Abstract
Herein, a ternary nanocomposite with TiO2 nanoparticles anchored on reduced graphene oxide (rGO)-encapsulated Fe3O4 spheres (Fe3O4@rGO@TiO2) is presented as a high efficient heterogeneous catalyst for photo-Fenton degradation of recalcitrant pollutants under neutral pH. Fe3O4@rGO@TiO2 was synthesized by depositing TiO2 nanoparticles on the surface of the Fe3O4 spheres wrapped by graphene oxide (GO) which was obtained by an electrostatic layer-by-layer method. This as-prepared catalyst reflected good ferromagnetism and superior stability which makes it convenient to be separated and recycled. Due to the synergic effects between the different components composed the catalyst, swift reduction of Fe3+ can be achieved to regenerate Fe2+. Fe3O4@rGO@TiO2 exhibited enhancing catalytic activity for the degradation of azo-dyes compared with Fe3O4, Fe3O4@SiO2@TiO2 or SiO2@rGO@TiO2, further conforming the rapid redox reaction between Fe2+ and Fe3+. All these merits indicate that the composite catalyst possesses great potential for visible-light driven destruction of organic compounds.
Recently, the ever-growing emission of dye wastewater from various industries such as textiles, printing, food and cosmetics has become a major threat to human and ecology owing to the toxicity and non-biodegradability1,2,3. Many methods such as adsorption, flocculation and chemical oxidation have been used to remove the persistent dyes released into aquatic environment. Compared with other methods, one of the chemical oxidation methods called Fenton reaction has attracted intensive attention due to its capability to destruct refractory organic pollutants and turn them into low-molecule-weight inorganic compounds4,5,6,7. Hydroxyl radicals (·OH), as the key intermediates of Fenton process, holds highly oxidative potential and can attack almost all the organic compounds in a non-selective way, leading finally to the mineral end-products7. However, high activity of the reaction between the ferrous ions and hydrogen peroxide are always limited to a pH value around 3. Moreover, homogeneous Fenton systems can generate a great deal of iron sludge which may cause secondary pollution and increase the operating costs8,9,10.
In order to deal with these issues, heterogeneous Fenton-like reactions based on solid catalysts have shown great promise to replace the iron salt-based homogeneous Fenton systems11,12. Currently, great attention has been paid to the heterogeneous Fenton-like catalysts such as zero-valent iron, iron-based materials and iron-containing materials13,14,15. Among them, magnetite (Fe3O4) nanoparticles have attracted considerable research interests because of its unique properties, including decent magnetic, electric, catalytic properties, biocompatibility and low toxicity. Nevertheless, the nano-scaled magnetite particles have a tendency to aggregate to form larger particles, reducing the original large specific surface area and dispersibility, which will finally undermine the catalytic activity. Therefore, it is essential to immobilize these nanoparticles onto supports or encapsulate them within protective layer to maintain their unique performances16,17. Besides, the catalytic efficiency of the Fe3O4 nanoparticles await further promotion considering the relatively low conversion rate between the Fe2+ and the Fe3+ when they are used as the Fenton catalysts. Rationally, hybridization of Fe3O4 with a speeding-up component is desirable to boost the conversion between Fe3+ and Fe2+. Herein, we choose TiO2 to fulfill this task. When the electrons in TiO2 are irradiated by UV-visible light, they can be excited from the valance band to the conduction band to generate electron-hole pairs18. The photoexcited electrons can quickly transport to Fe3+, accelerating the redox transformation between Fe(III) and Fe(II). Meanwhile, the holes generated simultaneously can also react with H2O to produce highly oxidative hydroxyl radicals. The as-generated radicals, together with the holes equipped with high oxidative potential, can mineralize the persistent organic pollutants nonselectively19. However, there are two aspects need to be concerned when utilizing TiO2 as a photo-assisted cocatalyst. First, the high energy of irradiating photons required as a result of the wide band gap (3.20 eV) of the anatase TiO2 restricts its photoactivity to the narrow light-response range of ultraviolet accounting for only about 3–5% of total sunlight. Second, the photo-induced electron-hole pairs suffer from high recombination rate, so the reactivity of the electron-hole pairs will rapidly diminish20,21. In order to solve these problems and strengthen the combination between TiO2 and Fe3O4, an effective interlayer should be introduced.
In recent decades, graphene has attracted tremendous attention due to its excellent electronic properties and promising applications in various fields22,23. In this work, we selected it as the support material to enhance the synergistic effect between TiO2 and Fe3O4, and configured a ternary-composite design of Fenton catalyst. The advantages of this design can be concluded as follows. First, when TiO2 was combined with graphene, the electrons in the valance band of TiO2 can be excited to conduction band under the irradiation of visible light24,25,26,27,28,29,30 and the existence of graphene will prolong the lifetime of the photoexcited electron-hole pairs and thus contributing to higher possibility of successful transfer of the electrons from the TiO2 to Fe3+/Fe2+ redox pair. Second, the graphene-wrapped Fe3O4 has better dispensability in aqueous medium. Besides, the presence of various oxygen-containing groups such as carboxyl, epoxides, alcohols, lactols31 on graphene due to the incomplete reduction of GO in the hydrothermal reaction endows graphene excellent adsorption capacity which will help remove the organic pollutants. In this study, we prepared reduced graphene oxide (rGO)-encapsulated Fe3O4 magnetic nanospheres which were eventually anchored with TiO2 nanoparticles. The effects of operating parameters such as catalyst dosage and H2O2 dosage on the degradation of methylene blue (MB) were investigated. The stability of the as-prepared catalyst was also studied. The results of this study indicate that the composite catalyst possesses great promise for visible-light driven destruction of organic compounds.
