Abstract
Cilia are microtubule based cellular projections that serve a wide variety of essential functions in animal cells. Defects in cilia structure or function have recently emerged as etiological mechanisms underpinning diverse human diseases. While many eukaryotic cells possess only one or two cilia, some cells, including those of many unicellular organisms, exhibit extensive multiciliation. In vertebrates, multiciliated cells (MCCs) are a specialized population of post-mitotic cells decorated with dozens of motile cilia that beat in a polarized and synchronized fashion to drive directed fluid flow across an epithelium. Dysfunction of human MCCs is associated with diseases of the brain, airway and reproductive tracts. Despite their importance, MCCs are relatively poorly studied and we are only beginning to understand the mechanisms underlying their development and function. Here, we briefly review the general phylogeny and physiology of multiciliation and detail our current understanding of the developmental and cellular events underlying the formation, maturation, and function of MCCs in vertebrates.
Introduction
Cilia are small, microtubule-based protrusions found across the eukaryotic lineage. Many unicellular organisms utilize motile cilia for locomotion, feeding, and sensation, and a subset of these organisms produce between dozens and thousands of cilia, a phenomenon called multiciliation. In vertebrates, including humans, most cells possess or are capable of generating single non-motile primary cilia, which serve as critical regulators of signal transduction during development and homeostasis (reviewed in [1]). However, some specialized vertebrate cells contain many dozens of cilia, which beat in a coordinated and polarized manner to drive directional fluid flow across tissues. These multiciliated cells (MCCs) are found, for example, in the spinal cord and ventricles of the adult brain, where they drive polarized fluid flow important for circulation of cerebrospinal fluid and neuronal migration [2]; in the airway, where they are important for protective mucus clearance [3]; and, in the oviduct/fallopian tubes where they are required for ovum transport [4].
While MCCs have clear roles in human health, and their dysfunction is etiologically linked with a number of diseases, they remain a relatively understudied population. Here, we review our current understanding of MCC biology, beginning with a brief overview of the phylogeny of multiciliation. We next discuss some specialized physiological concerns of these cells. Finally, we review transcriptional control of specification in vertebrate MCCs, and the specialized cell biological machinery that these cells employ.
Cilia structure and motility
General cilia structure and function have been extensively reviewed elsewhere (e.g. [1, 5, 6]), and so we provide only a brief introduction. Cilia are anchored at the cell surface by a modified centriole known as a basal body. The protrusive outgrowth of the cilium, known as the axoneme, extends from the basal body into the extracellular space. Axonemes exhibit a highly conserved, though not inviolable, architecture of nine microtubule doublets arranged circumferentially and enclosed within a specialized plasma membrane. Most motile cilia also contain two additional non-doublet microtubules known as the central pair, and these are required for productive and directional ciliary beating.
Ciliary motility is accomplished by the regulated action of outer and inner axonemal dynein arms, which slide adjacent doublets relative to one another. This sliding is constrained by protein bridges between adjacent doublets, and by the basal anchoring of the axoneme, which results in a bending motility (reviewed in [6, 7]). The ciliary beat cycle comprises two phases: 1) the effective stroke, wherein the cilia extends through an arc taking it perpendicular to the cell surface; and 2) the recovery stroke, during which the cilia remains bent and largely parallel to the cell body as it returns to its initial position. The bi-phasic nature of the ciliary stroke is an important consideration for effective motility in the essentially inertia-less environment in which cilia generally act [8]. Despite our understanding of the generalities of axonemal motility, the specific biophysical and molecular mechanisms underlying this action remain poorly understood [9].
Phylogeny of multiciliation
All extant eukaryotic lineages possess ciliated species, suggesting that the last eukaryotic common ancestor was in possession of at least one cilium, or that the evolution of the cilium provided an incredible competitive advantage during early eukaryotic evolution [10, 11]. Multiciliation, which for the purposes of this review we define as possession of more than two cilia/flagella by a single cell, has not been carefully catalogued. However it has been observed in unicellular eukaryotes including amebozoids (e.g. Multicilia marina [12]) and protists [13, 14] and in multicellular organism including many branches of metazoans, from carnivorous sponges to humans [15, 16], and even in the sperm of some plants including cycads, ferns, and some gymnosperms [17, 18].
