixed, with some studies finding adverse effects of high risk genotypes are more pronounced in women (Philibert et al. 2009; Villafuerte et al. 2012), and others in men (Enoch et al. 2006; Pescosolido et al. 2008). A number of other GxE effects involving GABRA2 have been identified, consistent with the disinhibition model of alcohol dependence described above. Studies have reported that in social environments characterized by high levels of social control, people were less apt to engage in maladaptive, deviant behavior irrespective of genotype, reducing the influence of GABRA2 (Dick et al. 2006a, 2009; Philibert et al. 2008). Conversely, when exposed to negative socialization or permissive social groups and contexts, patterns of drug and alcohol use reflected the full range of genetic variation.
Here we build upon previous research examining how GABRA2 interacts with life events (Pescosolido et al. 2008). However, we extend this prior research by exploring how this interaction is further moderated by gender. As predicted based on previous research in the area of gender and addiction, we find evidence for gender-specific GxE effects in a family-based sample of White Americans.
Methods
Subjects
Data are from the Collaborative Study on the Genetics of Alcoholism (COGA). Phase I data were collected at six venues between 1990 and 1999, and Phase II data between 1997 and 2004 (Edenberg et al. 2004). Only Phase II data were used in these analyses because key independent variables were not collected in Phase I. COGA relied on a complex, availability-based, family selection strategy (Reich 1996). In brief, probands with alcohol dependence were recruited at inpatient and outpatient treatment facilities through random selection procedures. After determining probands’ family distributions of alcohol dependence, densely affected nuclear and multigenerational families were identified and invited to participate. In addition, a group of control probands and their families were recruited from church congregations, large corporations, dental clinics, driver’s license bureaus, and health maintenance organizations to serve as community controls. Probands and first-, second-, and third-degree relatives who agreed to participate provided blood samples for DNA analysis, completed a structured psychiatric interview, and provided data on personality traits, family history, and psychosocial measures.
These selection procedures yielded a sample of 10,330 subjects at the Phase II assessment. Children and adolescents (n = 2,537) and adults without genetic information on GABRA2 (n = 4,853) were eliminated. Because risk for alcohol dependence associated with GABRA2 has been found to be weaker among adolescents relative to adults (Dick et al. 2006a), we conducted parallel analyses with an age-restricted sample of 25 or older. In addition, we ran analyses excluding lifetime non-drinkers, for whom the effects of GABRA2 genotype are also likely to be weak. Because dropping these cases did not affect our results, we retained the full sample. Respondents with genotype information were primarily from multiplex (i.e. densely affected) families and control families. Thus, families containing only one respondent with alcohol dependence were underrepresented in the genotyped subsample. Observations were also deleted if there were missing data on other study variables (n = 365).
In addition, initial descriptive statistics and bivariate analyses suggested large and significant differences in allele frequency between White and African American respondents in the COGA sample. Specifically, 53 % of African Americans carried the high-risk genotype on SNP rs279871 compared to only 32 % of Whites (X2 = 54.29; p < 0.001). Prior research demonstrated no significant association between GABRA2 variation and alcohol dependence in African American samples (Covault et al. 2007; Drgon et al. 2006; Enoch et al. 2010; Gelernter et al. 2009), and the same pattern is evident among African Americans in the current COGA analysis sample. Moreover, African Americans have two additional common haplotypes within the distal haplotype block, suggesting that rs279871 may not be an appropriate tag SNP for this population (Enoch 2008; Enoch et al. 2010). Consequently, the current analysis examining gender-specific GxE in alcohol dependence using SNP rs279871 was restricted to a sub-sample of Whites (294 African Americans were dropped from the analysis sample).
This yielded a final sample of 2,281 adult subjects (18 or older) from 461 families, with an average of 4.9 respondents per family. The sample contained more women (56 %) than men (44 %). Mean age was 40.4 years. About 57 % of respondents were currently married. Mean years of education was 13.6 and average household income was about $53K. See Table 1 for sample descriptive statistics.
Table 1.
GABRA2 genotype (SNP rs279871) and daily hassles and uplifts by gender and affection status (DSM-IV alcohol dependence), COGA (2,281)
| All | Affected n = 807 (36 %) | Unaffected n = 1,474 (65 %) | ||||
|---|---|---|---|---|---|---|
| n (%) | X2 | n (%) | X2 | n (%) | X2 | |
| High risk genotype | 724 (32) | 287 (36) | 437 (30) | 8.43** | ||
| Women | 403 (32) | 0.03 | 116 (34) | 2.51 | 378 (34) | 4.89* |
| Men | 321 (32) | 226 (40) | 161 (29) | |||
| Currently married | 1,555 (53) | 388 (48) | 902 (61) | 36.50*** | ||
| Women | 739 (58) | 2.18 | 132 (45) | 2.07 | 607 (62) | 0.57 |
| Men | 551 (55) | 256 (50) | 295 (60) | |||
| m(sd) | t | m(sd) | t | m(sd) | t | Range | |
|---|---|---|---|---|---|---|---|
| Age (years) | 40.56 (14.77) | 39.79 (12.96) | 40.97 (15.67) | 1.82 | 18.00–84.00 | ||
| Women | 40.30 (14.50) | 0.94 | 37.55 (10.70) | 3.76*** | 41.12 (15.37) | −0.52 | |
| Men | 40.88 (15.11) | 41.09 (13.95) | 40.67 (16.25) | ||||
| Education (years) | 13.58 (2.30) | 13.12 (2.28) | 13.83 (2.27) | 7.17*** | 4.00–17.00 | ||
| Women | 13.52 (2.22) | 1.45 | 13.04 (2.20) | 0.76 | 13.67 (2.21) | 4.08*** | |
| Men | 13.66 (2.40) | 13.17 (2.33) | 14.18 (2.37) | ||||
| HH income ($10K) | 53.33 (40.50) | 46.78 (39.09) | 56.93 (40.83) | 5.76*** | 0.50–175.00 | ||
| Women | 53.00 (39.50) | 0.45 | 43.68 (34.60) | 1.71 | 55.80 (40.46) | 1.50 | |
| Men | 53.77 (41.76) | 48.56 (41.38) | 59.17 (41.50) | ||||