Results
Characterization of the hybrids
The morphology and structure of the as-prepared catalysts were characterized by SEM and TEM. As shown in Fig. 1(a, d), the Fe3O4 nanoparticles have an average diameter of about 400 ± 20 nm and the surface is rough, which can be attributed to the fact that each Fe3O4 nanospheres are composed of many smaller particles32. Besides, the SAED graph of Fe3O4 shown in Fig. S1a illustrates that Fe3O4 is a polycrystallinity. The 3-Aminopropyltrimethoxysilane (APTMS) molecules that reacted with Fe3O4 particles endow the magnetic spheres amino groups representing electropositive which can help the Fe3O4 nanospheres combine with the GO containing a lot of negative charged groups on its surface and edges33. It can be seen in Fig. 1b that there exists obvious folds on the surface of the magnetic particles which accounts for the successfully integrating GO layers with the Fe3O4 particles, corresponding perfectly to the TEM images illustrated in Fig. 1e which shows that on the surface of the Fe3O4 particles do exist thin layers. After the magnetic particles were encapsulated into the silk-like GO layers, their diameters changed a little which can be observed in Fig. 1(b, e). The SEM and TEM images shown in Fig. 1(c, f) explains that the TiO2 particles were successfully anchored on the GO layers according to the hydrothermal and the size of the particles increased apparently compared to Fe3O4@GO.
Figure 1.
(a, d) SEM images of Fe3O4 nanoparticles prepared by solvothermal reaction. (b, e) SEM and TEM images of the GO wrapped Fe3O4 obtained by electrostatic interactions. (c, f) TEM images ofFe3O4@rGO@TiO2 synthesized by one-step hydrothermal reaction.
Figure 2a shows the XRD characterization of Fe3O4, APTMS-Fe3O4, GO-encapsulated Fe3O4 and Fe3O4@rGO@TiO2. As shown in Fig. 2a, characteristic diffraction peaks for Fe3O4 (2θ = 18.1°, 30°, 35.4°, 43.3°, 53.4°, 57.1° and 62.7°), which can be indexed to their indices (111), (220), (311), (400), (422), (511) and (440), were observed for the synthesized Fe3O4 nanoparticles. This result corresponds well to the PDF data (JCPDS file No. 19-0629) which shows that the magnetite diffraction peaks appeared at the same locations. Besides, this result is also in good consistence with the XRD characterization of Fe3O4 reported in the former literature34. After the modification by APTMS and GO, only the intensity of the obtained nanocomposites changed, which illustrated that the amount of the GO is too little to perform its crystallinity35. And the crystallinity of Fe3O4 modified by APTMS and GO seemed to just change a little. Two additional specific XRD diffraction peaks located at 2θ = 25.2° and 48° can be seen when the TiO2 nanoparticles were anchored on the surface of the GO-wrapped Fe3O4 nanospheres, which agree with the (101) and (200) planes (JCPDS file No. 21-1272) of anatase TiO2. Other characteristic peaks of anatase such as reported in the former literature36 can hardly be observed in Fig. 2a which may be attributed to the fact that these peaks may be close to the crystalline peaks of Fe3O4 that can hinder the anatase crystalline peaks.
Figure 2.
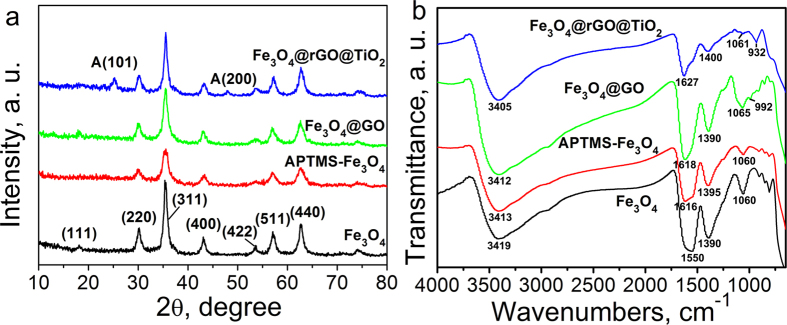
(a) XRD patterns and (b) FT-IR spectra of Fe3O4, APTMS-Fe3O4, Fe3O4@GO, Fe3O4@rGO@TiO2.
Figure 2b shows the FT-IR spectra of Fe3O4, APTMS-Fe3O4, GO-encapsulated Fe3O4 and Fe3O4@rGO@TiO2. It can be clearly seen from Fig. 2b that the peaks located at 3419 and 1550 cm−1 are due to the H-O-H stretching and the bending vibration of the free or adsorbed water, respectively37. After modified by APTMS and GO, a new absorption bands situated at 1616 cm−1 appeared in the FT-IR spectra, which can be indexed to C = O vibration, confirming the successful wrapping of GO on the Fe3O4 nanoparticles. In comparison with the FT-IR spectra of Fe3O4@GO, the intensity of the characteristic absorption peaks of the obtained Fe3O4@rGO@TiO2 located at around 1627, 1400 and 1065 cm−1 obviously diminished which can be ascribed to the reduction of GO. However, the absorption peaks located at around 1627, 1400 and 1065 cm−1 still existed, illustrating that the GO was not completely reduced. Moreover, the Raman spectrum shown in Fig. S2 can also account for the successful combination of Fe3O4, rGO and TiO2.