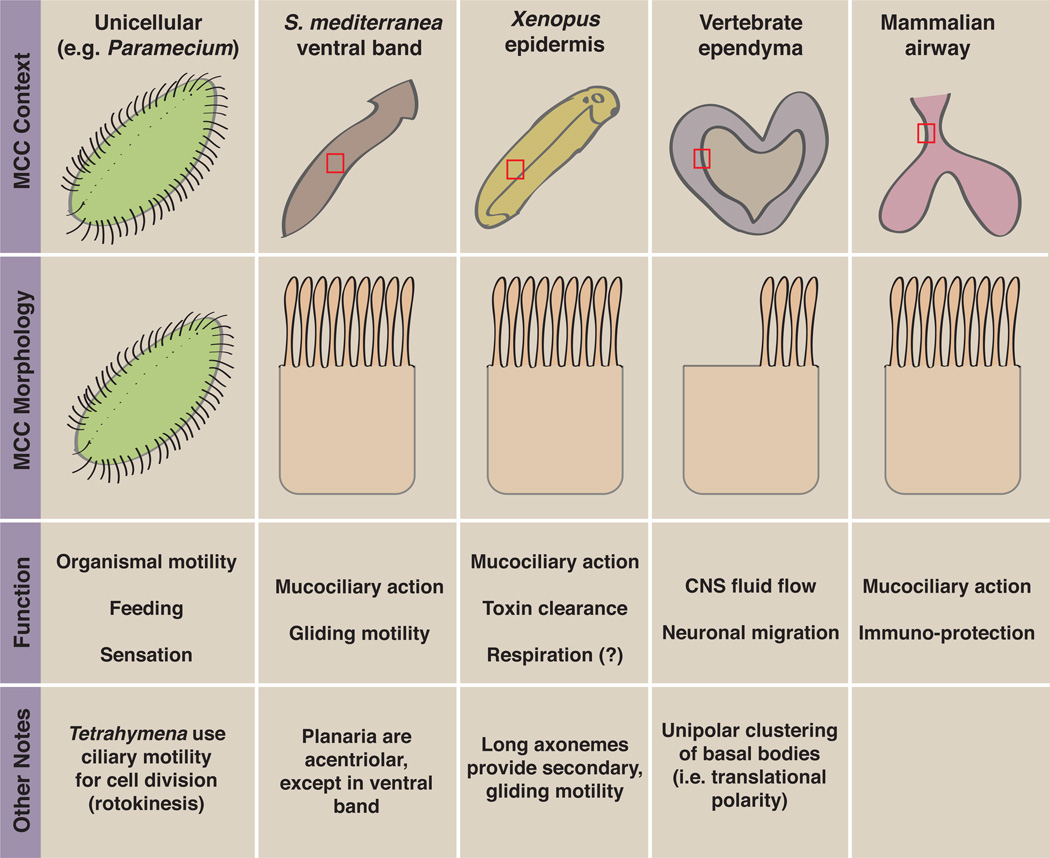
In vertebrate animals, MCCs are present in diverse tissues. In mammals, powerful genetics has been brought to bear on ependymal MCCs that line the brain ventricles and on the MCCs of the airway epithelium ([3, 19]; Fig. 1a, b; Fig. 2). Given their internal development, dynamic imaging of mammalian MCCs is challenging, but powerful primary culture approaches have now been established for both tissues [20, 21]. MCCs have also been extensively studied in amphibian embryos [22, 23], especially Xenopus, and the position of these cells on the epidermis of externally developing animals makes them highly amenable to live imaging ([24]; Fig. 1c; Fig. 2). Genetic controls on MCC development are strikingly similar between these three populations, though there are important differences as well (see below). Some aspects of MCCs have also been studied in the zebrafish kidney [25, 26], and the ventral band of planarians provides an additional model for study of metazoan MCCs (Fig. 2; [27]). Finally, aspects of multiciliation have been studied in the unicellular organisms Tetrahymena and Paramecium (e.g. [28–30]; Fig. 2). Interestingly, most observed cases of multiciliation result in the production of motile axonemes (with the notable exception of the olfactory cilia of mammalian species, which lack dynein arms and are therefore considered immotile despite having a 9+2 architecture [6, 31]). This suggests that multiciliation is a favorable solution to demand for local fluid flow--possibly due to its propensity for hydrodynamic coupling, as discussed in the following section.
Figure 1. Examples of vertebrate MCCs.
(a) Ependymal MCCs stained for acetylated tubulin in green and beta-catenin in magenta. Note the unipolar clustering of axonemes within each cell. Image courtesy of Shinya Ohata and Arturo Alvarez-Buylla. (b) SEM of a mouse tracheal epithelial MCC. Image courtesy of Eszter Vladar and Jeff Axelrod. (c) A Xenopus epidermal MCC expressing GFP-MAP7 and RFP-CLAMP to label the proximal and distal axoneme respectively.
Figure 2. Overview of selected MCC populations.
This figure shows the organismal context and morphology of some MCC populations, from unicellular organisms to specialized vertebrate tissues.
Physiology of multiciliated cells
The basic machinery and organization of cilia beating seems to be well conserved between eukaryotic organisms and between mono- and multi-ciliated cells, though some parameters such as beat frequency are under cellular control, and thus exhibit variability suited to their specific tasks (as reviewed in [6, 32]). Here, we focus briefly on some specialized physiological concerns of multiciliation.
Hydrodynamic forces, ciliary coupling, and metachrony
Cilia in aqueous environments function in what is essentially a low-Reynolds number environment--where viscous forces dominate and inertial forces are negligible--and are therefore susceptible to hydrodynamic considerations. Especially important are the effects of fluid-mediated interactions between neighboring cilia ([7, 33], and see [8] for a recent review). These interactions are thought to result in emergent phase coupling of neighboring axonemes, which can then act cooperatively to produce population level effects. One example is the phenomenon of metachrony observed in multiciliated populations (e.g. [14]), or in contexts where many cilia are spatially constrained even if they are not all from a single cell (e.g. in the colonial alga Volvox [34]).