| Daily hassles | 0.69 (0.40) | 0.76 (0.41) | 0.66 (0.40) | −5.77*** | 0.00–2.86 | ||
| Women | 0.71 (0.40) | −1.81 | 0.81 (0.39) | −2.78** | 0.68 (0.39) | −2.47* | |
| Men | 0.68 (0.41) | 0.73 (0.41) | 0.62 (0.40) | ||||
| Daily uplifts | 1.00 (0.45) | 0.97 (0.45) | 1.01 (0.46) | 2.07* | 0.00–2.92 | ||
| Women | 1.00 (0.43) | −0.46 | 0.98 (0.42) | −0.49 | 1.01 (0.43) | 0.54 | |
| Men | 0.99 (0.48) | 0.96 (0.47) | 1.02 (0.49) |
Table presents n (%) and X2 for categorical variables and mean (standard deviation) and t for continuous variables; Median hassles = 0.63, Possible range = 0–3; Median uplifts = 0.96, Possible range = 0–3
p < 0.05;
p < 0.01;
p < 0.001
Measures
The dependent variable was indexed by the assignment of subjects who met DSM-IV (American Psychiatric Association 1994) criteria for lifetime alcohol dependence. This was assessed using a diagnostic algorithm based on responses to the Semi-Structured Assessment for the Genetics of Alcoholism-II (Hesselbrock et al. 1999; Nurnberger et al. 2004). This outcome was chosen because this was the phenotype originally associated with GABRA2 in early COGA publications (Edenberg et al. 2004). Alcohol dependence was coded 1 if the respondent was classified as dependent according to these criteria, else 0. Thirty-six percent of the analysis sample met DSM-IV criteria for alcohol dependence.
Variables measuring gender, age, marital status, education, and household income were included in multivariate models as controls. Marital status was a binary variable (1 = currently married, respectively). Education and age were coded in years, and household income was coded in tens of thousands of dollars and logged to correct for positive skew. Age was later converted to tens of years to facilitate interpretation of odds ratios. Because alternative coding strategies for socio-demographic control variables (e.g. categorical education, additional marital status dummy variables, etc.) did not alter the effects of key variables of interest, the simplest forms were retained in final models.
Positive and negative experiences in everyday life were measured using a 49-item daily hassles and uplifts scale (DeLongis et al. 1982). These measures were intended to capture day-to-day events that cause chronic stress and might moderate the adverse effects of stress on health, respectively. The hassles scale included items indexing how much of a hassle or problem a particular activity/venue/person (e.g., work, finances, children, spouse, friends, etc.) had been in the last week. The uplifts scale measured how uplifting or pleasurable they had been. Response categories ranged from none or not applicable, coded 0, to a great deal, coded 3. In other words, a zero value on items comprising the hassles and uplifts scales may represent either a lack of stress or pleasure associated with a given activity or role, or not having participated in it. For example, someone who was not a parent would report a ‘‘0’’ (not applicable) when asked whether their children brought them pleasure and satisfaction, and likewise for those who were unemployed, unmarried, and uninvolved in church, clubs, and other voluntary organizations. In the case of some roles, this distinction was fairly straightforward (e.g., 96 % (n = 859) of those subjects who offered 0 responses when asked whether their spouse caused hassles and uplifts (n = 891) were currently unmarried). Other items (e.g., health, leisure time, neighbors) were universally applicable. Thus, lower values on the scales corresponded to reduced emotional reactions to existing relationships, roles, and environments and/or lack of integration into families, friendship groups, religious organizations, and the labor force. A respondent’s score on each scale is the sum for the scale divided by the number of non-missing items. The hassles (alpha = 0.91) and uplifts scales (alpha = 0.92) are both highly reliable.
DNA analyses
Genetic risk was indexed by a single item, high risk on the GABRA2 gene, identified via SNP genotyping and association analyses as being linked to alcohol dependence. Genotyping and association analyses are described in detail elsewhere (Edenberg et al. 2004). A total of 31 SNPs initially demonstrated significant association with alcohol dependence using the average Pedigree Disequilibrium Test (Martin et al. 2000). The SNP used here (rs279871) was chosen because it showed the strongest association with DSM-IV alcohol dependence in prior research, and has been used as a proxy for the LD block (Dick et al. 2006b, 2013; Edenberg et al. 2004). This SNP was also associated with increased risk for drug dependence in adulthood, as well as conduct disorder and externalizing behaviors in childhood (Agrawal et al. 2006; Dick et al. 2006a; Dick et al. 2009). In COGA data, the LD between rs279871 and the synonymous exonic coding variant rs279858 was complete (D’ = 1, r2 = 1; 1,000 genomes pilot 1 data for CEU). Increased risk for a clinical diagnosis of alcohol dependence was associated with carrying two copies of the high risk allele A at the SNP rs279871 at GABRA2 (Dick et al. 2006b; Edenberg et al. 2004). Participants were classified as having a high risk genotype (coded 1) if they were homozygous for the A allele, and 0 if they carried one or zero copies of this allele, consistent with previous research. Thirty-two percent of the analysis sample was classified as high-risk at GABRA2.
Statistical methods
Multivariate analyses of the influence of GABRA2 genotype and social factors on alcohol dependence were modeled with random-effects logistic regression models (Fitzmaurice et al. 2004; Rabe-Hesketh and Skrondal 2008) estimated with the xtlogit command in Stata (StataCorp 2011). Examining GxE effects using regression-based approaches have a number of advantages, including the ability to control for potential confounding variables that are correlated with both genotype and alcohol dependence, and the capacity to test moderation models (Waldman et al. 1999). Random effects models were used because they adjust for the lack of independence among observations resulting from having multiple individuals from the same family. These models reflected individuals (level-1) nested in families (level-2), and contained family-level (i.e. cluster-specific) random intercepts (Rabe-Hesketh and Skrondal 2008).
