Abstract
Increasing evidence supports an association between the skeleton and energy metabolism. These interactions are mediated by a variety of hormones, cytokines and nutrients. Here, the evidence for a role of osteocalcin in the regulation of glucose metabolism in humans is reviewed. Osteocalcin is a bone matrix protein that regulates hydroxyapatite size and shape through its vitamin-K-dependent γ-carboxylated form. In circulation, the concentration of osteocalcin is a measure of bone formation. The undercarboxylated form of osteocalcin is reported to be active in glucose metabolism in mice. Total serum osteocalcin concentrations in humans are inversely associated with measures of glucose metabolism; however, human data are inconclusive with regard to the role of uncarboxylated osteocalcin in glucose metabolism because most studies do not account for the influence of vitamin K on the proportion of undercarboxylated osteocalcin or differentiate between the total and uncarboxylated forms of osteocalcin. Furthermore, most human studies do not concomitantly measure other bone turnover markers to isolate the role of osteocalcin as a measure of bone formation from its effect on glucose metabolism. Carefully designed studies are required to define the role of osteocalcin and its carboxylated or undercarboxylated forms in the regulation of glucose metabolism in humans.
Introduction
Vitamin K was known to be essential for blood clotting; however, only in 1974 was vitamin K identified as a cofactor for the formation of γ-carboxyglutamic acid (Gla), a unique amino acid that is created by a post-translational modification of specific glutamic acid residues in a target protein. This process is inhibited by warfarin (Figure 1).1,2 Bone biologists actively seeking to identify noncollagen proteins in bone were aware that the fetal warfarin embryopathy often resulted in boney malformations; therefore, they searched for a calcium-binding protein containing γ-carboxyglutamic acid in bone. In 1977, osteocalcin (a 6 kD protein containing three γ-carboxyglutamic acid residues and made in osteoblasts and odontoblasts) was isolated and sequenced.3,4 Osteocalcin is one of the most abundant noncollagen proteins in bone, and its synthesis is exclusive to bone.
Figure 1.
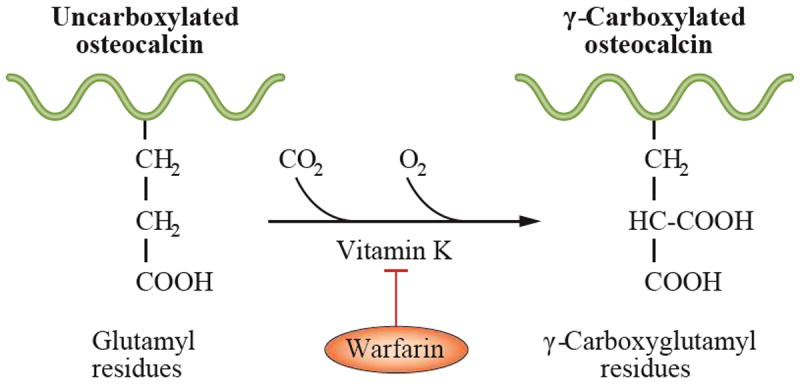
Vitamin K is required for the formation of γ-carboxyglutamic acid. γ-carboxyglutamic acid (Gla) is a unique amino acid that is created by a vitamin K-dependent post-translational modification of specific glutamic acid residues in all Gla-containing proteins, including osteocalcin. This process is inhibited by warfarin.
In 2007, Lee et al.5 suggested that in mice osteocalcin acts as a hormone to affect insulin sensitivity and energy expenditure. Osteocalcin was reported to improve glucose tolerance by increasing β-cell proliferation and insulin expression and secretion. Osteocalcin also increased insulin sensitivity in peripheral tissues, increased adiponectin expression and protected the mice from adiposity. The most surprising finding was that only the uncarboxylated or undercarboxylated forms of osteocalcin were active.5-12 Since those reports, a growing number of studies have linked serum osteocalcin concentrations to glucose homeostasis in humans. This research has led to speculation that serum osteocalcin concentrations have clinical utility as a risk marker for diabetes mellitus.
The bone–fat axis hypothesis has been extensively reviewed elsewhere.9,11,13-16 Here, the unique species-specific features of vitamin-K-dependent osteocalcin are reviewed and evidence for a role of osteocalcin in energy metabolism in humans is discussed.
Osteocalcin
A vitamin-K-dependent protein
To evaluate the role of osteocalcin in glucose metabolism and adiposity in humans, one needs to define the effect of vitamin K on the function of osteocalcin. Two major structural forms of vitamin K are present in nature, phylloquinone (also referred to as vitamin K1) and menaquinones (also referred to as vitamin K2).17 Phylloquinone is the most common dietary form of the vitamin and is found in green vegetables and vegetable oils (Table 1). All vitamin K forms share the common structure, 2-methyl-1,4-napthoquinone. This structure is the active site for this vitamin, which acts as an enzyme cofactor.18 Coincident with γ-carboxylation, the active quinol form of vitamin K is oxidized and subsequently recycled by a vitamin K epoxide.17 This recycling of vitamin K consumed during γ-carboxylation can be regarded as a pathway by which to preserve limited stores of vitamin K.
Table 1.
Phylloquinone content of commonly eaten foods148
| Food | Serving | Vitamin K (μg) |
|---|---|---|
| Olive oil | 1 tablespoon | 8.1 |
| Soybean oil | 1 tablespoon | 25.0 |
| Broccoli, cooked | 1 cup | 220 |
| Kale, raw | 1 cup | 547 |
| Spinach, raw | 1 cup | 145 |
| Leaf lettuce | 1 cup | 45.5 |
| Swiss chard, raw | 1 cup | 299 |
| Parsley, fresh | 1/4 cup | 246 |
Proteins that include γ-carboxyglutamic acid contain a propeptide recognition site that is essential for their binding to the vitamin-K-dependent carboxylase. After carboxylation, the propeptide is removed and the mature protein is secreted.19 Aside from the vitamin-K-dependent clotting factors, the requirement for vitamin-K-dependent γ-carboxylation to activate proteins has been demonstrated in many tissues for diverse biological functions. In the skeleton, matrix Gla protein (MGP) and Gla-rich protein (also known as UCMA) require the presence of γ-carboxyglutamic acid to function as potent inhibitors of calcification in cartilage and other soft tissues,20,21 whereas the γ-carboxyglutamic acid residues in osteocalcin are involved in the regulation of the size and shape of bone mineral.
Regulates crystal size and shape
Early studies to investigate the function of osteocalcin and other proteins containing γ-carboxyglutamic acid were designed based on the knowledge that the γ-carboxyglutamic acid residues in the vitamin-K-dependent clotting factors were essential in order to bind Ca2+ and interact with phospholipid membranes to facilitate the clotting cascade. Similarly, the γ-carboxyglutamic acid residues in osteocalcin bind free Ca2+ and hydroxyapatite.
Analysis by circular dichroism, nuclear magnetic resonance imaging and X-ray crystallography22-24 has revealed that osteocalcin is a globular protein comprised of three α-helices, a hydrophobic core, an unstructured N-terminus and an exposed C-terminus (Figure 2). All three γ-carboxyglutamic acid residues are found in the first helical region, and when bound to free calcium, facilitate a conformational change that aligns them in a complementary fashion to the calcium ions on the C-axis of the hydroxyapatite crystal lattice.
Figure 2.
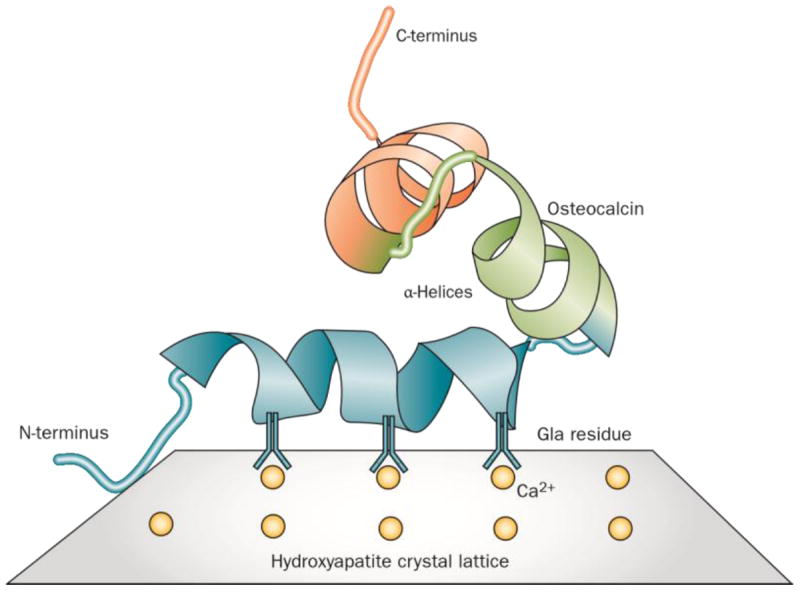
Direct structural analysis of osteocalcin by NMR imaging and X-ray crystallography predict a tight globular structure comprised of three α-helices, a C-terminal hydrophobic core and an unstructured N-terminus. All three γ-carboxyglutamic acid (Gla) residues are found in the first helical region. Osteocalcin amino acid sequences from all species share extensive amino homology at the central region containing the γ-carboxyglutamic acids but there is considerable sequence variation in other regions. The γ-carboxyglutamic acid residues are complementary to the calcium ions on the c-axis of the hydroxyapatite crystal lattice, and are positioned to control crystal size and shape within the constraints of the collagen fibril.