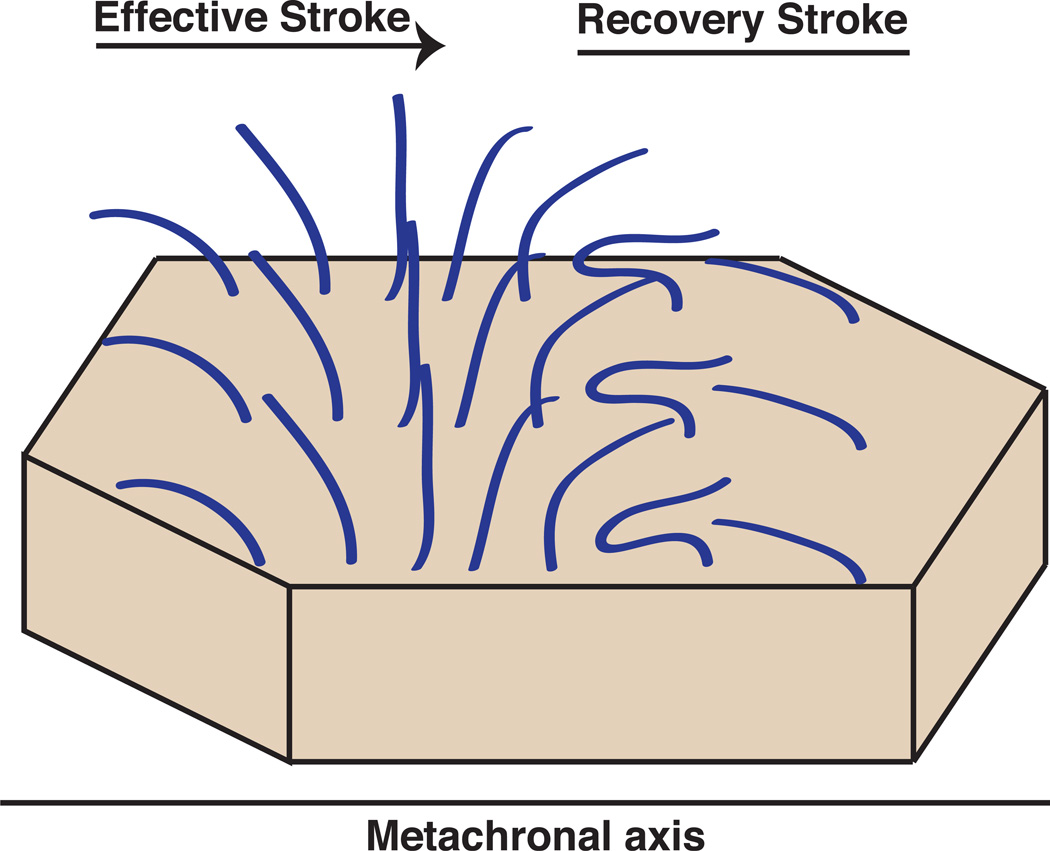
Cilia in a metachronic array are organized such that each cilium in a two-dimensional array will beat at the same frequency but in a phase-shifted manner with its neighbors along the axis of the effective stroke, and phase-synchronously with cilia in the perpendicular axis (Fig. 3). The net result of this process is a traveling wave of ciliary action across the array, which propels fluids in a concerted fashion [6, 7, 35]. Interestingly, mathematical models suggest that metachrony is an emergent property of hydrodynamically coupled ciliary arrays. Even if the array begins as purely synchronic, hydrodynamic interactions between cilia and incipient noise will steer the array toward metachrony [33, 35–37]. One reason for this might be that the load experienced by each cilium in a metachronal array is reduced, indicating that metachrony is energetically favorable and thus dominates over other beat organization paradigms [7, 33]. Further, mathematical models suggest that although ciliary beat frequency is reduced in metachronal arrays, as compared to arrays constrained to purely synchronous beating, bulk fluid flow is actually increased [36]. This likely reflects the fact that each sequential metachronal event is acting in concert to add impetus to fluid already in motion, as opposed to accelerating it from rest [7]. It seems, therefore, that multiciliation is advantageous to the generation of fluid flow, as even beyond the simple addition of more beating engines, the metachronic coordination of axonemes actually reduces the energetic burden on each cilium.
Figure 3. Schematic of metachronal organization.
Multiciliated cells often exhibit a specialized synchronization known as metachrony, which results in a traveling wave of coordinated ciliary organization across the surface of the cell. Here an array of axonemes is shown at a single time point. Note that axonemes are phase-shifted with respect to their neighbors along the axis of the effective stroke (the metachronal axis) but are in synchrony with neighbors along the perpendicular axis.
While metachrony has been the subject of intense interest in the modeling of ciliary array action, there have been few in vivo investigations of this phenomenon. The process is known to require the action of the regulatory dynein subunit LC1 in planarian ventral band MCCs [38]. Additionally, a recent study demonstrated that the metachronal wave is propagated intracellularly via short actin links between neighboring basal bodies [39], demonstrating that the phenomenon is not independent of cytoskeletal concerns. These studies reside at the intriguing intersection of environmental (i.e. hydrodynamic) and cellular control of a key process in MCC driven fluid flow, an area about which we know very little.
Mucociliary tissues
In vertebrates, mucociliary epithelia are important for protection and respiration and rely on the concerted action of MCCs to drive mucus clearance [3, 32, 40, 41]. Some special considerations of ciliary action are required in these epithelia as the fluid properties of mucus differ significantly from those of water. Mucus is a non-Newtonian viscoelastic fluid, which is secreted in a concentrated form and then undergoes hydration into a gel-like mesh. Once secreted, mucousal droplets coalesce above a layer of periciliary fluid (see [7] for review).