The magnetic properties of the as-prepared Fe3O4@rGO@TiO2 were tested by using a vibrating sample magnetometer at room temperature. As can been seen in Fig. 3, the saturation magnetization (Ms) value of Fe3O4 was 43.691 emu/g, while for Fe3O4@rGO@TiO2 it was just 34.202 emu/g, mainly due to the existence of the rGO wrapped on the surface of Fe3O4 and the subsequently anchored TiO2 nanoparticles38. The obtained catalysts can be quickly separated from solution under an external magnetic field because of their considerable Ms values, which will be beneficial for their reuse and boosting the overall water treatment efficiency in practical applications.
Figure 3.
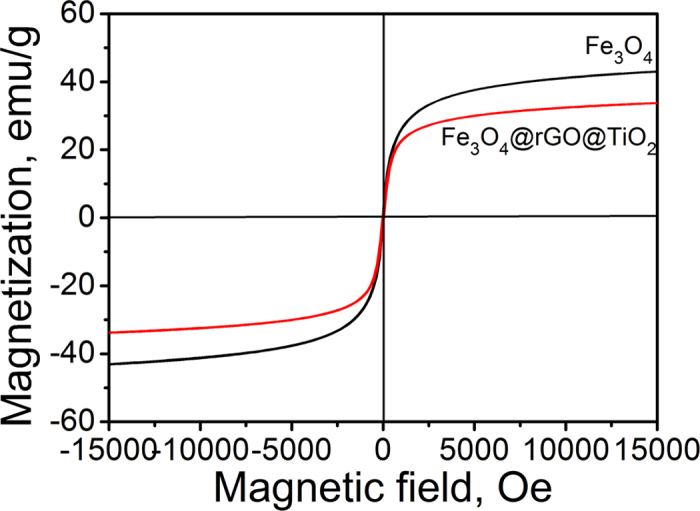
Room temperature magnetic hysteresis loops of Fe3O4 and Fe3O4@rGO@TiO2.
Photo-Fenton degradation activities of Fe3O4@rGO@TiO2
MB was chosen as the model organic pollutant to evaluate the degradation activities of the as-prepared hybrids. The concentration of MB was monitored by measuring the absorbance at a wavelength of 664 nm characteristic of MB. The suspension composed of MB and Fe3O4@rGO@TiO2 were stirred in the dark for about 30 min to achieve absorption-desorption equilibrium. The concentration of the MB was regarded as the initial concentration C0. Apparent degradation of MB was observed as soon as the photo-Fenton reaction was initiated by introducing illumination as well as H2O2 to the system.
The effect of the dose of the catalysts on the degradation activity in the Fenton process was illustrated in Fig. 4a. It can be concluded that the degradation accelerated as the amount of the catalyst increased from 0.1 to 1.5 g/L, but dropped with excessive dosage, as can be seen from the data of dosage of 2.0 g/L. This phenomenon can be ascribed to the reason that the number of the reactive sites can be increased when the amount of the composites were increasing. However, these nanoparticles may have a tendency to aggregate when their quantity is in excess, thus contributing to the decrease of the reactive sites. Besides, excess amount of Fe3O4 may exist as the scavenger of hydroxyl radicals38,39,40,41. In this study, the optimal amount of the catalyst was 1.5 g/L just as shown in Fig. 4a.
Figure 4.
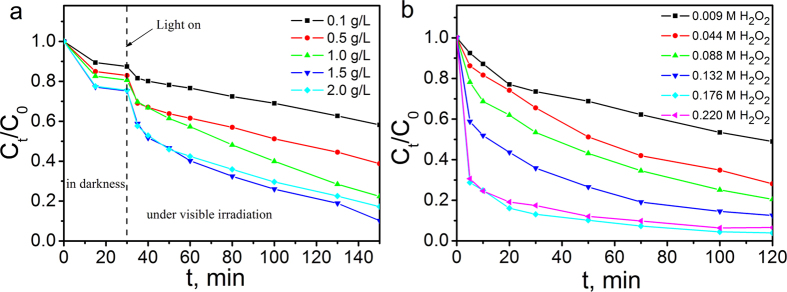
Photo-Fenton degradation of MB in the presence of Fe3O4@rGO@TiO2 at room temperature under neutral pH. (a) Effect of catalyst dosage on MB degradation (initial MB concentration, 10 mg/L; H2O2, 0.088 M); (b) Effect of H2O2 dosage on MB degradation(initial MB concentration, 10 mg/L; catalysts, 1.5 g/L).
The effect of amount of H2O2 on the degradation of MB in the Fenton-like system was also investigated in this work. The result illustrated in Fig. 4b clearly shows enhanced degradation activity as the amount of H2O2 increased, but demonstrates saturation and slight decrease when the concentration is beyond 0.176 M. As is well known that the generation rate of hydroxyl radicals (·OH) can be accelerated as more H2O2 is introduced to the Fenton system at the beginning, which is beneficial to the degradation of the organic dyes. Nevertheless, when the amount of H2O2 is achieving a critical point, the generated hydroxyl radicals (·OH) may react with the excessive H2O2 which is not initiated by the catalysts in time39,40. Therefore, the catalytic performance can only achieve a maximum effect when the utilization of H2O2 is optimal. This theory was totally accorded with the consequence illustrated in Fig. 4b. When the amount of H2O2 was over 0.176 M, it was distinctly seen that the degradation activity slightly decreased. To verify that the great degradation efficiency is due to the existence of the catalysts, different amount of H2O2 was used to remove MB under the same condition without any catalysts. The results are depicted in Fig. S3, showing that H2O2 just have slight effect on the degradation of MB.