The importance of the osteocalcin–hydroxyapatite interaction is illustrated by the fact that osteocalcin amino acid sequences from all animal species investigated share extensive amino acid sequence homology at the central γ-carboxyglutamic acid region. However, considerable sequence variation exists at other regions. Compared with humans, the overall protein sequence is conserved 80–95% in most species, but less in the frog and chicken (70%), mouse (60%) and bony fish (40%).24 The human osteocalcin gene is a single copy gene located at the distal long arm of chromosome 1. γ-carboxyglutamic acid is found at amino acid positions 17, 21 and 24 in human osteocalcin). By contrast, mice have a cluster of three osteocalcin genes in a 23 kb span oriented in the same transcriptional direction. Two of the genes (Bglap and Bglap2, also known as OG1 and OG2) are expressed only in bone, whilst a third gene, the osteocalcin-related gene (Bglap-rs1, also known as ORG), is expressed at low levels in brain, lung and kidney, but not in bone.25 Examination of the promoters of the human and rat gene reveal that they are very similar with respect to the organization of regulatory elements and their response to hormones and growth factors, whereas the mouse gene exhibits differences, particularly in response to 1,25-dihydroxyvitamin D3.26 The human and rat osteocalcin genes are dose-dependently upregulated by 1,25-dihydroxyvitamin D3, whereas the mouse osteocalcin gene is downregulated.26,27
The function of osteocalcin in bone is thought to be dictated by its structure. Immunolocalization studies show that the protein is distributed throughout the mineralized regions of bone matrix, dentin and calcified cartilage in rats.28 In vitro growth of the hydroxyapatite is inhibited by low concentrations of osteocalcin, which suggests that the protein’s function is related to the control of crystal morphology. In the bone of all species studied, osteocalcin first appears coincident with the onset of mineralization in utero, and its levels increase in tandem with hydroxyapatite deposition during skeletal growth.29,30 In rat and mouse osteoblast cultures, osteocalcin appears only after the extracellular matrix accumulates and begins to mineralize.31
In 1996, Ducy et al.32 generated an osteocalcin-deficient mouse model. Given the abundance of osteocalcin, its restriction to bone and specificity to hydroxyapatite, it was surprising that the mice had no overt phenotype.32 The mice were reported to be morphologically normal at birth, viable and fertile with no skeletal defects. By the age of 6 months, however, they exhibited a small increase in cortical thickness, a consequence of an increased bone formation rate, but had normal osteoclastic activity. Evaluation of the structural properties by whole bone biomechanical testing showed significant differences in bone strength. Bone mineral content was unaffected, but subsequent analysis of the crystal properties of hydroxyapatite showed altered mineral composition in the cortices of the bone of the osteocalcin knockout mice.33
Hydroxyapatite varies in crystallinity as a result of impurities, primarily carbonate ions, which accumulate with time as the crystal matures. In osteocalcin-deficient mice, the hydroxyapatite crystal showed less carbonate substitution, which suggested a less mature and/or remodelled mineral compared to that of the wild-type mice.33 Small angle x-ray scattering confirmed that the hydroxyapatite crystals were immature and also less thick and smaller in the osteocalcin-deficient mice than in the wild-type mice. Furthermore, the crystals in the osteocalcin-deficient mice were less well aligned along the collagen fibrils compared to those of the wild-type mice.34 Bone strength is a function of both bone quantity and quality, and in a mouse model of osteogenesis imperfecta, changes in optimal crystal size and orientation have been associated with increased brittleness.35
A marker of bone remodelling
The skeleton undergoes continuous remodelling (turnover) of bone, with removal of old bone by osteoclasts and coordinated replacement with new bone by osteoblasts. In the steady state, this coupling of bone formation and resorption maintains bone mass. The discovery of osteocalcin presented an opportunity for clinicians searching for noninvasive markers to aid in the management of patients with osteoporosis. Histomorphometric analysis and calcium kinetics demonstrated that serum osteocalcin levels were correlated with bone formation and osteoblast number.36-38 As a product of osteoblastic synthesis, osteocalcin has been used as a marker of bone formation. Furthermore, in normal rats, circulating osteocalcin was shown to originate primarily from new bone synthesis rather than from the breakdown of bone.39
The majority of commercially available osteocalcin assays measure the intact molecule and a large 1–43 fragment that is generated by tryptic activity in the circulation.40 Fragments of osteocalcin encompassing the mid-molecular region41 also circulate. These fragments are derived from the action of osteoclastic matrix metalloproteinases and cathepsin K during bone resorption,42,43 and are rapidly cleared in individuals with normal renal function. Although some single antibody assays have limited capacity to detect these fragments in serum, only urine-based mid-molecular assays have sufficient sensitivity and specificity to detect them.44
Currently, assays for bone formation include the N-terminal propeptide of type I collagen, bone-specific alkaline phosphatase, and intact or N-mid osteocalcin in serum. Resorption assays include collagen N-telopeptide, C-telopeptide, tartrate-resistant acid phosphatase and urinary mid-molecule osteocalcin. All have a circadian variation and respond to changes in bone formation and resorption accompanying growth, age, menopause, metabolic bone diseases and medications that affect bone turnover.45,46
γ-Carboxylation of osteocalcin
Undercarboxylation in humans
In most species, all three vitamin-K-dependent γ-carboxyglutamic acid sites in the osteocalcin molecule are fully carboxylated. However, in humans, osteocalcin in bone and serum is incompletely carboxylated (undercarboxylated osteocalcin). High-dose warfarin co-administered with high-dose vitamin K maintains adequate blood clotting but the osteocalcin is not fully carboxylated,47 which suggests that the liver sequesters vitamin K at the expense of bone. In vitro, carboxylation is an ordered process with Glu22 and Glu24 in osteocalcin being carboxylated first, followed by Glu17 in humans (corresponding to Glu13 in mice).48 Analysis of osteocalcin isolated from 20 human bone samples found carboxylation to be mean±SD 67±14, 88±9, and 93±4% at residues 17, 21 and 24, respectively,22 reflecting long-term variability in vitamin K availability to the bone. Circulating osteocalcin is similarly undercarboxylated. Estimates of the percentage of undercarboxylated osteocalcin by direct enzyme-linked immunosorbent assay (ELISA) or by differential binding to hydroxyapatite coupled to an immunoassay suggest that up to 50% of osteocalcin is undercarboxylated in serum of normal individuals, and that the percentage of undercarboxylated osteocalcin reflects current vitamin K intake.49 Perhaps as a direct consequence of this undercarboxylation, human osteocalcin concentrations in bone and in the circulation are only 20% of that found in other species.50
Response to vitamin K manipulation
In humans, the current recommendations for vitamin K are 90 to 120 μg per day based on median phylloquinone intakes.51 Some of the menaquinones are produced by gut flora in the large intestine, but the extent to which endogenous menaquinone production contributes to the daily requirement for vitamin K is not known in humans.52 However, a subclinical deficiency of vitamin K can be created within days by limiting dietary intakes of phylloquinone without a concomitant change in gut flora or menaquinone status.53 The degree to which a vitamin-K-dependent protein such as osteocalcin is carboxylated is used as a functional indicator of vitamin K status.
Biomarkers of vitamin K status are consistently altered in humans as human diets contain varying vitamin K content from day to day. Dietary restriction of phylloquinone to <35 μg per day causes rapid and marked increases in undercarboxylated osteocalcin (expressed as a percentage of the total osteocalcin that is not fully γ-carboxylated; percentage undercarboxylated osteocalcin)54 Conversely, supplementation with phylloquinone in doses from 250 μg per day for 14 days to 5,000 μg per day for 24 months markedly decreases the percentage of undercarboxylated osteocalcin to less than 10% (Figure 3).55-59 This response is consistent for the percentage of undercarboxylated osteocalcin regardless of the forms of vitamin K studied.56,60 The rapidity of the observed changes noted in the short-term studies suggests that the carboxylation of the Glu residues in osteocalcin may change in response to fluctuations in intakes of vitamin K on a daily basis in humans. Therefore, any discussion regarding the role of carboxylation of osteocalcin in energy metabolism in humans requires consideration of the vitamin K status at the time of measurement.
Figure 3.
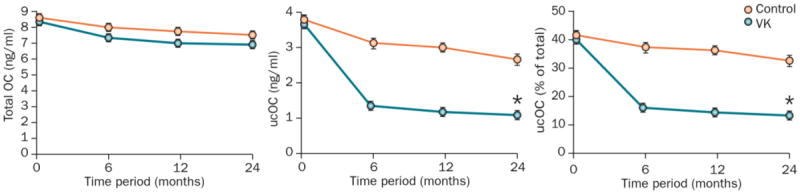
Response of all forms of osteocalcin to vitamin K supplementation in humans2-year changes(mean±SEM) in a ∣ serum total osteocalcin, b ∣ serum undercarboxylated osteocalcin and c ∣ percentage undercarboxylated osteocalcin in 396 men and women (age range 60–80 years) in response to 500 μg per day of vitamin K in the form of phylloquinone or no vitamin Ksupplementation (control) in a randomized, double-blind clinical trial.55 2-year changes in total osteocalcin concentrations did not differ between groups. By contrast, vitamin K supplementation decreased concentrations of undercarboxylated osteocalcin and percent undercarboxylated osteocalcin in the phylloquinone group (P<0.05) but not in the control group, which demonstrates the need for vitamin K status to be considered when discussing the role of undercarboxylated osteocalcin in energy metabolism in humans. Abbreviations: OC, osteocalcin; ucOC, undercarboxylated osteocalcin. VK, vitamin K
Correlation among osteocalcin measures
The percentage of undercarboxylated osteocalcin is influenced by vitamin K status whereas the serum total concentrations of osteocalcin are influenced by osteoblastic synthesis independent of vitamin K (Figure 4). This interdependence of the different available measures of circulating osteocalcin in humans has given rise to some confusion in the published literature regarding the predictive value of serum osteocalcin for the risk of type 2 diabetes mellitus (T2DM).