The viscoelastic properties of mucus require some specialization from the propulsive MCCs. First the comparative stiffness of mucus means that ciliary length tends to be limited to ~5–7um in the airway; longer cilia would exhibit significant back-bending upon contact with mucus, reducing the kinetic energy imparted to the mucosal layer [7, 41]. Cilia length must also be optimized to allow for the distal tip of the axoneme--which, given its dense protein matrix, is likely the stiffest region of the cilium--to specifically engage with the mucus [42–44]. Additionally, the tip domain has the highest perpendicular velocity of the axoneme during the effective stroke [41], and thus will be the most effective driver of mucus flow. Finally, the elasticity of mucus means that metachrony is particularly important, as cilia must be constantly engaging the mucousal gel and accelerating it in the direction of flow; cessation of this impulse would allow the mucus to release the imparted energy by back-expansion, impairing processive movement of the gel. In mucociliary tissues metachrony is propagated semi-locally over 2–3 cell diameters, with many such local events occurring across the tissue [41].
Mucociliary MCCs are also under physiological regulation at the level of beat frequency, and human airway cilia beat at sub-maximal frequency under homeostatic conditions. This frequency can be increased in response to stimuli, suggesting that beat frequency is actively modulated to meet physiological demands [32]. Physically, the cilia of mucociliary MCCs can be stimulated to beat faster, either by local application of mucus or other debris, or by the application of a mechanical probe. Molecularly, the beat frequency of these cells can be modulated by factors that influence levels of cAMP, cGMP, or intracellular Ca2+ (see [6, 7, 32] for reviews).
A final note: the organization of the mucociliary epithelium in the airway is a key concern for toxin clearance and infection prevention. The depth of the periciliary fluid must be strictly maintained, such that the mucosal gel is maintained at optimal height to receive the ciliary impulse. Chronic depletion of periciliary fluid results in a failure of mucus clearance and consequent dehydration, an etiological concern in respiratory diseases, including cystic fibrosis [45]. A recent investigation found that the periciliary layer is unexpectedly complex, acting as a semi-stiff macromolecular brush to occlude mucus from the periciliary region and provide a substrate for its movement by coordinated ciliary action [46].
Unicellular locomotion versus metazoan fluid flow
Given the breadth of organisms exhibiting multiciliation, it would be unreasonable to assert a single mode of action for all cases. The physiological needs of the cilia on a single-celled, mitotically cycling organism which requires a steerable beat waveform differ dramatically from those of cilia on terminally differentiated metazoan MCCs, which exhibit a strong requirement for polarized unidirectional beating across an epithelium. In this regard it is interesting to note that cilia from some, but not all, free-swimming unicellular organisms exhibit rotation of their central pair of microtubules (e.g. Paramecium [47]), which is one possible method of changing the direction of the effective stroke, and--thereby--of organismal steering. In contrast, the orientation of the central pair is fixed in many organisms, including mammals, resulting in purely unidirectional beating (discussed in [6]). The differences in multiciliation paradigms between unicellular and multicellular organisms is one of the most interesting open questions in MCC biology, and another about which we, unfortunately, know very little.
Transcriptional control of MCC specification and differentiation in vertebrates
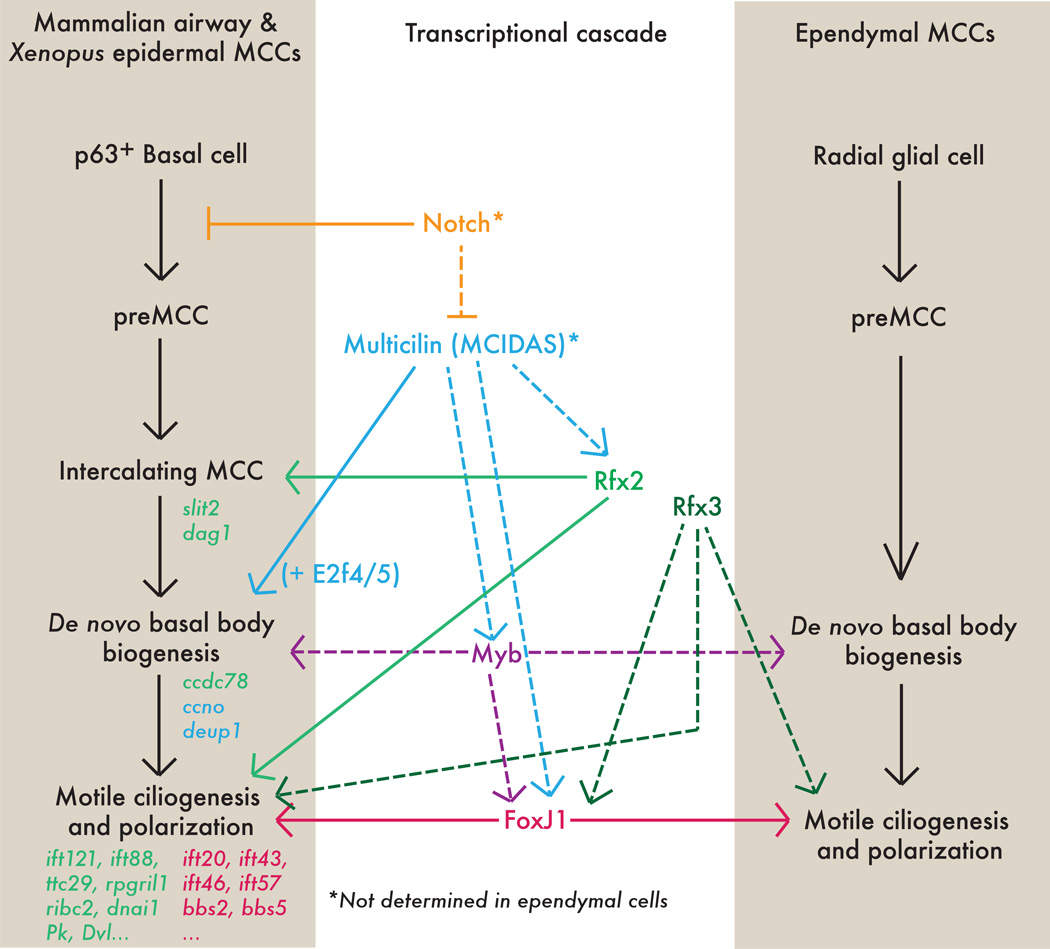
The production of a single cilium is a complex process under a number of transcriptional controls, and has been recently reviewed elsewhere [48]. However, MCCs face a different challenge entirely. The generation of dozens of cilia requires not only huge amounts of the basic ciliogenic machinery, but also specialized machinery for the robust production of basal bodies (modified centrioles), a process which in most cells is tightly regulated and is limited to only a single round of duplication. Thus, development of MCCs in vertebrates is under the control of a specialized regulatory hierarchy (Fig. 4).