A series of models using a pooled sample were conducted to assess whether the association of genotype and GxE interactions with alcohol dependence varied by gender. The first set of models examined genotype by gender interactions. Model 1 included only genotype; Model II added gender and control variables; and Model III added an interaction term for gender*genotype. The second set of models examined interactions between genotype and daily hassles and uplifts. Model I included genotype, gender, daily hassles, daily uplifts, and control variables; Model II added a two-way interaction term for genotype*uplifts; and Model III added a three-way interaction term for genotype*gender* uplifts. The three-way interaction model tested whether GxE effects differed by gender. Two additional models (not shown) included a two-way interaction term for genotype*hassles and a three-way interaction term for genotype*gender*hassles. Because there was no evidence of a GxE or GxExE effect of the hassles scale, these findings were reported in text, but not in tables or figures.
For two and three-way interaction models, group-specific odds ratios were reported in tables and text to facilitate interpretation. In logit models, Chow-type tests of the equality of coefficients across groups may be unreliable since they confound the magnitude of the effect for each group with group differences in residual variation (Allison 1999). Predicted probabilities across groups, however, are unaffected by the confounding of the slope coefficients and error variances (Long 2009). The statistical significance of differences in predicted probabilities were examined using Long’s delta method (Long 2009), providing a conservative assessment of the significance of moderating effects in logit models. Finally, interaction models were re-estimated using a subsample that excluded probands with alcohol dependence (n = 1,996) to test whether findings were robust in a non-clinical sample. Because of the reduction in statistical power associated with interaction modeling, adjustments for multiple testing were not made (Brookes et al. 2004). A post hoc estimate of statistical power based on simulation analysis suggests that with a 0.05 error probability, the observed effect sizes, and a high-risk genotype as common as SNP rs279871 at GABRA2, the sample is adequately powered (>0.80) to detect the observed gender- and genotype-specific effects of daily uplifts (Duncan and Keller 2011; Luan et al. 2001).
Results
Bivariate statistics
Bivariate statistics are presented in Table 1. Overall, 36 % of respondents in the analysis sample met criteria for alcohol dependence. About 36 % of subjects with alcohol dependence carried the high-risk genotype at GABRA2 compared to 30 % of those without alcohol dependence (X2 = 8.43, 1 df, p < 0.01). The percent of men and women in the sample with the high-risk genotype at GABRA2 was approximately equal (32 %). However, ignoring genotype, men were significantly more likely to have alcohol dependence than women (51 vs 23 %, X2 = 190.40, 1 df, p < 0.001). Compared to individuals without alcohol dependence, affected individuals reported higher mean daily hassles (0.76 vs 0.66, t = −5.77, p < 0.001) and lower mean daily uplifts (0.98 vs 1.01, t = 2.07, p < 0.05). Overall, mean negative and positive daily life events did not differ significantly by gender.
Multivariate models
Regression estimates are presented in Tables 2 and 3, and full logit models for the purposes of calculating predicted probabilities are provided in online supplementary material. As shown in Table 2, there was a significant main effect of GABRA2 such that carrying the high-risk genotype was associated with a 33 % increase in the estimated odds of alcohol dependence (p < 0.01). This odds ratio did not change in magnitude or significance when control variables were added to the model (Model II), suggesting that GABRA2 had a direct effect on alcohol dependence rather than an indirect effect through marital status, income, or other covariates. Holding other covariates constant, being a woman (OR = 0.21, p < 0.001) was associated with a significant decrease in the estimated odds of alcohol dependence, as was being currently married (OR = 0.71, p < 0.01). Also, additional years of education (OR = 0.88, p <0.001) and household income (OR = 0.88, p < 0.01) were associated with lower odds of alcohol dependence.
Table 2.
Random-effects logistic regression of alcohol dependence on GABRA2
| Model I OR (95 % CI) |
Model II OR (95 % CI) |
Model III OR (95 % CI) |
|
|---|---|---|---|
| High risk genotype | 1.33 (1.07–1.64)** | 1.31 (1.04–1.66)* | |
| Female | 0.21 (0.17–0.26)*** | 0.26 (0.20–0.34)*** | |
| Age (10 years) | 0.94 (0.87–1.01) | 0.94 (0.87–1.01) | |
| Education (years) | 0.88 (0.84–0.93)*** | 0.88 (0.84–0.93)*** | |
| Currently married | 0.71 (0.56–0.91)** | 0.70 (0.55–0.90)** | |
| Log of HH income ($10K) | 0.88 (0.80–0.96)** | 0.88 (0.81–0.96)** | |
| Interaction | |||
| High risk genotype* female | 0.94 (0.68–1.31) | ||
| High risk genotype* male | 1.83 (1.33–2.54)*** | ||
| Intra-class correlation | 0.18 | 0.22 | 0.22 |
| Wald X2 | 6.80** | 242.00*** | 247.18*** |
Genotype (SNP rs279871), gender, and socio-demographic controls, COGA (n = 2,281). Age units are tens of years to facilitate interpretation
OR odds ratio, CI confidence interval
p < 0.05;
p < 0.01;
p < 0.001
Table 3.