Figure 4.
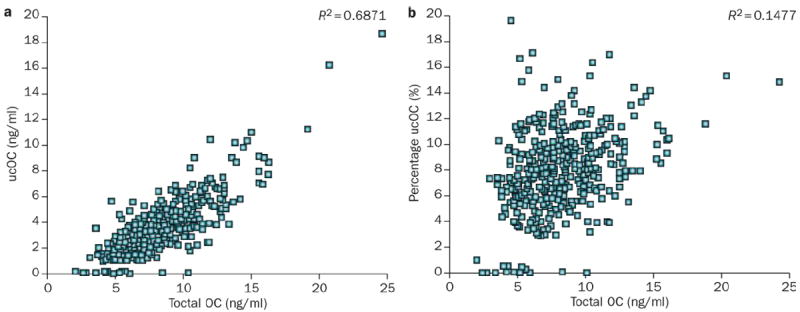
Correlation among osteocalcin measures. a ∣ Serum undercarboxylated osteocalcin is highly correlated with total serum concentrations of osteocalcin whereas b ∣ the serum percentage of undercarboxylated osteocalcin is not correlated to total serum concentrations of osteocalcin. The 426 men and women who participated were community-based older adults (age range 60–80 years) free of osteoporosis and cardiovascular disease at the time of enrolment in a randomized double-blind clinical trial examining the effect of vitamin K supplementation on bone health.55 Osteocalcin measures were made prior to vitamin K supplementation.55 Abbreviations: OC, osteocalcin; ucOC, undercarboxylated osteocalcin.
By use of either the current ELISA or hydroxyapatite binding assays, absolute concentrations of undercarboxylated osteocalcin are highly correlated with total osteocalcin, whereas the percentage of undercarboxylated osteocalcin is not (Figure 4). As reviewed elsewhere,61,62 for accurate interpretation of hydroxyapatite-binding assays, the assays require equivalent detection of both carboxylated and undercarboxylated forms of osteocalcin. Unfortunately, few studies in humans that used the ELISA assays have included the carboxylated forms and, hence, the percentage of undercarboxylated osteocalcin could not be calculated.
Osteocalcin and glucose metabolism
Overview
The existence of altered bone metabolism among patients with diabetes mellitus is a well-characterized phenomenon, although the mechanisms are not well understood.63 Bone mass is low in patients with type 1 diabetes mellitus (T1DM) but higher than normal in those with T2DM. Yet in both patients with T1DM and those with T2DM, increased fracture risk is observed at a given BMD as compared to that in individuals without diabetes mellitus.64-67 The effects of glucose toxicity on osteoblasts, low levels of insulin and insulin-like growth factor 1, altered vitamin D metabolism, low bone formation, raised levels of advanced glycation endproducts, increased cortical porosity, and biomechanical forces have all been implicated in the pathogenesis associated with changes in bone mass and fracture risk in diabetes mellitus, but these processes do not completely explain the clinical findings.68
Bone cells and adipocytes reside within the bone marrow and are thought to differentiate from a common precursor.69,70 Increased commitment of bone-marrow-derived mesenchymal stem cells to adipocytogenesis occurs at the expense of osteoblastogenesis, and high levels of marrow fat are associated with an increased risk of both osteoporosis and diabetes mellitus.71 A growing body of evidence shows that the pancreas and adipocytes secrete bone-active hormones.13-16 Adipose tissue, through leptin, regulates bone remodelling via the central nervous system.72-74 These relationships pose an important question, namely, are these relationships bidirectional such that bone plays a regulatory role in energy metabolism?
Genetic studies in mice
In a search for potential mediators of metabolism in mice, Lee et al.5 found two candidate genes for which protein biosynthesis is restricted to bone. The first of these, Esp (also known as Ptprv), is a gene expressed only in osteoblasts and Sertoli cells and encodes a transmembrane protein, tyrosine phosphatase (OST-PTP, also known as R-PTP-V). Both global and osteoblast-specific deletion of Esp produced animals that were lean, hypoglycaemic, and that had increased β-cell proliferation, insulin secretion and insulin sensitivity. When adipocytes from normal mice were grown in the presence of osteoblast-conditioned media from either wild-type mice or mice with global deletion of Esp, expression of adiponectin was increased by 40% and 100%, respectively. Likewise, insulin expression was increased in wild-type islets grown in the presence of osteoblast-conditioned media from wild-type mice (40%) or from mice with global deletion of Esp (100%). These results suggest that the osteoblast secretes a factor or factors that affect β cells and adipocytes and that OST-PTP regulates the activity of this factor. Because of its osteoblast-specific expression, a logical candidate for this factor was osteocalcin (Figure 5).
Figure 5.
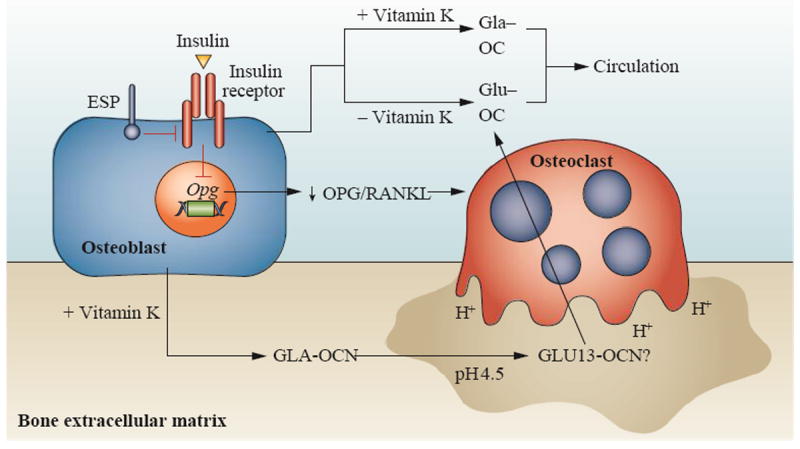
in human circulation would be the consequence of two separate processes: incomplete carboxylation of osteocalcin due to suboptimal vitamin K intake or decarboxylation during osteoclast resorption. Insulin signalling in osteoblasts limits production of osteoprotegerin, an inhibitor of osteoclast maturation. This facilitates osteoclast bone resorption, producing an acid environment that decarboxylates, and hence activates intact osteocalcin. OST-PTP/PTN 1+2 dephosphorylates the insulin receptor (insR) in osteoblasts leading to inhibition of insulin signaling. Abbreviations: cOC, carboxylated osteocalcin. OPG, osteoprotegerin. ucOC undercarboxylated osteocalcin
Lee et al. re-examined the phenotype of their osteocalcin-deficient mice,5 which had been described earlier32 Osteocalcin knockout mice were found to be obese with elevated glucose and lipid concentrations, reduced insulin levels, reduced numbers of β cells, and were glucose intolerant and insulin insensitive. However, in the original description of the osteocalcin knockout mouse,32 bone formation was elevated compared with wild-type mice, a finding which conflicts with other animal models of diabetes mellitus, in which bone formation is reduced.13 In contrast to what is observed in humans with T2DM, both insulin secretion and sensitivity were decreased in these mice, effects that were attributed to a decrease in adiponectin expression in adipose tissue.6 Overall, the phenotype was the exact opposite of that observed in the Esp−/− mice. Cultures of islets and adipocytes from wild-type mice with conditioned media from osteoblasts derived from osteocalcin knockout mice resulted in decreases in insulin and adiponectin (Figure 5). Furthermore, the metabolic phenotype was normalized in Esp−/− mice lacking one allele of osteocalcin, further supporting the notion that OST-PTP and osteocalcin are in the same pathway.
The circulating and expression levels of osteocalcin were normal in Esp−/− mice, which suggests that OST-PTP does not regulate the biosynthesis of osteocalcin, but rather regulates its metabolic function. Given that the only known modifiable aspect of osteocalcin is the post-translational carboxylation of three glutamic acid residues to γ-carboxyglutamic acid residues, Lee et al.5 showed that only uncarboxylated osteocalcin, but not carboxylated osteocalcin, induces expression of both adiponectin and insulin in islets. This finding presents a major paradigm shift given that all known vitamin-K-dependent proteins require the presence of γ-carboxyglutamic acid for function, including the carboxylating enzyme itself.18
To establish the role of osteocalcin in glucose metabolism, Ferron et al.6 implanted mice with osmotic minipumps containing uncarboxylated osteocalcin. Doses that delivered up to 3 ng/ml serum were given for 4 weeks resulted in low blood glucose levels and an increase in serum insulin levels. In these mice, the reported circulating level of undercarboxylated osteocalcin was 7 ng/ml,6 approximately 10% of the total circulating osteocalcin measured in adult mice fed standard rodent chows. At the doses given to the mice, the undercarboxylated osteocalcin increases only to 14%, which is within the intra-individual variation that is normal in humans consuming a varied diet. This highlights the need to compare the uncarboxylated or undercarboxylated osteocalcin to total osteocalcin in both human and animal studies to understand any relevant changes that are related to metabolism or vitamin K intake.