Figure 4. Transcriptional controls of multiciliogenesis.
The transcriptional cascade leading to the generation of MCC axonemal tufts is outlined for mucocilairy MCCs (i.e. the mammalian airway and the Xenopus epidermis) and for ependymal MCCs. Solid lines represent direct interactions, dashed lines represent indirect (or unknown) interactions. Selected known target genes are written in italics, and labeled with the color of the transcription factor controlling them. Asterisk indicates that connection is only known for mucuociliary cells.
At the top of this hierarchy is the specification of MCC precursors, and in the Xenopus epidermis, the mouse airway, and the zebrafish pronephros this process requires Notch/Delta signaling [25, 26, 49, 50]. Lateral inhibition by Notch and Delta is important, as overexpression of the Notch intracellular domain results in a reduced number of ciliated cells, and, conversely, repression of Notch signaling leads to an increased number of cells adopting an MCC fate [49–53]. The upstream control of Notch in this context is still unknown, though a recent report suggests that the activity of the pathway is mediated in part by the microRNA miR-449, which acts to regulate Notch and Delta-like1 levels [53]. Additionally, Notch appears to be downstream of hypoxia signaling in the context of MCC patterning, as human bronchial epithelial cultures grown in submersion or hypoxic conditions produces fewer MCCs than those grown at standard air-liquid interfaces, and this reduction is reversible by addition of the Notch inhibitor DAPT [54].
The central Notch target appears to be the recently discovered putative transcriptional cofactor Multicilin (also known as MCIDAS), which is required for multiciliogenesis in the MCCs of the Xenopus epidermis and in mouse airway [55]. Strikingly, ectopic Multicilin is able to induce multiciliation in other cell types; driving Multicilin expression in these cells causes them to exit the cell cycle and become apparently postmitotic, an important feature of MCCs. Subsequently, these ectopic proto-MCCs undergo significant de novo basal body biogenesis (see below). Multicilin overexpression also leads to the direct transcriptional activation of a number of ciliogenic genes, including other ciliary transcription factors (e.g. foxj1, myb) and more basal ciliary components (e.g. alpha-tubulin, tektin). The net result is the production of a ciliary tuft very much akin to those of true MCCs. Interestingly, Multicilin seems specific to the generation of a multiciliated fate, as the motile monocilia of the Xenopus gastrocoel roof plate (analogous to the mammalian node) are unaffected by loss of this factor [55]. Therefore, Multicilin is a major and specific regulator of MCC cell fate in vertebrates.
Multicilin itself is not a transcription factor, and so an open question is how it participates in the activation of ciliogenic genes. A recent study has shed light on the question, demonstrating that Multicilin binds specifically to the transcription factors E2f4 and E2f5 to promote the transcription of key genes in centriole replication (see below; [56]). Interestingly, this Multicilin/E2f complex does not appear to have a strong role in the production of axonemal structures, suggesting that Multicilin plays a role in multiple independent transcriptional events underlying multiciliation [56].
Downstream of Multicilin are several other transcription factors required for motile ciliogenesis, including multiple Rfx family members, C-Myb, and FoxJ1. The Rfx proteins --orthologues of the C. elegans transcriptional regulator of ciliogenesis Daf-19 [57]-- are broadly required for ciliogenesis in vertebrates, including in MCCs [58]. Rfx2 is required for differentiation of MCCs in the Xenopus epidermis [59] and likely in the zebrafish kidney [60], and a recent genome-wide survey revealed that direct targets of Rfx2 contribute to essentially all ciliary machinery, including genes required for cilia assembly (Intraflagellar Transport, etc.), cilia motility (dynein arms, etc.), and planar polarization of directional beating (Planar Cell Polarity proteins, etc.) [61]. Likewise, in mice, Rfx3 is required for motile multi-ciliogenesis in the airway and in the ependymal cells lining the brain ventricles; loss of Rfx3 function is associated with hydrocephalus [62–64]. The Myb transcription factor also has a role in the specification and/or elaboration of MCCs and appears to act downstream of Multicilin to promote de novo basal body biogenesis and ciliation [65]. In zebrafish, Myb activity is regulated by the microRNA miR-34b [66].