Stability of Fe3O4@rGO@TiO2
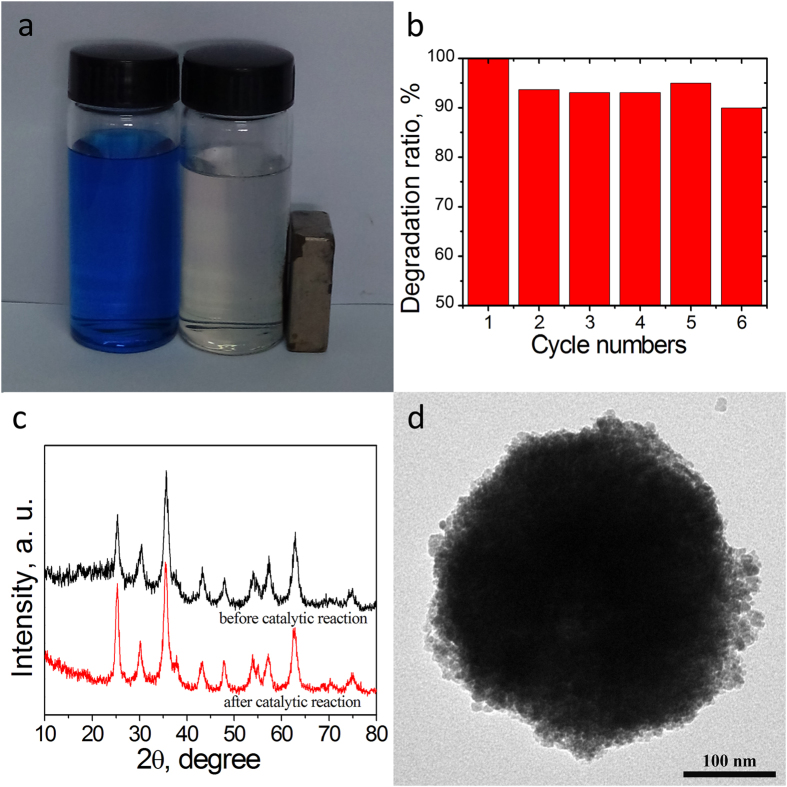
The stability of the catalytic materials is of great importance if the catalysts are to be practically applicable. The as-prepared catalyst Fe3O4@rGO@TiO2 can be easily separated from the MB solution by magnetic field and for reuse. Figure 5a shows the photograph comparing the MB solutions before and after the Fenton reaction, from which we can distinctly observe that MB almost totally discolored after the reaction. As depicted in Fig. 5b, the removal of MB during the first catalytic run could be achieved above 99.0% after 2 h. After six recycles for the catalytic degradation of MB, the catalytic activity of Fe3O4@rGO@TiO2 just slightly decreased. As can be seen in Fig. 5b, the degradation efficiency of Fe3O4@rGO@TiO2 can reach up to 93% after 2 h even if the catalysts had been utilized for several times, which indicates that this catalyst can maintain good stability. The TEM image of the Fe3O4@rGO@TiO2 which had been used for six times shown in Fig. 5d also proved the catalysts’ stability. The morphology of the as-prepared catalyst did not undergo obvious change even after several cycles. The XRD patterns (Fig. 5c) of the freshly prepared catalyst and the catalyst recycled after many times further illustrated the stability of the catalysts.
Figure 5.
(a) Photograph of MB before and after photo-Fenton reaction. (b) The cyclic utilization of the as-prepared Fe3O4@rGO@TiO2 hybrids for the degradation of MB with the addition of H2O2 and illumination at neutral pH and room temperature for 120 min. (c) XRD patterns of Fe3O4@rGO@TiO2 before and after six cycles. (d) TEM image of Fe3O4@rGO@TiO2 after six cycles.