Insulin signalling in osteoblasts
Ferron et al.7 considered OST-PTP as a regulatory factor of the enzymes of the vitamin K cycle but no tyrosine phosphorylation was found on either the enzymes or osteocalcin itself. However, substrate trapping showed that the insulin receptor in osteoblasts was a substrate for OST-PTP and that increased phosphorylation of the osteoblast insulin receptor is found in Esp−/− mice.7 Orthologues for all rodent protein tyrosine phosphatases have been found in humans, with the exception of OST-PTP. Ferron et al.7 employed substrate trapping to search for other protein tyrosine phosphatases that might compensate for this absence in humans. They showed that tyrosine-protein phosphatase nonreceptor type 1 (PTN1; also known as PTP-1B) was expressed in human osteoblasts. PTN1 is a ubiquitously expressed tyrosine phosphate and has been implicated in multiple signalling pathways.75 Subsequently, Zee et. al.76 demonstrated that tyrosine-protein phosphatase nonreceptor type 2 (PTN2; also known as TC-PTP) also regulates insulin receptor phosphorylation in the osteoblast of humans and is, in fact, more highly expressed in bone than either OST-PTP or PTN1 (Figure 5).
Insulin signalling in osteoblasts decreases the expression of osteoprotegerin, an inhibitor of osteoclast maturation, and hence activates bone resorption.7,8 Osteoclast bone resorption produces an acid environment which, as proposed by some researchers, may decarboxylate osteocalcin.15 Furthermore, in this model, decarboxylation occurs at only the first γ-carboxyglutamic acid residue at position 17 (or position 13 in mice) and is sufficient for osteocalcin hormonal action. The final regulation of this process is via leptin which, through the sympathetic tone, regulates the expression of Esp.74
This scenario raises several questions. First, it has been established that the kinetics of osteocalcin decarboxylation requires dry decarboxylation in vacuo to prevent hydrolytic side reactions that can occur in solution and produce multiple osteocalcin fragments.77 Furthermore, when human osteoclasts are cultured on bovine bone, only a small amount of intact osteocalcin is released during acidification. When the matrix osteocalcin is subsequently degraded enzymatically, fragments of osteocalcin are produced.44 These observations are consistent with clinical studies that show that urinary osteocalcin fragments are associated with bone resorption.78 Secondly, the requirement for bone resorption to activate matrix-bound osteocalcin during bone resorption is inconsistent with results of Lee et al.5 in which conditioned media from osteoblasts for wild-type Esp knockout mice affected the expression of adiponectin and insulin. Finally, undercarboxylated osteocalcin in human circulation could be the consequence of two separate processes: incomplete carboxylation of osteocalcin due to suboptimal vitamin K intake or decarboxylation during osteoclast resorption (Figure 5).
G-coupled protein receptor
The final element necessary to complete a putative endocrine loop mediated by osteocalcin was the identification of a tissue-specific receptor. This identification was achieved in an indirect way. On the basis of the fact that OST-PTP was expressed in osteoblasts and Leydig cells of the testes, Oury et al.79 showed that uncarboxylated osteocalcin also regulated male fertility. Furthermore, the researchers showed that the binding of osteocalcin in vitro to a G-protein-coupled receptor (GPRC6a) in isolated mice Leydig cells was associated with a reduction in the biosynthesis of testosterone.79 If this scenario is consistent in humans, then vitamin K intake would have to be considered a modifier of male fertility.
Ample evidence exists for the involvement of GPRC6A in the regulation of biological processes in humans. GPRC6A is a seven transmembrane receptor; the domain of GPRC6A that mediates signalling contains a wide range of L-alpha-amino acids, predominantly the basic amino acids, arginine, lysine and orthinine80 Of the human family C receptors, of which GPRC6A is a member, the calcium-sensing receptor is the closest homologue.80 GPRC6A is widely expressed in brain and peripheral tissues of humans, including kidney, skeletal muscle, testis and leucocytes.81 GPRC6A was shown to be directly activated by high concentrations of Ca2+[ed should read Ca2+ to indicate the two ions], which was augmented by osteocalcin,82 but the form used was fully carboxylated. Mice lacking GPRC6A have been produced by two separate laboratories. Wellendorph et al.83 found that knockout mice were viable and fertile, developed normally and exhibited no significant differences in body weight or skeletal manifestations compared with their wild-type littermates. By contrast, Pi et al.84 reported a complex metabolic phenotype, decreased BMD and impaired mineralization. Given the wide expression of the receptor, the question remains how osteocalcin functions as a cell-specific ligand. Perhaps a coreceptor is required for tissue specificity, as seen for FGF-23 (another bone-derived factor) and its coreceptor, Klotho.85
Bone turnover and glucose metabolism
Bone biopsy data in humans show, as in mice, that formation rate in cortical bone is low in patients with diabetes mellitus.86 These studies are corroborated in humans by measures of bone turnover, including serum total osteocalcin and urinary N-telopeptide, which are lower in patients with T1DM87,88 and T2DM88 than in individuals without diabetes mellitus. Conversely, interventions that improve glycemic control are associated with a concomitant increase in total osteocalcin serum concentrations in patients with T1DM89 and T2DM.90,91 These changes occur in parallel to changes in other measures of bone formation and resorption, which implies that a single measurement of total osteocalcin does not confirm its independent hormonal role. Furthermore, in studies that measure only undercarboxylated forms of osteocalcin, interpretation of results are complicated by the high correlation between undercarboxylated and total osteocalcin concentrations (Figure 4). Few studies, however, have measured multiple forms of osteocalcin in response to improvement in glycemic control. In one study of improved glycemic control, primarily achieved through dietary modification, total osteocalcin levels increased but levels of the undercarboxylated form of osteocalcin did not change.
Epidemiological studies
A plethora of studies have examined cross-sectional associations between serum concentrations of different forms of osteocalcin and various measures of glucose metabolism and adiposity among nondiabetic individuals. In reviewing cross-sectional studies of nondiabetic adults and children, some of which are summarized in Supplementary Table 1 online92-104 total osteocalcin serum concentrations are, in general, inversely associated with measures of glycaemia, consistent with the hypothesis that either osteocalcin influences β-cell function and insulin sensitivity or that increased glucose levels affect bone turnover.5,6 Similarly, total osteocalcin serum concentrations appear to be inversely associated with measures of adiposity, such as percentage body fat and BMI (Supplementary Table 1 online). However, some researchers have reported a positive association between serum total osteocalcin concentrations and insulin sensitivity index among lean, but not obese, adult men.105
Whereas an abundance of studies have included serum total osteocalcin, few studies have measured serum undercarboxylated osteocalcin. Of those that have, an overall lack of association was found between the undercarboxylated form of osteocalcin and measures of glucose metabolism or adiposity. A few studies reported inverse association in subgroups, such as among the obese96 and among male adolescents.101 In one longitudinal study in older men (55–80 years) at risk of cardiovascular disease and not taking antidiabetic medication, baseline concentrations of total osteocalcin, but not undercarboxylated osteocalcin, were positively associated with a 2-year change in fasting insulin levels and insulin resistance, as captured by the homeostasis model assessment of insulin resistance (HOMA-IR).106 In another 3-year study of older men and women (60–80 years), concentrations of carboxylated osteocalcin were associated with a decrease in fasting insulin and HOMA-IR but neither total nor undercarboxylated osteocalcin was associated with changes in HOMA-IR.94 Similarly, baseline concentrations of total osteocalcin, undercarboxylated osteocalcin and percentage of undercarboxylated osteocalcin did not predict development of diabetes mellitus among community-based adults.107
The regulation of insulin sensitivity by osteocalcin has been proposed to act through an effect on an adipocyte-derived hormone, adiponectin.5 This hormone, which decreases as fat mass increases, is recognized to be an important regulator of insulin sensitivity.108 In the past 5 years, it has been proposed that leptin also has an indirect role in osteocalcin’s hormonal action.5,74,109 In the few cross-sectional studies that measured adiponectin and total osteocalcin serum concentrations, adiponectin was positively associated with total osteocalcin in adults,94 but inversely associated among subgroups, including Asian-American children.101 Neither the uncarboxylated form of osteocalcin nor N-telopeptide levels were associated with adiponectin levels in adults.94 Similarly, levels of total osteocalcin were not associated with leptin concentrations in adults with or without diabetes mellitus.110 The data are currently too sparse and equivocal to draw conclusions.
The overall lack of association of undercarboxylated osteocalcin and glucose metabolism in humans is in contrast to the mouse models in which the uncarboxylated form of osteocalcin is the active hormonal form. If total osteocalcin, but not undercarboxylated osteocalcin, is inversely correlated with glucose metabolism and adiposity in humans, it becomes important to discern if the osteocalcin protein is mediating this effect or if it is an independent indication of an impaired osteoblast, as suggested by studies in an insulin-resistant T2DM rat model.111 Unfortunately, most studies do not include independent measures of bone formation and resorption, which limits our ability to address the question of osteocalcin as a mediator or marker. Of the few studies that included multiple bone turnover markers, there did not appear to be any consistent trends and indeed, the preponderance of studies that include bone resorption markers reported no association between bone resorption and glucose metabolism measures (supplemental Table 1).