Multicilin, Rfx3 and Myb are all required for the optimal expression of FoxJ1 [55, 62, 63, 65], which governs the articulation of motile cilia--including those of MCCs--in evolutionarily distant organisms [67–73]. Loss of FoxJ1 in mice leads to loss of motile cilia, including those of airway and ependymal MCCs, but not of primary cilia [67]. Intriguingly, ectopic overexpression of FoxJ1 in the non-ciliated cells of the Xenopus embryonic epidermis leads to the generation of one or two motile cilia. FoxJ1 alone, however, is not capable of generating an ectopic MCC tuft, likely because it does not lead to de novo production of basal bodies [73].
It should be noted here that mucociliary and ependymal MCCs arise from differing developmental trajectories. Mucociliary MCCs in the mammalian airway and the Xenopus epidermis are an intercalating population; i.e. they are specified in a deep layer of the epithelium and subsequently undergo an apical migration (sometimes referred to as radial intercalation) into the surface layer [51–53, 74, 75]. Control of intercalation and ciliogenesis appear to be tightly coordinated in MCCs, and recent evidence suggests that Rfx2 regulates both processes [61]. Contrastingly, ependymal MCCs differentiate in situ from radial glial precursors [76]. Despite these important differences, ependymal and mucociliary MCCs share significant portions of the transcriptional cascade of multiciliogenesis including, FoxJ1, Rfx3, and Myb [59, 62, 63, 65, 67, 72]. This suggests that tissue-specific upstream signals may converge on a unified downstream multiciliation cassette as an iterable solution to the complex cell biological problem of generating many dozens of axonemes, as detailed below.
The cell biology of multiciliogenesis
Recent advances in understanding the developmental programs underlying MCC specification have coincided with renewed investigation of the mechanisms required for the generation and coordination of dozens of motile axonemes in a single cell. These unique cell-biological challenges are considered below.
De novo/acentriolar generation of basal bodies
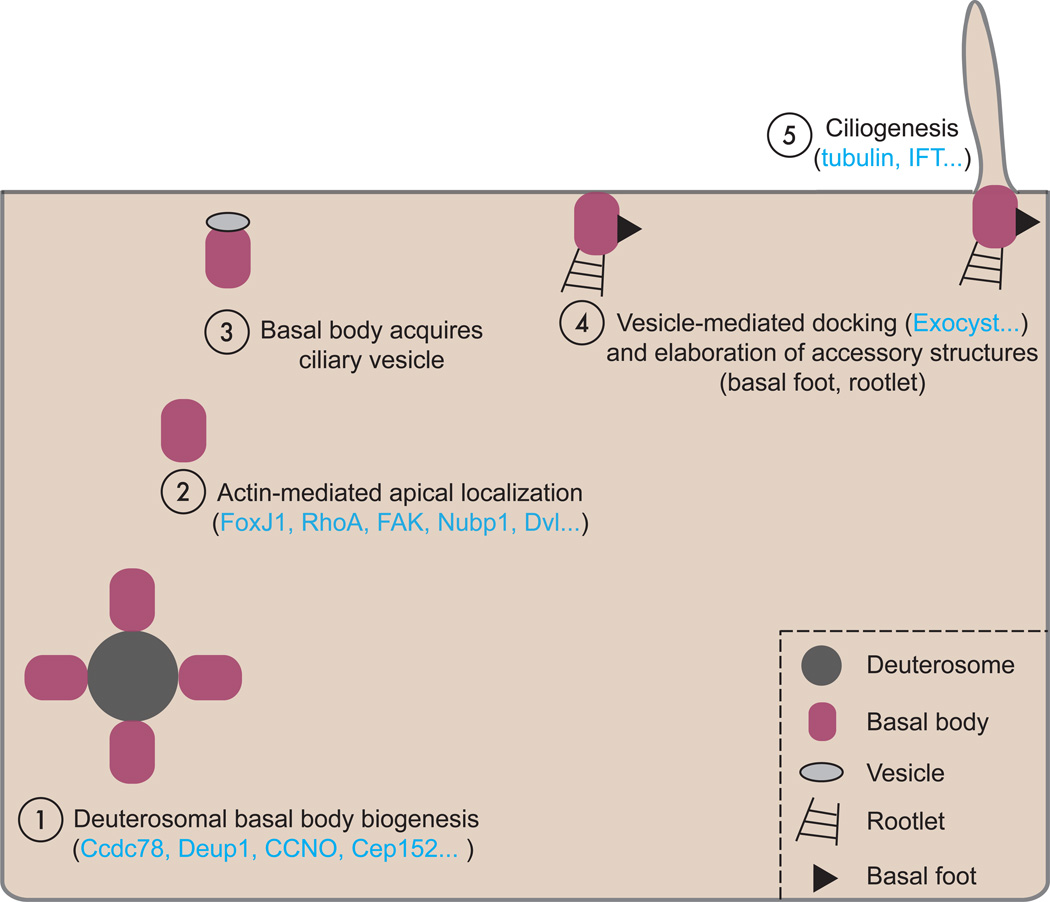
One major difference between monociliated and multiciliated vertebrate cells is the process of de novo basal body generation (Fig. 5). Unlike cycling, mono-ciliated cells where one centriole of the pair becomes the basal body and gives rise to the cilium, terminally differentiated MCCs require the generation of many dozens of basal bodies (~150 on average in a Xenopus epidermal MCC) [77–81]. The first insights to the process came from early electron microscopy work in various multiciliated tissues including the Xenopus epidermis [78], the mammalian lung [77], and the avian trachea [79] and oviduct [80]. Interestingly, while mother centriole dependent duplication does occur in these cells, many, if not most, of the basal bodies in these cells are not templated by existing centrioles, but rather by an indistinct, electron opaque cytoplasmic structure, termed the “deuterosome” by Sorokin [77]. This method of basal body production is sometimes referred to as “acentriolar” basal body biogenesis, but here we will strive to use the more common term “de novo” basal body biogenesis.