Discussion
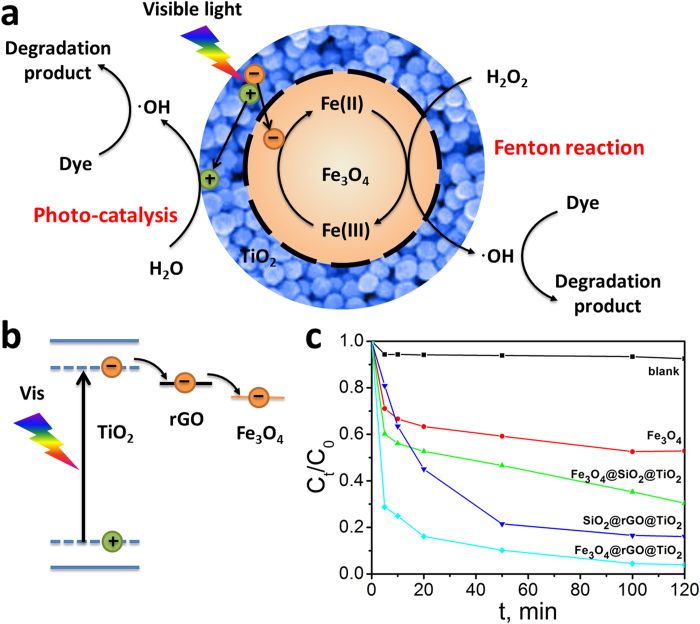
One essential concern on the present Fenton system is that which component has the main effect on decomposition of H2O2 into hydroxyl radicals. To verify the contributions from different components to the degradation of MB, several different materials including Fe3O4, Fe3O4@SiO2@TiO2, SiO2@rGO@TiO2 and Fe3O4@rGO@TiO2, were chosen to act as the catalysts. The degradation processes under different conditions are shown in Fig. 6c. It can be obviously seen from the five degradation curves that Fe3O4@rGO@TiO2 shows the best degradation performance. The degradation process was quick at first when Fe3O4 was used as the catalyst. However, the reaction rate decreased after 10 min, which can be attributed to the reason that the ferrous ions existed in Fe3O4 had been almost totally consumed at beginning and the conversion rate of ferric to ferrous is far less than the ferric ions’ consumption rate36,37,41,42. It can also draw a conclusion from Fig. 6c that when SiO2@rGO@TiO2 or Fe3O4@SiO2@TiO2, whose structure and morphology is similar to Fe3O4@rGO@TiO2 except for the component, were utilized as the catalysts to decompose MB, their performance is not as efficient as Fe3O4@rGO@TiO2. It may be ascribed to the synergic effect between the three different components. The assumed mechanism can be summarized into three aspects and has been illustrated in Fig. 6(a, b). (1) Fe3O4, the core of Fe3O4@rGO@TiO2, is the major component used to react with H2O2 to generate hydroxyl radicals (•OH) to finally decompose MB. Besides, the ferromagnetic of Fe3O4 make the catalysts facilely separable from the solution for subsequent usage. (2) The existence of rGO not only endows the catalysts good adsorption of MB which may be beneficial to the degradation of MB, but also endues the catalysts with extended usage of the solar energy. Besides, the band gap of TiO2 can be narrowed from 3.2 to 2.8 eV when TiO2 was combined with GO20 (see supplementary information Fig. S4 for certification) so that the valence electrons of TiO2 can be also excited to the conduction band state only under visible light irradiation24,25,26,27,28,29,30,31. (3) The presence of TiO2 may also have a great effect on the degradation of MB. When irradiated by the visible light (λ > 400 nm), TiO2 anchored on GO can be excited to generate photo-induced electrons and holes, and the existence of GO may be able to transport the photo-induced electrons43 to inner Fe3+ to help the Fe3+ to be reduced to Fe2+. Furthermore, the rapid transfer rate of the photo-generated electrons from TiO2 to Fe3+ prolong the life time of the photo-generated holes whose oxidative potential is also high enough to degrade most organic pollutants. The opportune isolation of the photo-generated electrons and holes effectively avoids their recombination so that the residue holes may be able to straightly react with the organic pollutants to make the organics degrade44. All the advantages brought by these components make the catalysts composed of Fe3O4, GO and TiO2 perform better catalytic activity compared with the bare Fe3O4, SiO2@rGO@TiO2 and Fe3O4@SiO2@TiO2 nanoparticles, illustrating that the catalysts cannot manifest good degradation efficiency unless they hold all of the three components.
Figure 6.
(a, b) Suggested mechanism for the photo-Fenton degradation of MB by Fe3O4@rGO@TiO2 at room temperature and neutral pH. (c) Photo-Fenton degradation of MB at room temperature and neutral pH by different catalysts (blank, Fe3O4, Fe3O4@SiO2@TiO2, SiO2@rGO@TiO2 and Fe3O4@rGO@TiO2).
In summary, the sphere-like ternary Fe3O4@rGO@TiO2 as an efficient heterogeneous Fenton-like catalyst was successfully synthesized in our work. The GO was wrapped on Fe3O4 nanospheres by using an electrostatic layer-by-layer method. The TiO2 nanoparticles were anchored on the surface of Fe3O4@GO and the GO was incompletely reduced to rGO through the hydrothermal reaction simultaneously. The experiments of degrading MB confirmed that the obtained catalysts can perform great catalytic activity even if it was used in the neutral pH and irradiated by visible light. And the catalysts could still exhibit high catalytic activity even though it had been used for many times. The comparison of the catalytic activity among Fe3O4, Fe3O4@SiO2@TiO2, SiO2@rGO@TiO2 and Fe3O4@rGO@TiO2 showed that the three components composed the catalysts possessed synergic effects which may be beneficial to the degradation of the recalcitrant organics.
Methods
Materials and preparation
All other chemical reagents were purchased from Shanghai Chemical Reagent Co. All chemicals were used without any purification. Ultrapure water (18 MUcm) was used for all experiments.
Graphene oxide (GO) was prepared from natural graphite by a modified Hummers method45. In a typical procedure, 2 g natural flake graphite, 2 g of NaNO3, together with 96 mL of concentrated H2SO4 were mixed at 0 °C. Then, 12 g of KMnO4 was gradually added to the obtained mixture and continuously stirred for 90 min while keeping the temperature at 0 °C. Thereafter, the mixture was heated to 50 °C and stirred for 2 h. 30 mL of concentrated HNO3 was slowly added to the above mixture and keeping stirring at 50 °C for about 2 h. Then, distilled water (80 mL) was slowly dropped into the resulting solution to dilute the mixture, and the stirring continued for 1 h at 95 °C. Finally, 10 mL of H2O2 (30%) were added to react with the residual KMnO4. The graphite oxide deposit was collected from the graphite oxide suspension by centrifugation at 10000 rpm for 15 min, and washed with distilled water for several times to remove the residual ions. Then the obtained graphene oxide suspension was dialyzed for two weeks to get the final products.