The lack of human evidence to support an association of bone resorption with glucose metabolism is inconsistent with the hypothesis that uncarboxylated osteocalcin is the consequence of decarboxylation during osteoclastic resorption in mice,15 but consistent with the lack of effect observed in rats.111 Not enough studies have included bone formation measures other than osteocalcin to differentiate between an osteocalcin effect per se or specific changes in bone formation that relate to glucose metabolism. Certainly, confirmation through changes in multiple bone formation markers would be consistent with the known effects of insulin on the osteoblast.12 In fact, in mice, insulin signalling stimulates the synthesis not only of osteocalcin but also of other major matrix proteins, as well as MGP, in osteoblasts.8
Comparisons among observational studies are problematic because most are post-secondary analyses of studies that were not designed to examine the role of osteocalcin on glucose metabolism. Furthermore, neither longitudinal nor cross-sectional studies take into account the many factors that independently influence osteocalcin serum concentrations in humans, such as age, diet, ethnicity and sex. The majority of studies have measured total osteocalcin as the exclusive measure of bone formation. Similarly, many researchers have extrapolated the findings of their studies (using total osteocalcin serum concentrations in humans) as providing support of the mouse models that identified uncarboxylated osteocalcin as the hormonal form. Few studies directly measured both the total and the undercarboxylated forms of osteocalcin, which precludes forming conclusions regarding the importance of osteocalcin carboxylation in glucose metabolism. Similarly, very few studies used assays that measure the percentage of undercarboxylated osteocalcin, which would address any concern of the high correlation between total and undercarboxylated osteocalcin concentrations (Figure 4).
The influence of bone active agents
The notion that osteocalcin is activated by osteoclastic resorption of the matrix led to the question of whether agents that target the osteoclast would also increase the risk of diabetes mellitus. A study of elderly patients with T1DM found that those who were taking the bisphosphonate alendronate had a reduced insulin requirement compared with those receiving only calcium and vitamin D.112 In a large population based study in Denmark, a reduced risk of T2DM was found in individuals receiving alendronate, etidronate and raloxifine.113 The conclusions are indirect, as no markers of bone turnover were measured in these studies. However, markers of bone resorption and bone formation, including osteocalcin, are equally decreased in women with and without diabetes mellitus during anti-resorptive therapy.114 Although these studies would conflict with the hypothesis that reduction in uncarboxylated osteocalcin is detrimental to glucose metabolism, positive effects of the bisphosphonates and raloxifene on circulating lipids have been noted, potentially confounding these observations.115,116
A study published in 2011 evaluated the relationship between 3-month changes in bone turnover and 12-month measures of body weight, fat mass, and levels of adiponectin, leptin and glucose in participants treated with either parathyroid hormone 1–84 (PTH1–84) or alendronate.117 As expected, both total and undercarboxylated osteocalcin increased with PTH1–84 and decreased with alendronate treatment. 3-month changes in undercarboxylated osteocalcin were negatively associated with 12-month changes in body weight and fat mass, and positively with 12-month changes in adiponectin in those individuals receiving PTH1–84, consistent with the proposed mouse model of osteocalcin and glucose metabolism.6 Unfortunately, no other information was provided regarding potential relationship with total osteocalcin, other markers of bone formation and resorption, or other known clinical outcomes of PTH therapy, such as levels of calcium or 1,25-dihydroxyvitamin D3.
Vitamin K and glucose metabolism
The capacity to experimentally manipulate the percentage of osteocalcin that is carboxylated without affecting bone turnover through the use of vitamin K has been well established in humans.54 Therefore, manipulation of vitamin K provides an ideal model for testing the hypothesized link between uncarboxylated osteocalcin and glucose metabolism in humans.
High vitamin K intakes in a free-living population are associated with a low percentage of undercarboxylated osteocalcin,118 and are also associated with reduced insulin resistance.119-121 These observational studies infer that a low percentage of uncarboxylated osteocalcin actually improves glucose metabolism in humans. As major sources of phylloquinone in the diet are green leafy vegetables, high phylloquinone intakes are generally associated with healthier lifestyle and dietary habits,122 which may independently contribute to the reduced insulin resistance.123 Therefore, it is critical to isolate the effect of vitamin K manipulation on carboxylation of osteocalcin and its subsequent effect on glucose metabolism in clinical trials. Short-term supplementation of supraphysiological doses of menaquinone-4 (vitamin K2) improved acute insulin response after an oral glucose load in individuals with a high percentage of undercarboxylated osteocalcin at baseline.124 In studies using high doses of menaquinone-4, no effect on body weight was observed despite the reduction in the percentage of undercarboxylated osteocalcin.125,126 In those studies that measured glucose metabolism, administration of both menaquinone-4 and phylloquinone reduced the percentage of undercarboxylated osteocalcin and HOMA-IR and improved insulin in men (Table 2).120,125 Similar findings, however, were not noted in women.120,127 The role of vitamin K in regulating insulin sensitivity still requires more systematic investigation in humans.
Table 2.
Response of osteocalcin forms and measures of glucose metabolism to vitamin K supplementation
| Form of vitamin K | Daily dose | Duration | Change in treatment group compared with control group | Body weight | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Total osteocalcin | Undercarboxylated osteocalcin | % Undercarboxylated osteocalcin | Glucose metabolism | |||||
| Menaquinone-4 | 30 mg | 4 weeks | Not indicated | Decrease | Decrease | No change in glucose; increase in insulin sensitivity index | No change | 125 |
| Menaquinone-4 | 45 mg | 36 months | Not indicated | Decrease | Decrease | Not indicated | No change | 126 |
| Phylloquinone | 1 mg | 12 months | No change | Decrease | Decrease | No change in glucose, HOMA-IR or insulin | NR | 127 |
| Phylloquinone | 500 μg | 36 months | No change | Decrease | Decrease | No change in glucose; reduction in HOMA-IR and insulin in men only | NR | 120 |
Abbreviation: HOMA-IR, homeostasis model assessment of insulin resistance; NR, not reported.
Warfarin
On the basis of the presence of osteocalcin and other vitamin-K-dependent proteins in bone, patients receiving warfarin have been compared with age-matched individuals not receiving warfarin to explore the physiological effects of vitamin K antagonism and deficiency on bone mass, with varying levels of success128 Warfarin is among the 15 most widely prescribed drugs in the USA,129 and is used for the prevention and treatment of thrombosis.130 Of importance to the role of osteocalcin in energy metabolism is the interruption of the carboxylation reaction for all vitamin-K-dependent proteins in response to warfarin treatment, including osteocalcin (Figure 1). Carboxylation of osteocalcin is dramatically decreased by warfarin in both mice and humans.62,131 In mice, the first γ-carboxyglutamic acid residue (Glu13) is the most sensitive to warfarin131 because, as in humans, it is least likely to be carboxylated when vitamin K availability in the osteoblast is reduced.47
With regard to glucose metabolism, one case study reported hypoglycaemia in a premature infant exposed to warfarin in utero.132 However, the infant had neonatal hepatopathy, which may have impaired glucose independent of undercarboxylated osteocalcin. No other reports of hypoglycaemia exist in an otherwise large body of literature of case studies of warfarin embryopathy. One of the major challenges in studying the role of undercarboxylated osteocalcin in glucose metabolism among patients using warfarin is the potential interactions with glucose-lowering drugs that are independent of the carboxylation of osteocalcin.133,134 There are no reports of changes in insulin resistance in patients treated with warfarin alone. Therefore, it is unlikely that warfarin studies will provide insight into the role of osteocalcin in energy metabolism.
Exercise and weight loss
Physical inactivity decreases BMD and increases insulin resistance.135 Levinger et al. proposed the hypothesis that the increase in glucose disposal rate associated with exercise is driven by the strain of the exercise on the bone, which increases bone formation and insulin action through uncertain mechanisms.136 Resistance training increases measures of bone formation whilst transiently suppressing measures of bone resorption.137 However, the current data are inconsistent, with resistance training associated with either no effect138-140 or an increase105,139,141 in total serum osteocalcin concentrations. In one study of resistance training in combination with dietary counselling for weight loss, an increase in serum osteocalcin concentrations was observed but these changes were not statistically associated with changes in insulin resistance or circulating adiponectin levels.105
Only a few exercise studies have actually directly measured the undercarboxylated form of osteocalcin. Among obese men, acute aerobic activity resulted in an increase in the levels of total and uncarboxylated osteocalcin and adiponectin.136 In a subgroup analysis of participants with diabetes mellitus, the acute exercise increased the undercarboxylated osteocalcin by 4%, and this change was correlated with a decrease in glucose levels. By comparison, an increase in vitamin K intake of an amount that is equivalent to two servings of green, leafy vegetables, can decrease the percentage of undercarboxylated osteocalcin by ~15% through reduction in the undercarboxylated form of osteocalcin but not the total osteocalcin form.54 This finding would suggest that the well-documented daily variations in vitamin K intake,142 which cause larger changes in uncarboxylated osteocalcin than those achieved though exercise, would result in rapid and large fluctuations in glucose concentrations. Therefore, it is unclear if this is an independent osteocalcin effect on glucose metabolism or if osteocalcin is a marker for the short-term changes in bone turnover created by acute exercise.