Figure 5. A schematic representation of de novo basal body biogenesis.
Since its characterization by electron microscopy over 40 years ago, the molecular nature of the deuterosome has remained elusive. Recently, however, a pair of exciting studies reported the first known deuterosomal molecules. The first reports that the coiled-coil domain containing protein Ccdc78 localizes to deuterosomes in the cytoplasm of Xenopus MCCs and in mouse tracheal epithelial cultures (MTECs), and knockdown leads to a reduction in centriole number [81]. Importantly, this key regulator of de novo centriole biogenesis at deuterosomes acts by Cep152-mediated recruitment of Plk4 and SAS-6, key regulators of centriole duplication in cycling cells ([81] and see [82] for a review of centriole duplication).
Another recent report identified Deup1 (previously Ccdc67) as a mediator of de novo basal body biogenesis [83]. Deup1 localizes in a punctate distribution co-local with ongoing de novo centriole amplification in MTECs, and loss of Deup1 leads to a reduction in the number of deuterosomes and defects in de novo basal body amplification. Strikingly, overexpression of Deup1 in non-MCC cells is sufficient to elicit the formation of ring-like structures co-local with ectopic centriole amplification. Further, the paralog of Deup1, Cep63, appears to specifically govern mother centriole dependent amplification, suggesting that the two proteins may play antagonistic roles in the organization of centriole amplification. In support of this, Deup1 and Cep63 compete for binding to Cep152, suggesting that there may be competition between centriolar and de novo amplification [83].
All together, these data suggest that Deup1 and Ccdc78 are key mediators of deuterosomal biogenesis and function [81, 83]. A common thread in these two studies is that both Ccdc78 and Deup1 are required for the localization of Cep152 to the deuterosome. Importantly, Cep152 is also required for mother centriole dependent replication, as it is a central regulator of centriole structure biogenesis [82]. It is interesting, then, that overexpression of Deup1 is sufficient to drive centriole biogenesis, whereas that of Ccdc78 is not [81, 83]. These data suggest additional layers of regulation governing de novo production of basal bodies, though the specifics remain unclear. Transcriptionally, Deup1 is downstream of the Multcilin/E2f complex [56], whereas Ccdc78 is downstream of Multicilin (but apparently independent of E2F4/5 [56, 81]) and FoxJ1 [73], and is a direct target of Rfx2 [61]. It will be of interest to discover if and how these two factors are interconnected.
The importance of understanding deuterosome formation and function is underscored by the discovery of human patients with mutations in cyclin O (CCNO), a critical regulator of de novo centriole biogenesis in human and mouse respiratory MCCs, as well as Xenopus epidermal MCCs [84]. CCNO is expressed downstream of Multicilin/E2f4 [56, 84] and is required for de novo basal body biogenesis and docking (see below), and, therefore, the generation of axonemal tufts. Interestingly, CCNO deficient cells are still capable of mono- or bi-ciliation, suggesting that this factor is specifically important in MCCs. Moreover, human patients with mutations in CCNO experience severe and progressive respiratory symptoms, but exhibit normal left-right patterning (situs solitus), supporting a specific function for CCNO in multiciliation [84].
Together, the above studies demonstrate the importance of de novo deuterosomal basal body biogenesis. The identification of the first bona fide components of this long intractable structure opens the door to sophisticated proteomic and cell biological analysis of a process central to MCC function. Finally, and from an evolutionary perspective, it is noteworthy that all metazoans in which the question has been asked rely--at least in part--on cytoplasmic de novo basal body biogenesis for multiciliation [65, 77, 81, 83–85]. In fact planarians, which otherwise entirely lack centrioles, undergo de novo basal body biogenesis in their ventral band MCCs [86]. Conversely, many unicellular multiciliated organisms, such as Tetrahymena and Paramecium, undergo basal body replication at the cell cortex in a manner somewhat reminiscent of centriolar duplication during the cell cycle [13, 30, 87]. However, some organisms from other eukaryotic lineages, such as the protist N. gruberi and the oomycete Phytophthora, are capable of undergoing acentriolar cytoplasmic centriole biogenesis (reviewed in [88]). It is therefore unclear where in evolution the association of multiciliation and de novo centriole biogenesis occurred. Comparative phylogenetic and mechanistic studies of centriole replication, basal body biogenesis and multiciliation gene cassettes in metazoans, unicellular multiciliates, and plants will be interesting in terms of understanding the basal innovations and phylogenetic relationships of multiciliation.
Basal body migration and docking
Following de novo centriole formation in the cytoplasm, nascent basal bodies must undergo migration and vesicle-mediated fusion with the apical surface of the MCC; simultaneously they must acquire a number of accessory structures required for ciliogenesis (Fig. 5; reviewed in [89–91]). Early experiments in the quail oviduct using pharmacological agents to perturb cytoskeletal elements showed that basal body docking depended upon actin filament assembly but not microtubule polymerization [85, 92, 93]. More recently, molecular controls on this process have been discovered.