Preparation of GO encapsulated Fe3O4
1.299 g of FeCl3, 0.5 g of trisodium citrate, and 2.0 g of NaAc were dissolved in 40 mL of ethylene glycol with magnetic stirring. The homogeneous yellow solution was then transformed into a 100 mL Teflon-lined stainless-steel autoclave, heated at 200 °C for about 10 h, and then cooled to room temperature. The obtained black products were washed by ethanol and distilled water for three times, respectively. 0.5 g of the obtained Fe3O4 was homogeneous dispersed in isopropyl alcohol solution by ultrasonic for 30 min. Afterwards, 0.5 mL of APTMS were added to the above mixture and refluxed at 80 °C for 24 h. The products shown in Fig. 7a were washing by ethanol for several times and then dried in a vacuum oven. Finally, 100 mL homogeneous aqueous solution of the APTMS modified Fe3O4 (APTMS-Fe3O4) was mixed with 150 mL of 0.5 mg/mL GO for about 30 min under mechanical stirring to get GO wrapped Fe3O4 (Fe3O4@GO) nanospheres just like the image illustrated in Fig. 7b.
Figure 7.
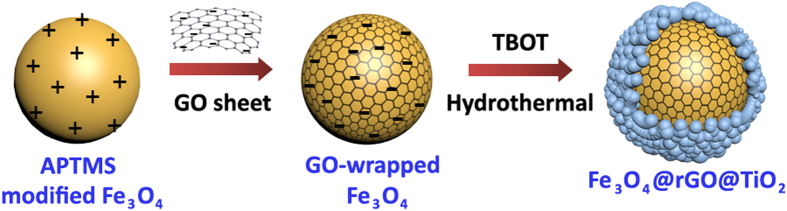
Schematic illustration of synthesis steps for Fe3O4@rGO@TiO2 hybrid. (a) Fe3O4 modified by APTMS. (b) Synthesis step of GO wrapped Fe3O4. The hybrid was synthesized through electrostatic interactions. (c) Synthesis step of Fe3O4@rGO@TiO2.The hybrid was synthesized through one step hydrothermal GO reduction and TiO2 crystallization.
Synthesis of Fe3O4@rGO@TiO2
30 mg of the as-prepared Fe3O4@GO were dispersed in 25 mL isopropyl alcohol by ultrasonic for 30 min. 100 μL tetrabutyl titanate (TBOT) were slowly dropped into the mixture and continuously stirred for another 30 min. Then 1 mL distilled water was added dropwise into the above solution. After keeping stirring for 30 min, the mixture was transformed into a 50 mL Teflon-lined stainless-steel autoclave, heated at 180 °C for 8 h, then cooled to temperature. The end products shown in Fig. 7c were rinsed by ethanol and distilled water for three times, respectively. After dried at 70 °C under vacuum, a grizzly ternary composite with TiO2 nanoparticles decorating on the surface of the GO-wrapped Fe3O4 sphere was obtained.
Degradation of MB by heterogeneous photo-Fenton reaction
The photo-Fenton activity of the as-prepared catalysts was evaluated by photodegradation of MB in aqueous solution under visible irradiation. A 300 W UV-vis lamp equipped with a λ > 400 nm cut off filter which covered the window of the Xenon lamp to absorb UV light and allow visible light to pass through was used as a light source46. All catalytic reactions were conducted in a 100 mL radius flask with constant mechanical agitation at room temperature. For the degradation of MB, desired amount of the as-prepared catalysts were added into the 50 mL aqueous solution containing 10 mg/L MB. Before illumination, the suspension without any H2O2 was sufficiently stirred for 30 min to reach adsorption-desorption equilibrium between the catalysts and MB so that the adsorption in the dark can be discounted. The lamp was turned on while a certain amount of H2O2 was adding to the mixed solution. About 5 mL aliquots were withdrawn at given time intervals and the catalysts were collected by magnetic separation. The concentration of the remnant MB was determined by testing the absorbance of the supertant at 664 nm by UV-vis spectroscopy.
Characterizations
To demonstrate the surface morphology and structure of the as-prepared catalysts, the samples were examined by scanning electron microscopy (SEM) using a JEOL SM-6360LV microscope equipped with an energy dispersive X-ray analyzer (EDX). Transmission electron microscopy (TEM) observation was achieved with a JEOL 2011 microscope (Japan) operated at an acceleration voltage of 200 kV. All the samples were suspended in the anhydrous ethyl alcohol and dropped on a carbon-coated copper grid, followed by drying at room temperature overnight. Power X-ray diffraction (XRD) measurements was performed on a X-ray diffractometer (RIGAKU, D/MAX 2550VB/PC, Japan) with CuKα radiation to verify the crystalline structure of the catalysts. The UV-vis spectra were recorded on a UV-vis spectrometer (UNICO UV-2102PC) at room temperature. The magnetization curve of the product was measured with a vibrating sample magnetometer (LAKE SHORE, 7407).
Author Contributions
X.L.Y. and W.C. conceived and designed the experiments. W.C. analyzed results and wrote the manuscript. Y.H.Z. and J.F.H. advised W.C. and reviewed the manuscript. Y.Z. and C.Z.L. reviewed the manuscript. All authors discussed the results and commented on the manuscript.
Additional Information
How to cite this article: Yang, X. et al. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4@rGO@TiO2-catalyzed photo-Fenton system. Sci. Rep. 5, 10632; doi: 10.1038/srep10632 (2015).