Random-effects logistic regression of alcohol dependence on the interaction of GABRA2 genotype (SNP rs279871), gender, and daily uplifts, COGA (n = 2,281)
| Model I OR (95 % CI) |
Model II OR (95 % CI) |
Model III OR (95 % CI) |
|
|---|---|---|---|
| High risk genotype | 1.29 (1.02–1.62)* | 1.66 (0.96–2.84) | |
| Female | 0.20 (0.16–0.25)*** | 0.20 (0.16–0.25)*** | 0.26 (0.14–0.48)*** |
| Age (10 years) | 0.97 (0.90–1.05) | 0.97 (0.90–1.05) | 0.97 (0.89–1.05) |
| Education (years) | 0.87 (0.83–0.92)*** | 0.87 (0.83–0.92)*** | 0.87 (0.83–0.92)*** |
| Currently married | 0.68 (0.53–0.87)** | 0.68 (0.53–0.87)** | 0.67 (0.52–0.86)** |
| Log of HH income ($10K) | 0.91 (0.83–0.99)* | 0.91 (0.83–0.99)* | 0.91 (0.83–0.996)* |
| Daily hassles | 2.45 (1.86–3.23)*** | 2.46 (1.86–3.24)*** | 2.47 (1.87–3.26)*** |
| Daily uplifts | 0.85 (0.67–1.08) | ||
| Interactions | |||
| Uplifts* high risk genotype | 0.77 (0.47–1.27) | ||
| Uplifts* low risk genotype | 0.92 (0.69–1.23) | ||
| High risk genotype* female | 0.79 (0.35–1.77) | ||
| High risk genotype* male | 3.30 (1.54–7.06)*** | ||
| Uplifts* female* high risk genotype | 1.02 (0.56–1.87) | ||
| Uplifts* male* high risk genotype | 0.51 (0.28–0.91)* | ||
| Uplifts* female* low risk genotype | 0.90 (0.58–1.38) | ||
| Uplifts* male* low risk genotype | 0.94 (0.64–1.37) | ||
| Intra-class correlation | 0.23 | 0.23 | 0.23 |
| Wald X2 | 263.18*** | 263.87*** | 269.92*** |
OR odds ratio, CI confidence interval
p < 0.05;
p < 0.01;
p < 0.001
As shown in Model III, there was a significant interaction between gender and genotype (p < 0.05) such that carrying the high-risk genotype on GABRA2 had no significant effect on the odds of alcohol dependence among women (OR = 0.94), but was associated with a substantial increase in odds among men (OR = 1.83, p < 0.001). The predicted probability of alcohol dependence for men with the low-risk genotype was 0.46, compared to 0.61 for men with the high-risk genotype (Z = 3.54, p < 0.01)—a change in predicted probability of 0.15. In contrast, the predicted probability of alcohol dependence for women was 0.17 for those with the low-risk genotype and 0.18 for those with the high-risk genotype. This pattern was very similar in the subsample that excluded probands with alcohol dependence; the change in predicted probability of alcohol dependence associated with genotype for men was 0.13 (Z = 3.02, p < 0.01) and there was no change for women.
There were significant associations between daily life events and alcohol dependence (Table 3, Model I). Additional daily hassles were associated with a strong increase in the odds of alcohol dependence (OR = 2.45, p <0.001). Two-way GxE interactions between daily hassles and genotype (not shown) and daily uplifts and genotype were not statistically significant (Model II). The three-way interaction between gender, daily hassles, and genotype was not statistically significant (not shown). However, the addition of a three-way interaction term in Model III reflected a statistically significant moderation of risk for alcohol dependence by higher levels of daily uplifts only for men who were high risk at GABRA2 (p < 0.05). Specifically, for men with the high-risk genotype, additional positive daily experiences were associated with a significant decrease in risk for alcohol dependence (OR = 0.51, p < 0.05). However, for men with the low-risk genotype (OR = 0.94) and women with both the high-(OR = 1.02) and low-risk (OR = 0.90) genotypes, uplifts had no significant relationship to the estimated odds of alcohol dependence.
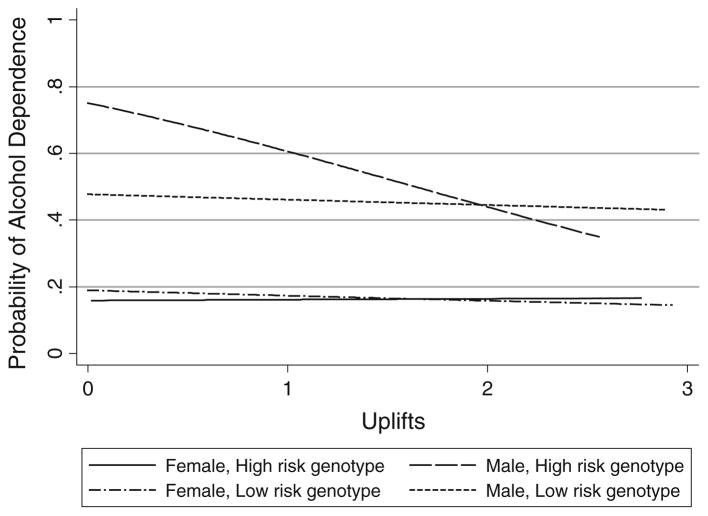
Figure 1 shows predicted probabilities of alcohol dependence from the three-way interaction model presented in Model III of Table 3. There was a strong negative relationship between positive daily life events and alcohol dependence for men with the high-risk genotype at GABRA2 such that the predicted probability of alcohol dependence at the lowest level of uplifts (0) was 0.77, compared to 0.35 at the highest observed level of uplifts in this group (2.5). The difference in predicted probabilities of alcohol dependence at 0 uplifts for men with the low- and high-risk genotypes (0.49 vs 0.76) was statistically significant (Z = 3.35, p < 0.01), as was the difference at 1 uplift (0.47 vs 0.62, Z = 3.62, p < 0.01). However, differences in predicted probabilities at 2 uplifts (where the lines cross) or more were not statistically significant. In contrast to the pattern observed for men, the differences in predicted probabilities were not significant at any level of uplifts for women.
Fig. 1.
Predicted probability of alcohol dependence as a function of daily uplifts by GABRA2 genotype (SNP rs279871) and gender1, COGA (n = 2,281). 1n by group: female, high-risk = 403; female, low-risk = 873; male, high-risk = 321; male, low-risk = 684
The three-way interaction results were very similar in the subsample that did not include probands with alcohol dependence (n = 1,996). The difference in predicted probabilities of alcohol dependence associated with genotype for men was 0.22 at 0 uplifts (Z = 2.47, p < 0.01) and 0.14 at 2 uplifts (Z = 3.32, p < 0.01), but never achieved statistical significance for women. The figure of predicted probabilities looked nearly identical to the one presented here except for a modest intercept shift associated with removing a substantial proportion of respondents with alcohol dependence from the sample (available upon request).