In another study, obese children who engaged in a 6-month exercise programme had an increase in insulin levels with a concomitant increase in serum concentrations of both total and undercarboxylated osteocalcin.143 By contrast, no associations between insulin levels and serum concentrations of total or undercarboxylated osteocalcin were found among obese children not participating in the exercise program. This result led the authors to conclude that there was a regulatory loop in which an increase in osteocalcin formation in response to exercise would stimulate insulin secretion up to a currently undefined level, upon which the high insulin level would then exert a negative effect on osteocalcin secretion.
Currently the human data are inconclusive regarding the role of undercarboxylated osteocalcin in improving glucose metabolism in response to exercise. Furthermore, the study designs used do not differentiate changes in total osteocalcin concentrations that occur in response to changes in bone formation regardless of causality from those changes in total osteocalcin concentrations that may be responsible for direct effects on insulin action through feed-forward loops.
Conclusions
The well-established observation that diabetes mellitus is associated with altered bone metabolism has stimulated tremendous interest in terms of identifying potential mechanisms that link adipose tissue and the skeleton. The hypothesis that osteocalcin, a vitamin-K-dependent protein produced in the osteoblast, acts as a hormone to affect insulin sensitivity and energy expenditure was first proposed based on a series of studies conducted in in vivo and in vitro knockout mice models. These models specified that the uncarboxylated form of osteocalcin had the hormonal function. Since that time, a surge of reports in humans have used post hoc correlative analysis to examine associations between osteocalcin and glucose metabolism in studies with a variety of study designs.
Species-specific differences in osteocalcin that exist challenge the extrapolation of findings from the mouse model to humans. In most species, osteocalcin is fully carboxylated, whereas in humans osteocalcin in bone and serum is incompletely carboxylated, and the degree of carboxylation is determined by vitamin K availability in the diet. The concentration of osteocalcin in human bone and blood is only 20% of that observed in other species. Whereas the mouse model has tremendous value in studies of diabetes mellitus, there are examples of genetically modified mouse models for which one cannot extrapolate directly from mouse to humans.144 Osteocalcin may be a protein that would fall in this category and caution needs to be used in the interpretation of the current literature.
Overall, a lack of consensus exists among current literature to support a unique effect of undercarboxylated osteocalcin on the regulation of glucose metabolism or on measures of adiposity in humans. However, few studies have measured the undercarboxylated form of osteocalcin. Furthermore, the interpretation of the effect of uncarboxylated osteocalcin is confounded by its high correlation to total osteocalcin when measured using hydroxyapatite-binding assays or specific immunoassays, which reduces its utility as an independent biomarker. Manipulation of vitamin K intake can alter the proportion the osteocalcin that is undercarboxylated without altering bone turnover. Interestingly, a high intake of vitamin K, which results in a low proportion of osteocalcin that is undercarboxylated, has been reported to reduce insulin resistance, which is the opposite to what is proposed based on the mouse model.
Another limitation of human studies to date is the reliance on a single biomarker of bone turnover. Serum concentrations of total osteocalcin are established measures of bone formation. However, in the absence of other bone turnover markers, one cannot isolate the putative role of osteocalcin in glucose metabolism from its role as a measure of bone formation. In fact, several studies in the past few years show that the relationship between adipose tissue and the skeleton is complex, involving not only pancreatic and adipose tissue hormones but also nutritional factors and enteric hormones.145-147 It seems likely that additional new factors will be added to the repertoire of agents that connect bone and energy metabolism. In the interim, further studies are required to precisely define the effect of insulin on osteoclast activity and the nature of osteocalcin receptor binding in humans.
Key points.
Osteocalcin is a calcium-binding bone matrix protein that contains the vitamin-K-dependent amino acid, γ-carboxyglutamic acid; circulating osteocalcin concentrations are a measure of bone formation
Studies in mice show that osteocalcin acts as a hormone to affect insulin sensitivity and energy expenditure; only the undercarboxylated form of osteocalcin is active
Human dietary intake of vitamin K is suboptimal in contrast to that in mice and, as a consequence, both bone and serum osteocalcin is undercarboxylated in humans
Most human studies examining the association between serum osteocalcin and measures of glucose metabolism do not differentiate between the total and undercarboxylated forms or take into account vitamin K intake
Most human studies also do not measure other bone turnover markers to isolate the effect of osteocalcin on bone turnover from its effect on glucose metabolism
In mice, the uncarboxylated form of osteocalcin is linked to glucose homeostasis, whereas in humans the data are inconclusive
Acknowledgments
Supported by the USDA, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, and NIH DK69341, AG14759, AR38460 and P30 DK04735. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Footnotes
Author contributions
SL. Booth, A. Centi and C. Gundberg researching data for the article, provided a substantial contribution to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. S. R. Smith provided a substantial contribution to discussion of content and reviewed and/or edited the manuscript before submission
Supplementary information is linked to the online version of the paper at www.nature.com/nrendo.
Competing interests
The authors declare no competing interests.
Contributor Information
Sarah L. Booth, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, 711 Washington Street, Boston, MA 02111, USA
Amanda Centi, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, 711 Washington Street, Boston, MA 02111, USA.
Steven R. Smith, Translational Research Institute of Metabolism and Diabetes, Florida Hospital, Sanford Burnham Institute, 301 E. Princeton Street, Orlando, FL 32804, USA
Caren Gundberg, Orthopaedics and Rehabilitation, Yale School of Medicine, 333 Cedar Street, New Haven, CT 06520-8071, USA.
References
- 1.Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci USA. 1974;71:2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suttie JW. The biochemical basis of warfarin therapy. Adv Exp Med Biol. 1987;214:3–16. doi: 10.1007/978-1-4757-5985-3_2. [DOI] [PubMed] [Google Scholar]
- 3.Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA. 1975;72:3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci USA. 1976;73:1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferron M, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulzele K, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen CJ, Motyl KJ. No bones about it: insulin modulates skeletal remodeling. Cell. 2010;142:198–200. doi: 10.1016/j.cell.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Vella A. Carbohydrate metabolism and the skeleton: picking a bone with the beta-cell. J Clin Endocrinol Metab. 2011;96:1269–1271. doi: 10.1210/jc.2010-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motyl KJ, McCabe LR, Schwartz AV. Bone and glucose metabolism: a two-way street. Arch Biochem Biophys. 2010;503:2–10. doi: 10.1016/j.abb.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan TR, Duque G, Keech AC, Herrmann M. An Old Friend in a New Light: The Role of Osteocalcin in Energy Metabolism. Cardiovasc Ther. doi: 10.1111/j.1755-5922.2011.00300.x. http://dx.doi.org/10.1111/j.1755-5922.2011.00300.x. [DOI] [PubMed]
- 13.Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 14.Kawai M, Devlin MJ, Rosen CJ. Fat targets for skeletal health. Nat Rev Rheumatol. 2009;5:365–372. doi: 10.1038/nrrheum.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3:182–195. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rishavy MA, Berkner KL. Vitamin K oxygenation, glutamate carboxylation, and processivity: Defining the three critical facest of catalysis by the vitamin K-dependent carboxylase. Adv Nutr. 2012;3:135–148. doi: 10.3945/an.111.001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkner KL. The vitamin K-dependent carboxylase. Annu Rev Nutr. 2005;25:127–149. doi: 10.1146/annurev.nutr.25.050304.092713. [DOI] [PubMed] [Google Scholar]
- 20.Viegas CS, et al. GLA-rich protein (GRP): A new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J Biol Chem. 2008;283:36655–36664. doi: 10.1074/jbc.M802761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proudfoot D, Shanahan CM. Molecular mechanisms mediating vascular calcification: role of matrix Gla protein. Nephrology (Carlton) 2006;11:455–461. doi: 10.1111/j.1440-1797.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 22.Dowd TL, Rosen JF, Li L, Gundberg CM. The three-dimensional structure of bovine calcium ion-bound osteocalcin using 1H NMR spectroscopy. Biochemistry. 2003;42:7769–7779. doi: 10.1021/bi034470s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauschka PV, Carr SA. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982;21:2538–2547. doi: 10.1021/bi00539a038. [DOI] [PubMed] [Google Scholar]
- 24.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 25.Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem. 1994;269:1183–1190. [PubMed] [Google Scholar]