Supplementary Material
Supplementary Figures 1-6
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21471056, 21236003, 21206042, and 21176083), the Basic Research Program of Shanghai (13NM1400700, 13NM1400701), and the Fundamental Research Funds for the Central Universities.
References
- Chen Y. Z., Li N., Zhang Y. & Zhang L. D. Novel low-cost Fenton-like layered Fe-titanate catalyst: Preparation, characterization and application for degradation of organic colorants. J. Colloid Interface Sci. 422, 9–15 (2014). [DOI] [PubMed] [Google Scholar]
- Rache M. L. et al. Azo-dye orange II degradation by the heterogeneous Fenton-like process using a zeolite Y-Fe catalyst-kinetics with a model based on the Fermi’s equation. Appl. Catal., B 146, 192–200 (2014). [Google Scholar]
- Lin Y. et al. Ternary graphene-TiO2-Fe3O4 nanocomposite as a recollectable photocatalyst with enhanced durability. Eur. J. Inorg. Chem. 28, 4439–4444 (2012). [Google Scholar]
- Yu F., Ma J. & Han S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 4, 5326; 10.1038/srep05326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponou J. et al. Evaluation of the flocculation and de-flocculation performance and mechanism of polymer flocculants. Water Sci. Technol. 69, 1249–1258 (2014). [DOI] [PubMed] [Google Scholar]
- Li L. L., Li X. J., Duan H. M., W X. J., L C. N. Removal of Congo Red by magnetic mesoporous titanium dioxide-graphene oxide core-shell microspheres for water purification. Dalton Trans. 43, 8431–8438 (2014). [DOI] [PubMed] [Google Scholar]
- Yang X. J., Xu X. M., Xu J. &Han Y. F. Iron oxychloride (FeOCl): an efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 135, 16058–16061 (2013). [DOI] [PubMed] [Google Scholar]
- Han T. T., Qu L. L., Luo Z. J., Wu X. Y. & Zhang D. X. Enhancement of hydroxyl radical generation of a solid state photo-Fenton reagent based on magnetite/carboxylate-rich carbon composites by embedding carbon nanotubes as electron transfer channels. New J. Chem. 38, 942–948 (2014). [Google Scholar]
- Ai Z. H. et al. Fe@ Fe2O3 core-shell nanowires as the iron reagent. 2. An efficient and reusable sono-Fenton system working at neutral pH. J. Phys. Chem. C111, 7430–7436 (2007). [Google Scholar]
- Li Z. J., Ali G., Kim H. J., Yoo S. H. & Cho S. O. LiFePO4 microcrystals as an efficient heterogeneous Fenton-like catalyst in degradation of rhodamine 6G. Nanoscale Res. Lett. 9, 276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. L., Xiao D. X., Guo Y. G., Wang Z. H. & Liu J. S. A novel photosensitized Fenton reaction catalyzed by sandwiched iron in synthetic nontronite. RSC Adv. 4, 12958–12963 (2014). [Google Scholar]
- Hu X. B. et al. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nanoFe3O4/MWCNTs in aqueous solution. Appl. Catal., B 107, 274–283 (2011). [Google Scholar]
- Xu L. J. & Wang J. L. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 46, 10145–10153 (2012). [DOI] [PubMed] [Google Scholar]
- Liu L. J., Zhang G. L., Wang L., Huang T. & Qin L. Highly active S-modified ZnFe2O4 heterogeneous catalyst and its photo-Fenton behavior under UV–visible irradiation. Ind. Eng. Chem. Res. 50, 7219–7227 (2011). [Google Scholar]
- Feng J. Y., Hu X. J. & Yue P. L. Discoloration and mineralization of Orange II using different heterogeneous catalysts containing Fe: a comparative study. Environ. Sci. Technol. 38, 5773–5778 (2004). [DOI] [PubMed] [Google Scholar]
- Zubir N. A., Yacou C., Motuzas J., Zhang X. W. & da Costa J. C. D. Structural and functional investigation of graphene oxide-Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 4, 4594; 10.1038/srep04594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. C. et al. Preparation and characterization of magnetic porous carbon microspheres for removal of methylene blue by heterogeneous Fenton reaction. ACS Appl. Mater. Interfaces 6, 7275–7285 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou K. F., Zhu Y. H., Yang X. L., Jiang X. & Li C. Z. Preparation of graphene-TiO2 composites with enhanced photocatalytic activity. New J. Chem. 35, 353–359 (2011). [Google Scholar]
- Shao X., Lu W. C., Zhang R. & Pan F. Enhanced photocatalytic activity of TiO2-C hybrid aerogels for methylene blue degradation. Sci. Rep. 3, 3018; 10.1038/srep03018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., You K. H. & Park C. B. Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv. Mater. 24, 1084–1088 (2012). [DOI] [PubMed] [Google Scholar]
- Liu H. et al. A green and direct synthesis of graphene oxide encapsulated TiO2 core/shell structures with enhanced photoactivity. Chem. Eng. J. 230, 279–285 (2013). [Google Scholar]
- Wang P. et al. Dye-sensitization-induced visible-light reduction of graphene oxide for the enhanced TiO2 photocatalytic performance. ACS Appl. Mater. Interfaces 5, 2924–2929 (2013). [DOI] [PubMed] [Google Scholar]