Discussion
Summary of results
These analyses extended previous findings which identified significant GxE associations between GABRA2 and characteristics of the social environment, but suggested that this gene confers risk for alcohol dependence only among men (Pescosolido et al. 2008). The present study was conducted to explore the relationships between alcohol dependence, gender, GABRA2 genotype, and everyday negative and positive experiences in social domains such as family, work, and community. A series of regressions examined main effects of gender, genotype, and negative and positive daily life events, as well as two-way and three-way interactions between (1) gender and genotype; (2) daily hassles/uplifts and genotype; and (3) gender, genotype, and daily hassles/uplifts. Significant main effects of gender and genotype were demonstrated, consistent with previous research using COGA data (Dick et al. 2006a, 2006b; Pescosolido et al. 2008). In addition, everyday hassles were associated with increased risk of alcohol dependence, while positive daily experiences were negatively related.
Models with two-way interaction terms indicated that GABRA2 had a significant effect on alcohol dependence among men, but no influence among women. Also, no significant moderation of genotype by daily hassles or uplifts was identified when effects were held constant across gender, leading to the erroneous conclusion that there were no GxE effects. In contrast, when gender-specific GxE effects were examined (a GxExE effect), a strong and significant negative association between positive daily experiences on risk for alcohol dependence emerged, but only among men with the high-risk genotype at GABRA2. These findings were present in the full COGA sample, and in a subsample that excluded probands with alcohol dependence.
Theoretical implications
Because of the cross-sectional nature of these data, multiple explanations for the association between positive daily experiences and alcohol dependence among men with the high-risk genotype are possible. It is likely that alcohol dependence would lead to a decrease in positive daily experiences for affected individuals (i.e. reverse causation). Likewise, individuals with the high risk genotype might experience fewer positive daily events than those with the low risk genotype because of personality or temperamental characteristics associated with that genotype. We note that there is no association between alcohol dependence and positive daily events for women. If alcohol dependence caused lower levels of positive daily experiences in this sample, this pattern is inconsistent with existing research suggesting that women with alcohol dependence experienced more alcohol related problems than men (Fillmore et al. 1997; Martino et al. 2004; Nolen-Hoeksema and Hilt 2006). However, it is possible that personality characteristics associated with the high risk genotype are gender-specific.
One potential explanatory concept is the existence of an endogenous neurophysiological endophenotype such as central nervous system disinhibition (Porjesz and Rangaswamy 2007). That is, if the mechanism underlying the association between GABRA2 and alcohol dependence is an endophenotype, individuals with the high-risk genotype might be susceptible both to alcohol dependence and to social conditions like divorce, unemployment, and social isolation that are associated with lower levels of positive daily experiences (i.e. a confounding effect). Based on prior research, the disinhibition phenotype is expected to be present to a greater degree in men than women (Cross et al. 2011), but we do note that the high-risk genotype is not associated with lower levels of daily uplifts among women or men without alcohol dependence in the COGA sample. That said, we cannot rule out the possibility that a neurophysiological endophenotype may have contributed to the etiological pathways that produce the observed patterns.
Our results on gender-specific GxE effects are consistent with the disinhibition explanation of alcohol use and the etiology of alcohol dependence. Features of the social environment, including family ties and religion, have been shown to influence alcohol phenotypes, constraining alcohol misuse and opportunities for drinking (Dick et al. 2009; Koopmans et al. 1999; Nilsson et al. 2005). On average, men with high scores on the daily uplifts scale probably perceive themselves as more financially stable, happier in their jobs, and more fulfilled in their relationships and family life compared to those with low scores. The existence of pleasurable and identity-affirming relationships and responsibilities in day-to-day life likely contributes to a sense of wellbeing and social integration. In other words, though this explanation is speculative, it is possible that these men have much to lose by engaging in problem drinking behaviors. In contrast, men reporting the lowest value for items on the uplifts scale had fewer employment, social, and family obligations and relationships than those reporting higher values. Those who are less involved with or responsible to others do not benefit from the social control functions that social integration provides. For instance, men who are genetically predisposed to behavioral undercontrol may nonetheless be motivated to avoid drinking socially after work and on weekends if they have loving wives and children waiting at home. On the other hand, among men without these external mechanisms of control, the high-risk genotype at GABRA2 is likely to be fully expressed.
Conversely, the GxE effect may not be active among women because they are less likely exhibit internal behavioral undercontrol than men, and their drinking behaviors tend to be less influenced by impulsivity traits (Cross et al. 2011; Rutledge and Sher 2001a). In other words, the disinhibition pathway may be a less relevant mechanism of alcohol misuse in women, effectively eliminating the effects of the interaction between GABRA2 and daily uplifts that is observable in men. Alternatively, feminine gender norms and the stigma associated with excessive drinking for women may serve as more uniform agents of social control against alcohol misuse (Keefe 1994; Nolen-Hoeksema and Hilt 2006), affecting women equally or in ways that do not correlate with genotype or daily uplifts. If true, these gendered forms of social control would suppress the expression of GABRA2 even among those with low levels of positive daily experiences and high genetic risk.
In sum, as suggested by research on gender and addiction, the GxExE findings identified here may be attributable to gender differences in the form and potency of mechanisms underlying disinhibition pathways to alcohol dependence. Men and women differ, on average, in the types of roles they occupy, the social norms associated with those roles, and social constraints and opportunities (Loscocco and Spitze 2007; Simon 1992, 1995). Consequently, etiological pathways involving interactions with the social environment cannot reasonably be assumed to be equal across gender. It may be that the environmental variables that are most likely to trigger or suppress the expression of genetic predisposition to disease are those that have the greatest significance for individuals, and these are likely to vary systematically by gender in our stratified society.