- 26.Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci USA. 1989;86:4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian J, et al. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci USA. 1989;86:1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boivin G, et al. Localization of endogenous osteocalcin in neonatal rat bone and its absence in articular cartilage: effect of warfarin treatment. Virchows Arch A Pathol Anat Histopathol. 1990;417:505–512. doi: 10.1007/BF01625731. [DOI] [PubMed] [Google Scholar]
- 29.Price PA, Lothringer JW, Baukol SA, Reddi AH. Developmental appearance of the vitamin K-dependent protein of bone during calcification. Analysis of mineralizing tissues in human, calf, and rat. J Biol Chem. 1981;256:3781–3784. [PubMed] [Google Scholar]
- 30.Hauschka PV, Reid ML. Timed appearance of a calcium-binding protein containing gamma-carboxyglutamic acid in developing chick bone. Dev Biol. 1978;65:426–434. doi: 10.1016/0012-1606(78)90038-6. [DOI] [PubMed] [Google Scholar]
- 31.Owen TA, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 32.Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 33.Boskey AL, et al. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 34.Poundarik A, Gundberg C, Vashishth D. Non-collageneous proteins influence bone mineral size, shape and orientation: A SAXS study. J Bone Miner Res. 2011;26(Suppl):S36. [Google Scholar]
- 35.Fratzl P, Paris O, Klaushofer K, Landis WJ. Bone mineralization in an osteogenesis imperfecta mouse model studied by small-angle x-ray scattering. J Clin Invest. 1996;97:396–402. doi: 10.1172/JCI118428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JP, Delmas PD, Arlot M, Meunier PJ. Active bone turnover of the cortico-endosteal envelope in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1987;64:954–959. doi: 10.1210/jcem-64-5-954. [DOI] [PubMed] [Google Scholar]
- 37.Brown JP, et al. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984;1:1091–1093. doi: 10.1016/s0140-6736(84)92506-6. [DOI] [PubMed] [Google Scholar]
- 38.Eastell R, et al. Bone formation rate in older normal women: concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J Clin Endocrinol Metab. 1988;67:741–748. doi: 10.1210/jcem-67-4-741. [DOI] [PubMed] [Google Scholar]
- 39.Price PA, Williamson MK, Lothringer JW. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981;256:12760–12766. [PubMed] [Google Scholar]
- 40.Garnero P, et al. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992;7:1389–1398. doi: 10.1002/jbmr.5650071206. [DOI] [PubMed] [Google Scholar]
- 41.Ivaska KK, et al. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem. 2004;279:18361–18369. doi: 10.1074/jbc.M314324200. [DOI] [PubMed] [Google Scholar]
- 42.Gundberg CM, Weinstein RS. Multiple immunoreactive forms of osteocalcin in uremic serum. J Clin Invest. 1986;77:1762–1767. doi: 10.1172/JCI112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor AK, et al. Multiple osteocalcin fragments in human urine and serum as detected by a midmolecule osteocalcin radioimmunoassay. J Clin Endocrinol Metab. 1990;70:467–472. doi: 10.1210/jcem-70-2-467. [DOI] [PubMed] [Google Scholar]
- 44.Ivaska KK, et al. Urinary osteocalcin as a marker of bone metabolism. Clin Chem. 2005;51:618–628. doi: 10.1373/clinchem.2004.043901. [DOI] [PubMed] [Google Scholar]
- 45.Eastell R, Hannon RA. Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc. 2008;67:157–162. doi: 10.1017/S002966510800699X. [DOI] [PubMed] [Google Scholar]
- 46.Looker AC, et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int. 2000;11:467–480. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 47.Price PA, Williamson MK. Effects of warfarin on bone. Studies on the vitamin K-dependent protein of rat bone. J Biol Chem. 1981;256:12754–12759. [PubMed] [Google Scholar]
- 48.Benton ME, Price PA, Suttie JW. Multi-site-specificity of the vitamin K-dependent carboxylase: in vitro carboxylation of des-gamma-carboxylated bone Gla protein and Des-gamma- carboxylated pro bone Gla protein. Biochemistry. 1995;34:9541–9551. doi: 10.1021/bi00029a031. [DOI] [PubMed] [Google Scholar]
- 49.Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm. 2008;78:1–22. doi: 10.1016/S0083-6729(07)00001-5. [DOI] [PubMed] [Google Scholar]
- 50.Cairns JR, Price PA. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J Bone Miner Res. 1994;9:1989–1997. doi: 10.1002/jbmr.5650091220. [DOI] [PubMed] [Google Scholar]
- 51.Food and Nutrition Board & Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press; Washington, D.C.: 2001. [PubMed] [Google Scholar]
- 52.Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399–417. doi: 10.1146/annurev.nu.15.070195.002151. [DOI] [PubMed] [Google Scholar]
- 53.Booth SL, et al. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133:2565–2569. doi: 10.1093/jn/133.8.2565. [DOI] [PubMed] [Google Scholar]
- 54.Truong JT, et al. Age group and sex do not influence responses of vitamin K biomarkers to changes in dietary vitamin K. J Nutr. 2012;142:936–941. doi: 10.3945/jn.111.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Booth SL, et al. Effect of Vitamin K Supplementation on Bone Loss in Elderly Men and Women. J Clin Endocrinol Metab. 2008;93:1217–1223. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binkley N, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res. 2009;24:983–991. doi: 10.1359/JBMR.081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung AM, et al. Vitamin K Supplementation in Postmenopausal Women with Osteopenia (ECKO Trial): A Randomized Controlled Trial. PLoS Med. 2008;5:e196. doi: 10.1371/journal.pmed.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Binkley NC, Krueger DC, Engelke JA, Foley AL, Suttie JW. Vitamin K supplementation reduces serum concentrations of under-gamma- carboxylated osteocalcin in healthy young and elderly adults. Am J Clin Nutr. 2000;72:1523–1528. doi: 10.1093/ajcn/72.6.1523. [DOI] [PubMed] [Google Scholar]
- 59.Bolton-Smith C, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22:509–519. doi: 10.1359/jbmr.070116. [DOI] [PubMed] [Google Scholar]
- 60.van Summeren MJ, et al. The effect of menaquinone-7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. Br J Nutr. 2009;102:1171–1178. doi: 10.1017/S0007114509382100. [DOI] [PubMed] [Google Scholar]
- 61.Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: Friend or foe? Adv Nutr. 2012;3:149–157. doi: 10.3945/an.112.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- 63.Adami S. Bone health in diabetes: considerations for clinical management. Curr Med Res Opin. 2009;25:1057–1072. doi: 10.1185/03007990902801147. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz AV, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metabol. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 65.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 66.Bonds DE, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 67.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz AV, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35:1525–1531. doi: 10.2337/dc11-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takada I, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 70.Gimble JM, Nuttall ME. The relationship between adipose tissue and bone metabolism. Clin Biochem. 2012;45:874–879. doi: 10.1016/j.clinbiochem.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Griffith JF, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–951. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 72.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 73.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008;473:231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hinoi E, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yip SC, Saha S, Chernoff J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem Sci. 2010;35:442–449. doi: 10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zee T, Settembre C, Levine RL, Karsenty G. T-cell protein tyrosine phosphatase regulates bone resorption and whole-body insulin sensitivity through its expression in osteoblasts. Mol Cell Biol. 2012;32:1080–1088. doi: 10.1128/MCB.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poser JW, Price PA. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979;254:431–436. [PubMed] [Google Scholar]
- 78.Kumm J, Ivaska KK, Rohtla K, Vaananen K, Tamm A. Urinary osteocalcin and other markers of bone metabolism: the effect of risedronate therapy. Scand J Clin Lab Invest. 2008;68:459–463. doi: 10.1080/00365510701832237. [DOI] [PubMed] [Google Scholar]
- 79.Oury F, et al. Endocrine Regulation of Male Fertility by the Skeleton. Cell. 2011 doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wellendorph P, et al. Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- 81.Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J Biol Chem. 2000;275:3256–3263. doi: 10.1074/jbc.275.5.3256. [DOI] [PubMed] [Google Scholar]
- 83.Wellendorph P, et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol. 2009;42:215–223. doi: 10.1677/JME-08-0149. [DOI] [PubMed] [Google Scholar]
- 84.Pi M, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 86.Krakauer JC, et al. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 87.Pater A, Sypniewska G, Pilecki O. Biochemical markers of bone cell activity in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2010;23:81–86. doi: 10.1515/jpem.2010.23.1-2.81. [DOI] [PubMed] [Google Scholar]
- 88.Gerdhem P, Isaksson A, Akesson K, Obrant KJ. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporosis Int. 2005;16:1506–1512. doi: 10.1007/s00198-005-1877-5. [DOI] [PubMed] [Google Scholar]
- 89.Rosato MT, Schneider SH, Shapses SA. Bone turnover and insulin-like growth factor I levels increase after improved glycemic control in noninsulin-dependent diabetes mellitus. Calcif Tissue Int l. 1998;63:107–111. doi: 10.1007/s002239900498. [DOI] [PubMed] [Google Scholar]
- 90.Bao YQ, et al. Relationship between serum osteocalcin and glycaemic variability in Type 2 diabetes. Clin Exp Pharmacol Physiol. 2011;38:50–54. doi: 10.1111/j.1440-1681.2010.05463.x. [DOI] [PubMed] [Google Scholar]
- 91.Kanazawa I, et al. Adiponectin is associated with changes in bone markers furing glycemic control in type 2 diabetes mellitus. J Clin Endocrinol Metabol. 2009;94:3013–3037. doi: 10.1210/jc.2008-2187. [DOI] [PubMed] [Google Scholar]