- Lu J., Deng C. H., Zhang X. M., Yang P. Y. Synthesis of Fe3O4/graphene/TiO2 composites for the highly selective enrichment of phosphopeptides from biological samples. ACS Appl. Mater. Interfaces 5, 7330–7334 (2013). [DOI] [PubMed] [Google Scholar]
- Tan L. L., Chai S. P. & Mohamed A. R. Synthesis and applications of graphene-based TiO2 photocatalysts. ChemSusChem , 5, 1868–1882 (2012). [DOI] [PubMed] [Google Scholar]
- Zhao D. L., Sheng G. D., Chen C. L. & Wang X. K. Enhanced photocatalytic degradation of methylene blue under visible irradiation on graphene@TiO2 dyade structure. Appl. Catal., B 111, 303–308 (2012). [Google Scholar]
- Jiang B. J. et al. In situ growth of TiO2 in interlayers of expanded graphite for the fabrication of TiO2-graphene with enhanced photocatalytic activity. Chem. Eur. J. 17, 8379–8387 (2011). [DOI] [PubMed] [Google Scholar]
- Štengl V., Bakardjieva S., Grygar T. M., Bludská J. & Kormunda M. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J. 7, 41–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M. S. A. S., Park A. R., Zhang K., Park J. H. & Yoo P. J. Green synthesis of biphasic TiO2-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 4, 3893–3901 (2012). [DOI] [PubMed] [Google Scholar]
- Song P., Zhang X. Y., Sun M. X., Cui X. L. & Lin Y. H. Graphene oxide modified TiO2 nanotube arrays: Enhanced visible light photoelectrochemical properties. Nanoscale 4, 1800–1804 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Tang Z. R., Fu X. Z. & Xu Y. J. TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 4, 7303–7314 (2010). [DOI] [PubMed] [Google Scholar]
- Hummers J. W. S. & Offeman R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958). [Google Scholar]
- Shen J. H., Zhu Y. H., Zhou K. F., Yang X. L. & Li C. Z. Tailored anisotropic magnetic conductive film assembled from graphene-encapsulated multifunctional magnetic composite microspheres. J. Mater. Chem. 22, 545–550 (2012). [Google Scholar]
- Yang S. B., Feng X. L., Ivanovici S., Mullen K. Fabrication of graphene-encapsulated oxide nanoparticles: towards high-performance anode materials for lithium storage. Angew. Chem. Int. Ed. 49, 8408–8411 (2010). [DOI] [PubMed] [Google Scholar]
- Lin S. X., Shen C. M., Lu D. B., Wang C. M. & Gao H. J. Synthesis of Pt nanoparticles anchored on graphene-encapsulated Fe3O4 magnetic nanospheres and their use as catalysts for methanol oxidation. Carbon 53, 112–119 (2013). [Google Scholar]
- Jiang G. D. et al. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 49, 2693–2701 (2011). [Google Scholar]
- Wang Q., Tian S. L. & Ning P. Degradation mechanism of methylene blue in a heterogeneous Fenton-like reaction catalyzed by ferrocene. Ind. Eng. Chem. Res . 53, 643–649 (2013). [Google Scholar]
- Carra I., Malato S., Santos-Juanes L., Lopez J. L. C. & Perez J. A. S. Study of iron sources and hydrogen peroxide supply in the photo-Fenton process using acetaminophen as model contaminant. J. Chem. Technol. Biotechnol. 88, 636–643 (2013). [Google Scholar]
- Bai S. et al. One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 50, 2337–2346 (2012). [Google Scholar]
- Kuang Y., Wang Q. P., Chen Z. L., Megharaj M. & Naidu R. Heterogeneous Fenton-like oxidation of monochlorobenzene using green synthesis of iron nanoparticles. J. Colloid Interface Sci. 410, 67–73 (2013). [DOI] [PubMed] [Google Scholar]
- Hadjltaief H. B., Da Costa P., Galvez M. E. & Ben Zina M. Influence of operational parameters in the heterogeneous photo-Fenton discoloration of wastewaters in the presence of an iron-pillared clay. Ind. Eng. Chem. Res. 52, 16656–16665 (2013). [Google Scholar]
- Garrido-Ramrez E. G., Theng B. K. G. & Mora M. L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions-a review. Appl. Clay Sci. 47, 182–192 (2010). [Google Scholar]
- Guo L. Q., Chen F., Fan X. Q., Cai W. D. & Zhang J. L. S-doped alpha-Fe2O3 as a highly active heterogeneous Fenton-like catalyst towards the degradation of acid orange 7 and phenol. Appl. Catal., B 96, 162–168 (2010). [Google Scholar]
- Park Y., Kang S. H. & Choi W. Exfoliated and reorganized graphite oxide on titania nanoparticles as an auxiliary co-catalyst for photocatalytic solar conversion. Phys. Chem. Chem. Phys. 13, 9425–9431 (2011). [DOI] [PubMed] [Google Scholar]
- Liu L. M., Yang W. Y., Li Q., Gao S. A. & Shang J. K. Synthesis of Cu2O nanospheres decorated with TiO2 nanoislands, their enhanced photoactivity and stability under visible light illumination, and their post-illumination catalytic memory. ACS Appl. Mater. Interfaces 6, 5629–5639 (2014). [DOI] [PubMed] [Google Scholar]
- Yang X. L. et al. Tailored graphene-encapsulated mesoporous Co3O4 composite microspheres for high-performance lithium ion batteries. J. Mater. Chem. 22, 17278–17283 (2012). [Google Scholar]
- Shen J. H., Zhu Y. H., Yang X. L. & Li C. Z. Magnetic composite microspheres with exposed {001} faceted TiO2 shells: a highly active and selective visible-light photocatalyst. J. Mater. Chem. 22, 13341–13347 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6