Methodological implications
It is noteworthy from a methodological standpoint that the complex nature of these findings emerged only when gender-specific interactions were examined. The two-way interactions between genotype and the hassles and uplifts scales revealed no significant moderating effects of social environment, underestimating the influence of both genotype and social environment. Moreover, the two-way interactions masked the most important finding—namely, that the gendered nature of etiological pathways produced very different GxE patterns in alcohol dependence for men and women. Thus, gender differences in GxE effectively washed out this effect, leading to an underestimation of this effect and a Type II error. These results underscore the critical need to consider gender-specific pathways in GxE research, supporting the need for complex models that move beyond the interaction of genotype and only one indicator of the social environment (Shanahan and Hofer 2005). In other words, the role of the environment in moderating the phenotypic expression of genotype is complicated by genetic or social heterogeneity that influences gene–environment interactions (i.e., GxExE).
Evidence even for two-way interactions between gender and genotype has been slow to accumulate in the genetics literature, and replicability is often constrained by methodological issues. When gender-specific genetic effects are examined, the typical strategy is to split the sample and run analyses separately for men and women (Harrison and Tunbridge 2007; Ober et al. 2008). Among the significant limitations of this method is the high likelihood of inferential errors, particularly if the variance or sample size differs across groups (e.g. Ono et al. 2004; Stein et al. 2005). That is, when making group comparisons by estimating separate models, a significant effect of genotype in one gender and an insignificant effect in the other does not necessarily mean that the difference in effect size is statistically significant (Brambor et al. 2006). Moreover, splitting the analysis sample to roughly half its full size reduces statistical power to detect significance, particularly when effects are small (as most SNP effects are). For example, a regression with a main effect powered at 80 % (the accepted minimum level) has only 29 % power to detect an interaction effect of the same size (Brookes et al. 2004). When adjustments are made for multiple testing, statistical power is reduced even further, making it very difficult to detect and replicate significant gender-genotype interactions.
Introducing interaction terms to test group-specific effects overcomes many of the problems associated with running separate analyses by gender. However, these are frequently used incorrectly (Brambor et al. 2006), particularly when employing regression models for binary outcomes common in the biomedical sciences (e.g., the presence or absence of a disease; Allison 1999; Long 2009). Compared to two-way interaction models, three-way interactions are even less well-powered and more difficult to implement and interpret, compounding the problems described above. These issues make it difficult, from a practical standpoint, to examine gender-specific GxE interactions. Given that interaction terms are seldom used at all in the biological or psychological sciences (Patsopoulos et al. 2007), opportunities for interdisciplinary research, cross-fertilization, and replication of these complex effects are currently extremely limited.
Limitations
A number of limitations of GxE research using candidate genes (cGxE) have been identified (Duncan and Keller 2011). Notably, publication bias against null GxE results inflates the number of published false positives—a problem that is amplified by the increased power requirements of interaction models relative to main effects. In addition, poorly-understood genotype-to-phenotype pathways render theorized mechanisms of GxE tenuous (Duncan and Keller 2011). Because the sample used in the current study is large and there is substantial variation on the candidate genotype and the outcome of interest (i.e. alcohol dependence) in both genders, concerns about adequate power are minimized. Moreover, the pathways through which GABRA2 may affect substance abuse and dependence (i.e. impulsivity and stress response) have been theorized and tested (e.g. Dick et al. 2013; Edenberg et al. 2004; Villafuerte et al. 2012, 2013), and these are consistent with the GxE effect presented in this study and GxE effects in previous research (e.g. Dick et al. 2001, 2009, 2013; Nilsson et al. 2005; Pescosolido et al. 2008; Rose et al. 2001). However, because cGxE research poses methodological challenges that limit the replicability of our findings, we view our contribution as largely theoretical. We hope that this research will generate methodological innovation and new hypotheses about the complex interplay between genes, social status, and social environments (Caspi et al. 2010).
In addition, findings from these cross-sectional data are associational, and causality cannot be determined. Though we provide a rationale supporting the presence of a causal pathway that is grounded in the existing literature (e.g. Dick et al. 2013; Edenberg et al. 2004; Villafuerte et al. 2012, 2013), the implications for etiological theories of alcohol dependence are speculative. This study should be replicated in the future using longitudinal samples that measure both genetics and social experiences prior to the development of alcohol dependence.
Also, the GxExE effects examined do not reflect the full complexity of either genetic or social mechanisms in alcohol dependence, and not all theories of addiction have been addressed here. Only one candidate gene out of dozens that have been implicated in genetic predisposition to alcohol dependence was tested, and GABRA2 may be subject to regulation by other genes not examined in this analysis (Enoch et al. 2010; Young-Wolff et al. 2011). Likewise, variables tapping into positive and negative life events do not capture stress and social experiences in adequate depth or breadth. For example, the uplifts scale does not distinguish absence of pleasure from lack of experience in a given role, potentially conflating low social embeddedness and unpleasurable participation (e.g., being unemployed may have different consequences for alcohol use than being employed but dissatisfied with your job). Additionally, uplifts and hassles were reported for the past week, improving accuracy of recall but introducing uncertainty about the degree to which the past week was typical of respondents’ experiences. For these reasons, the GxExE effect reported may be difficult to replicate without a large sample and more precise measurement over longer periods of time. Finally, the extent to which the GxE effect demonstrated in men is consistent with theories of social control and behavioral disinhibition cannot be adequately tested with the COGA data. Thus, future research should systematically explore associations between family and employment characteristics, feelings of social integration and life satisfaction, identity, and alcohol use.
Conclusion
Despite a substantial body of literature underscoring the relevance of gendered etiological pathways in alcohol dependence, gender differences are often unaddressed within the existing body of GxE research. The neglect of gender, both theoretically and methodologically, is a major limitation of existing GxE research. Results presented here illustrate that if ignored, gender-differentiated GxE effects can increase the likelihood of null findings and obscure complex interactions between genetic predisposition and gendered environments and experiences. To realize the full potential of GxE research going forward, it is critical to integrate theories and methodologies from diverse social science and biomedical disciplines.