- 92.Saleem U, Mosley TH, Jr, Kullo IJ. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2010;30:1474–1478. doi: 10.1161/ATVBAHA.110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827–832. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shea MK, et al. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90:1230–1235. doi: 10.3945/ajcn.2009.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25:768–772. doi: 10.1002/dmrr.1045. [DOI] [PubMed] [Google Scholar]
- 96.Foresta C, et al. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab. 2010;95:3502–3506. doi: 10.1210/jc.2009-2557. [DOI] [PubMed] [Google Scholar]
- 97.Shea MK, et al. Adulthood obesity is positively associated with adipose tissue concentrations of vitamin K and inversely associated with circulating indicators of vitamin K status in men and women. J Nutr. 2010;140:1029–1034. doi: 10.3945/jn.109.118380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pitroda AP, Harris SS, Dawson-Hughes B. The association of adiposity with parathyroid hormone in healthy older adults. Endocrine. 2009;36:218–223. doi: 10.1007/s12020-009-9231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iki M, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos nt. 2012;23:761–770. doi: 10.1007/s00198-011-1600-7. [DOI] [PubMed] [Google Scholar]
- 100.Gravenstein KS, et al. Cross-sectional evidence of a signaling pathway from bone homeostasis to glucose metabolism. J Clin Endocrinol Metab. 2011;96:E884–890. doi: 10.1210/jc.2010-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boucher-Berry C, et al. Vitamin D, osteocalcin and risk for adiposity as co-morbidities in middle school children. J Bone Miner Res. 2011 doi: 10.1002/jbmr.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Misra M, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metabol. 2007;92:2046–2052. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- 103.Pollock NK, et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J Clin Endocrinol Metabol. 2011;96:E1092–E1099. doi: 10.1210/jc.2010-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iglesias P, et al. Serum concentrations of osteocalcin, procollagen type 1 N-terminal propeptide and beta-CrossLaps in obese subjects with varying degrees of glucose tolerance. Clin Endocrinol. 2011;75:184–188. doi: 10.1111/j.1365-2265.2011.04035.x. [DOI] [PubMed] [Google Scholar]
- 105.Fernandez-Real JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94:237–245. doi: 10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- 106.Bullo M, Moreno-Navarrete JM, Fernandez-Real JM, Salas-Salvado J. Total and undercarboxylated osteocalcin predict changes in insulin sensitivity and beta cell function in elderly men at high cardiovascular risk. Am J Clin Nutr. 2012;95:249–255. doi: 10.3945/ajcn.111.016642. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz AV, et al. Undercarboxylated osteocalcin does not predict development of diabetes in older adults in the Health, Aging and Body Composition Study. J Bone Miner Res. 2011;26:S69. [Google Scholar]
- 108.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 109.Shi Y, et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee YJ, et al. Serum osteocalcin is inversely associated with adipocyte-specific fatty acid-binding protein in the Korean metabolic syndrome research initiatives. Diabetes Care. 2010;33:e90. doi: 10.2337/dc10-0071. [DOI] [PubMed] [Google Scholar]
- 111.Hamann C, et al. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am J Physiol Endocrinol Metab. 2011;301:E1220–1228. doi: 10.1152/ajpendo.00378.2011. [DOI] [PubMed] [Google Scholar]
- 112.Maugeri D, et al. Alendronate reduces the daily consumption of insulin (DCI) in patients with senile type I diabetes and osteoporosis. Arch Gerontol Geriatr. 2002;34:117–122. doi: 10.1016/s0167-4943(01)00202-3. [DOI] [PubMed] [Google Scholar]
- 113.Vestergaard P. Risk of newly diagnosed type 2 diabetes is reduced in users of alendronate. Calcif Tiss Int. 2011;89:265–270. doi: 10.1007/s00223-011-9515-z. [DOI] [PubMed] [Google Scholar]
- 114.Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27:1547–1553. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]
- 115.Luckman SP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J BoneMin Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 116.Gluck O, Maricic M. Skeletal and nonskeletal effects of raloxifene. Curr Osteoporos Rep. 2003;1:123–128. doi: 10.1007/s11914-996-0007-4. [DOI] [PubMed] [Google Scholar]
- 117.Schafer AL, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1-84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study) J Clin Endocrinol Metab. 2011;96:E1982–1989. doi: 10.1210/jc.2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sokoll LJ, et al. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma- carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am J Clin Nutr. 1997;65:779–784. doi: 10.1093/ajcn/65.3.779. [DOI] [PubMed] [Google Scholar]
- 119.Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr. 2008;88:210–215. doi: 10.1093/ajcn/88.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshida M, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31:2092–2096. doi: 10.2337/dc08-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beulens J, B DE, S I, Spijkerman A, vdS YT. Dietary phylloquinone and menaquinones intake and risk of type 2 diabetes. Diabetes Care. 2010;33:1699–1705. doi: 10.2337/dc09-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Erkkila AT, Booth SL. Vitamin K intake and atherosclerosis. Curr Opin Lipidol. 2008;19:39–42. doi: 10.1097/MOL.0b013e3282f1c57f. [DOI] [PubMed] [Google Scholar]
- 123.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000;19:259–263. doi: 10.1054/clnu.2000.0102. [DOI] [PubMed] [Google Scholar]
- 125.Choi HJ, et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care. 2011;34:e147. doi: 10.2337/dc11-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knapen MH, et al. Association of vitamin K status with adiponectin and body composition in healthy subjects: uncarboxylated osteocalcin is not associated with fat mass and body weight. Br J Nutr. 2011;108:1017–1024. doi: 10.1017/S000711451100626X. [DOI] [PubMed] [Google Scholar]
- 127.Kumar R, Binkley N, Vella A. Effect of phylloquinone supplementation on glucose homeostasis in humans. Am J Clin Nutr. 2010;92:1528–1532. doi: 10.3945/ajcn.2010.30108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Booth SL. Warfarin use and fracture risk. Nutr Rev. 2000;58:20–22. doi: 10.1111/j.1753-4887.2000.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 129.Cropp JS, Bussey HI. A review of enzyme induction of warfarin metabolism with recommendations for patient management. Pharmacotherapy. 1997;17:917–928. [PubMed] [Google Scholar]
- 130.Mega JL. A new era for anticoagulation in atrial fibrillation. N Engl J Med. 2011;365:1052–1054. doi: 10.1056/NEJMe1109748. [DOI] [PubMed] [Google Scholar]
- 131.Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res Commun. 2010;397:691–696. doi: 10.1016/j.bbrc.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hetzel PG, Glanzmann R, Hasler PW, Ladewick A, Buhrer C. Coumarin embryopathy in an extremely low birth weight infant associated with neonatal hepatitis and ocular malformations. Eur J Pediatr. 2006;165:358–360. doi: 10.1007/s00431-005-0064-1. [DOI] [PubMed] [Google Scholar]
- 133.Scheen AJ. Drug interactions of clinical importance with antihyperglycaemic agents: an update. Drug Saf. 2005;28:601–631. doi: 10.2165/00002018-200528070-00004. [DOI] [PubMed] [Google Scholar]
- 134.Soon D, et al. Effect of exenatide on the pharmacokinetics and pharmacodynamics of warfarin in healthy Asian men. J Clin Pharmacol. 2006;46:1179–1187. doi: 10.1177/0091270006291622. [DOI] [PubMed] [Google Scholar]
- 135.Thyfault JP, Booth FW. Lack of regular physical exercise or too much inactivity. Curr Opin Clin Nutr Metab Care. 2011;14:374–378. doi: 10.1097/MCO.0b013e3283468e69. [DOI] [PubMed] [Google Scholar]
- 136.Levinger I, et al. The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos Int. 2011 doi: 10.1007/s00198-010-1370-7. [DOI] [PubMed] [Google Scholar]
- 137.Fujimura R, et al. Effect of resistance exercise training on bone formation and resorption in young male subjects assessed by biomarkers of bone metabolism. J Bone Miner Res. 1997;12:656–662. doi: 10.1359/jbmr.1997.12.4.656. [DOI] [PubMed] [Google Scholar]
- 138.Yarasheski KE, Campbell JA, Kohrt WM. Effect of resistance exercise and growth hormone on bone density in older men. Clin Endocrinol (Oxf) 1997;47:223–229. doi: 10.1046/j.1365-2265.1997.2461060.x. [DOI] [PubMed] [Google Scholar]
- 139.Schroeder ET, Hawkins SA, Jaque SV. Musculoskeletal adaptations to 16 weeks of eccentric progressive resistance training in young women. J Strength Cond Res. 2004;18:227–235. doi: 10.1519/R-13443.1. [DOI] [PubMed] [Google Scholar]
- 140.Judge JO, et al. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos Int. 2005;16:1096–1108. doi: 10.1007/s00198-004-1816-x. [DOI] [PubMed] [Google Scholar]
- 141.Adami S, et al. Physical activity and bone turnover markers: a cross-sectional and a longitudinal study. Calcif Tissue Int l. 2008;83:388–392. doi: 10.1007/s00223-008-9184-8. [DOI] [PubMed] [Google Scholar]
- 142.Booth SL, et al. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J Nutr. 1997;127:587–592. doi: 10.1093/jn/127.4.587. [DOI] [PubMed] [Google Scholar]
- 143.Rochefort GY, et al. Osteocalcin-insulin relationship in obese children: a role for the skeleton in energy metabolism. Clin Endocrinol (Oxf) 2011;75:265–270. doi: 10.1111/j.1365-2265.2011.04031.x. [DOI] [PubMed] [Google Scholar]
- 144.Ashcroft FM, Rorsman P. Diabetes Mellitus and the beta Cell: The Last Ten Years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yoshikawa Y, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Mineral Res. 2011;26:2012–2025. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei W, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci USA. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clowes JA, Khosla S, Eastell R. Potential role of pancreatic and enteric hormones in regulating bone turnover. J Bone Miner Res. 2005;20:1497–1506. doi: 10.1359/JBMR.050524. [DOI] [PubMed] [Google Scholar]
- 148. [03.30.12]; https:www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/SR24/nutrlist/sr24a430.