Supplementary Material
Acknowledgments
This research was funded in part by a grant from the Peter F. McManus Charitable Trust. The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes ten different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice, K. Bucholz); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy), Howard University (R. Taylor) and Virginia Commonwealth University (D. Dick). A. Parsian and M. Reilly are the NIAAA Staff Collaborators. This national collaborative study is supported by NIH Grant U10 AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA). Funding support for GWAS genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the National Institute on Alcohol Abuse and Alcoholism, the NIH GEI (U01HG004438), and the NIH contract ‘‘High throughput genotyping for studying the genetic contributions to human disease’’ (HHSN268200782096C). The authors thank Kim Doheny and Elizabeth Pugh from CIDR and Justin Paschall from the NCBI dbGaP staff for valuable assistance with genotyping and quality control in developing the dataset available at dbGaP.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10519-013-9607-9) contains supplementary material, which is available to authorized users.
Contributor Information
Brea L. Perry, Email: breaperry@uky.edu, Department of Sociology, University of Kentucky, 1515 Patterson Office Tower, Lexington, KY 40506-0027, USA
Bernice A. Pescosolido, Department of Sociology, Indiana University, Bloomington, IN, USA
Kathleen Bucholz, Department of Psychiatry, Washington University in St. Louis, St. Louis, MO, USA.
Howard Edenberg, Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN, USA.
John Kramer, Department of Psychiatry, University of Iowa College of Medicine, Iowa City, IA, USA.
Samuel Kuperman, Department of Psychiatry, University of Iowa College of Medicine, Iowa City, IA, USA.
Marc Alan Schuckit, Department of Psychiatry, University of California, San Diego, CA, USA.
John I. Nurnberger, Jr., Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN, USA
References
- Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, Nurnberger JI, Crowe R, Kuperman S, Schuckit MA, Begleiter H, Porjesz B, Dick DM. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- Allison P. Comparing logit and probit coefficients across groups. Sociol Methods Res. 1999;28:186–208. [Google Scholar]
- Bancroft A. Drugs intoxication and society. Polity; Cambridge: 2009. [Google Scholar]
- Bearman PS, Brückner H. Opposite-sex twins and adolescent same-sex attraction. Am J Sociol. 2002;107:1179–1205. [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Bird CE, Rieker PP. Gender and health: the effects of contrained choices and social policies. Cambridge University Press; New York: 2008. [Google Scholar]
- Brambor T, Clark WR, Golder M. Understanding interaction models: improving empirical analyses. Polit Anal. 2006;14:63–82. [Google Scholar]
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses: power and sample size for the interaction test. J Clin Epidemiol. 2004;57:229–236. doi: 10.1016/j.jclinepi.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Begg D, Dickson N, Langley J, Silva PA. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Gelernter J, et al. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2007;33(4):837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137:97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Coyne JC, Dakof G, Folkman S, Lazarus RS. Relationship of daily hassles, uplifts, and major life events to health status. Health Psychol. 1982;1:119–136. [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene–environment interactions: socioregional moderation of alcohol use. J Abnorm Psychol. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- Dick D, Bierut L, Hinrichs A, Fox L, Bucholz K, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Xuei X, Edenberg H, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006a;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Schuckit MA, Bierut L, Hinrichs A, Fox L, Mullaney J, Cloninger CR, Hesselbrock V, Nurnberger JI, Almasy L, Foroud T, Porjesz B, Edenberg H, Begleiter H. Marital status, alcohol dependence, and GABRA2: evidence for gene–environment correlation and interaction. J Stud Alcohol. 2006b;67:185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, Pettit GS, Bates JE. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Arch Gen Psychiatry. 2009;66:649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Review: understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse S, Porjesz B, Schuckit M, Rangaswamy M, Hesselbrock V, Edenberg H, Nurnberger J, Agrawal A, Bierut L, Wang J, Bucholz K, Kuperman S, Kramer J. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Res Hum Genet. 2013;16:661–669. doi: 10.1017/thg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgon T, D’Addario C, et al. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B. 2006;141(8):854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168(10):1041. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li T-K, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the [alpha]2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitle D, Taylor J, Eitle TM. Heavy episodic alcohol use in emerging adulthood: the role of early risk factors and young adult social roles. J Drug Issues. 2010;40:295–320. [Google Scholar]
- Enoch M-A. The role of GABAA receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B. 2006;141B:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Hodgkinson CA, Yuan Q, Shen P-H, Goldman D, Roy A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67:20–27. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore KM. Women’s drinking across the adult life course as compared to men’s. Brit J Addict. 1987;82(7):801–811. doi: 10.1111/j.1360-0443.1987.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird N, Ware J. Applied longitudinal analysis. Wiley; New York: 2004. [Google Scholar]
- Gelernter J, Kranzler HR, et al. Dense genomewide linkage scan for alcohol dependence in African Americans: significant linkage on chromosome 10. Biol Psychiatry. 2009;65(2):111–115. doi: 10.1016/j.biopsych.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomberg ESL. Alcohol abuse: age and gender differences. In: Wilsnack RW, Wilsnack SC, editors. Gender and alcohol: individual and social perspectives. Rutgers Center of Alcohol Studies; New Brunswick: 1997. pp. 225–244. [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Guo G. The linking of sociology and biology. Soc Forces. 2006;85:145–150. [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2007;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Heath AC, Nelson EC. Effects of the interaction between genotype and environment. Research into the genetic epidemiology of alcohol dependence. Alcohol Res Health. 2002;26:193–201. [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA-a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Horwitz AV, White HR. Gender role orientations and styles of pathology among adolescents. J Health Soc Behav. 1987;28:158–170. [PubMed] [Google Scholar]
- Huselid RF, Cooper ML. Gender roles as mediators of sex differences in adolescent alcohol use and abuse. J Health Soc Behav. 1992;33:348–362. [PubMed] [Google Scholar]
- Keefe K. Perceptions of normative social pressure and attitudes toward alcohol use: changes during adolescence. J Stud Alcohol. 1994;55:46–54. doi: 10.15288/jsa.1994.55.46. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, van Baal GCM, Boomsma DI. The influence of religion on alcohol use initiation: evidence for genotype X environment interaction. Behav Genet. 1999;29:445–453. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Landrine H, Bardwell S, Dean T. Gender expectations for alcohol use: a study of the significance of the masculine role. Sex Roles. 1988;19:703–712. [Google Scholar]
- Lewis MA, Neighbors C. Gender-specific misperceptions of college student drinking norms. Psychol Addict Behav. 2004;18:334–339. doi: 10.1037/0893-164X.18.4.334. [DOI] [PubMed] [Google Scholar]
- Long JS. Group comparisons in logit and probit using predicted probabilities. Indiana University; 2009. pp. 1–25. [Google Scholar]
- Loscocco K, Spitze G. Gender patterns in provider role attitudes and behavior. J Fam Issues. 2007;28(7):934–954. [Google Scholar]
- Luan J, Wong M, et al. Sample size determination for studies of gene–environment interaction. Int J Epidemiol. 2001;30(5):1035–1040. doi: 10.1093/ije/30.5.1035. [DOI] [PubMed] [Google Scholar]
- Martino SC, Collins RL, Ellickson PL. Substance use and vulnerability to sexual and physical aggression: a longitudinal study of young adults. Violence vict. 2004;19(5):521–540. doi: 10.1891/vivi.19.5.521.63684. [DOI] [PubMed] [Google Scholar]
- Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet. 2000;67:146–154. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene–environment interactions in psychopathology. Perspect Psychol Sci. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Damberg M, Alm PO, Öhrvik J, Leppert J, Lindström L, Oreland L. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcoholism. 2005;29:564–570. doi: 10.1097/01.alc.0000159112.98941.b0. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J Gen Psychol. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Shirakawa O, Nushida H, Ueno Y, Maeda K. Association between catechol-O-methyltransferase functional polymorphism and male suicide completers. Neuropsychopharmacology. 2004;29:1374–1377. doi: 10.1038/sj.npp.1300470. [DOI] [PubMed] [Google Scholar]
- Patsopoulos NA, Tatsioni A, Ioannidis JPA. Claims of sex differences. J Am Med Assoc. 2007;298:880–893. doi: 10.1001/jama.298.8.880. [DOI] [PubMed] [Google Scholar]
- Pescosolido BA, Perry BL, Long JS, Martin JK, Nurnberger JI, Jr, Hesselbrock V. Under the influence of genetics: how transdisciplinarity leads us to rethink social pathways to illness. Am J Sociol. 2008;114:S171–S201. doi: 10.1086/592209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B. 2008;147B:543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Gunter TD, Beach SRH, Brody GH, Hollenbeck N, Andersen A, Adams W. Role of GABRA2 on risk for alcohol, nicotine, and cannabis dependence in the Iowa Adoption Studies. Psychiatr Genet. 2009;19:91–98. doi: 10.1097/YPG.1090b1013e3283208026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using stata. Stata; College Station: 2008. [Google Scholar]
- Reich T. A genomic survey of alcohol dependence and related phenotypes: results from the Collaborative Study on the Genetics of Alcoholism (COGA) Alcoholism. 1996;20:133a–137a. doi: 10.1111/j.1530-0277.1996.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Ricciardelli LA, Connor JP, Williams RJ, Young RM. Gender stereotypes and drinking cognitions as indicators of moderate and high risk drinking among young women and men. Drug Alcohol Depend. 2001;61:129–136. doi: 10.1016/s0376-8716(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene–environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcoholism. 2001;25:637–643. [PubMed] [Google Scholar]
- Rutledge PC, Sher KJ. Heavy drinking from the freshman year into early young adulthood: the roles of stress, tension-reduction drinking motives, gender and personality. J Stud Alcohol. 2001;62:457–466. doi: 10.15288/jsa.2001.62.457. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Klee L, Ames G. Review and analysis of literature on indicators of women’s drinking problems. Br J Addict. 1990;85:179–192. doi: 10.1111/j.1360-0443.1990.tb03069.x. [DOI] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene–environment interactions: retrospect and prospect. J Gerontol B. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Shanahan Michael J, Vaisey S, Erickson Lance D, Smolen A. Environmental contingencies and genetic propensities: social capital, educational continuation, and dopamine receptor gene DRD2. Am J Sociol. 2008;114:S260–S286. doi: 10.1086/592204. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Simon RW. Parental role strains, salience of parental identity andgender differences in psychological distress. J Health Soc Behav. 1992;33(1):25–35. [PubMed] [Google Scholar]
- Simon RW. Gender, multiple roles, role meaning, and mental health. J Health Soc Behav. 1995;36(2):182–194. [PubMed] [Google Scholar]
- Singer BH, Ryff CD. New horizons in health: an Integrative approach. National Academy Press; Washington: 2001. [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. StataCorp LP; College Station: 2011. [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, Reczek C. Social relationships and health behavior across life course. Annu Rev Sociol. 2010;36:139–157. doi: 10.1146/annurev-soc-070308-120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2012;17(5):511–519. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Strumba V, Stoltenberg SF, Zucker RA, Burmeister M. Impulsiveness mediates the association between GABRA2 SNPs and lifetime alcohol problems. Genes Brain Behav. 2013;12:525–531. doi: 10.1111/gbb.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, Robinson BF, Rhee SH. A logistic regression extension of the transmission disequilibrium test for continuous traits: application to linkage disequilibrium between alcoholism and the candidate genes DRD2 and ADH3. Genet Epidemiol. 1999;17(Suppl 1):S379–S384. doi: 10.1002/gepi.1370170764. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, et al. The influence of gene–environment interactions on alcohol consumption and alcohol use disorders: a comprehensive review. Clin Psychol Rev. 2011;31(5):800–816. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol–disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Dev Perspect. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: common bisocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.