For example, FoxJ1 has been found to govern apical basal body docking by controlling actin assembly via the actin regulator ezrin and the small GTPase RhoA [69, 71, 94]. The mechanisms of Rho-mediated actin assembly remain unclear in MCCs, but there is mounting evidence that components of the Planar Cell Polarity (PCP) signaling system are involved. PCP proteins are well-known regulators of Rho activation [95] and disruption of either the core PCP protein Dishevelled (Dvl) or the PCP effector protein Inturned (Intu) leads to loss of apical actin and a failure of basal body docking in Xenopus MCCs [96, 97]. In addition, the putative small GTPase Rsg1 --a known binding partner for the PCP effector protein Fuz [98]-- is also required for basal body docking in Xenopus [99]. This role for PCP proteins is not restricted to Xenopus, as the core PCP proteins Celsr2 and Celsr3 are essential for basal body docking in mouse ependymal cells [100], and mice lacking Vangl2 show variable defects in MCC ciliogenesis in the airway [101].
Further elucidation of the role for actin networks in basal body docking comes from a recent study of focal adhesion complex proteins in Xenopus MCCs, where knockdown of Focal Adhesion Kinase (Fak) disrupted basal body to actin network connections and led to a failure of apical basal body migration [102]. In another study, Nucleotide binding protein 1 (Nubp1) was shown to regulate an internal actin network that anchored basal bodies to the cell cortex during MCC intercalation. Disruption of Nubp1 led to a disorganized actin mesh, and a failure of basal body migration without disrupting the localization or activation of Rho [103].
Rotational polarization of multiple cilia within a single cell
Another key aspect of MCC development is the polarization of cilia. In order for MCCs to effectively generate fluid flow, all of the axonemes within a cell must beat in a synchronized and polarized fashion, a property referred to as rotational polarization [104, 105]. Nascent MCCs show only a weak polarization, with many axonemes not yet properly oriented. As the MCC matures these axonemes are progressively reoriented until all the axonemes of the cell beat in a largely unidirectional fashion [106, 107]. This reorientation of cilia is accomplished through a positive feedback mechanism, where the weak, but directional, flow of the early axonemal tuft directs the progressive reinforcement of cilia into the correct orientation [106]. This was first demonstrated in an elegant study where fluid flow was externally reversed across explanted Xenopus MCCs, which caused cilia to reorient opposite to their normal direction in response. Such reorientation only occurred when cilia were motile, as experimental ablation of dynein arms and other key motility components led to a general failure in axonemal polarization [106]. Interestingly, the requirement for axonemal motility may not be conserved in the mammalian trachea, as immotile cilia do not impair basal body polarization in this tissue [108]. However, subsequent studies found that a similar mechanism is at work in mouse ependymal cells [21], suggesting that it is not species specific.
Cytoskeletal organization is also a key regulator of ciliary polarity in MCCs. Early electron microscopy work showed that basal bodies are closely associated with both actin and microtubule networks, and early Cytochalasin D (cyto D) experiments suggested that the actin network is important for ciliary polarity [85, 92, 93]. More recently, pharmacological experiments demonstrated differential roles for actin and microtubule networks in the refinement of cilia polarity. In Xenopus MCCs, apical actin is localized in two distinct populations, an apical-most actin meshwork, and a sub-apical set of actin links between neighboring cilia [39]. Doses of cyto D that specifically perturb the sub-apical population lead to global defects in cilia polarity within an MCC. That is, the cilia of the MCC still exhibit an initially biased polarity, but fail to undergo refinement [39]. Further, Dishevelled/Active RhoA/actin activity is required for polarization of cilia, in addition to its role in basal body docking [97]. In contrast to the global refinement defects resulting from disruption of actin networks, treatment of MCCs with the microtubule de-polymerizer nocadazole leads to a disruption of local polarity; i.e. neighboring cilia are oriented randomly with respect to one another, ablating even the modest polarization bias of early MCCs [39]. An excellent candidate for upstream control of cytoskeletal polarity is the PCP pathway, as Dvl, Celsr2, and Celsr3 control the rotational polarity of basal bodies in MCCs [97, 100]. An interesting finding is that basal feet appear to be required for rotational polarization, as basal bodies Odf2 mutants lack basal feet and are incapable of undergoing rotational polarization, even in the presence of appropriate PCP cues [109].
Mature rotational polarity is actively maintained, as demonstrated by a recent study of the coiled-coil protein Bbof1 (also known as Ccdc176), a Foxj1 target gene [110]. Loss of function of Bbof1 does not interfere with the initial weak polarization of immature MCCs, but does lead to a failure to refine cilia polarity. Bbof1 knockdown cells are capable of undergoing flow-mediated reorientation to achieve strong polarity similar to that of mature MCCs, however they are incapable of maintaining that polarity once artificial flow is no longer applied. Interestingly, Bbof1 overexpression leads to the premature orientation of basal bodies, even in the context of disrupted actin or microtubule networks. This suggests that Bbof1 may, in some way, link neighboring basal bodies and lock their orientation with respect to one another [110]. Such a function would impart a resistance to individual basal body reorientation, providing a mechanism for resistance to local hydrodynamic disruptions, including cases where axonemal beat asymmetry is lost [111].
Tissue level polarization of MCCs
In addition to intracellular rotational polarization, where the many cilia on a single MCC establish a refined and unidirectional polarity relative to one another, MCCs within a tissue must also establish so-called “tissue-level” polarization with the other MCCs in that tissue, so that coordinated beating can lead to directed and productive fluid flow across the epithelium (see [105]). In Xenopus, MCCs polarize after they intercalate and do so in response to established polarity cues, as epidermal regions with disrupted PCP signaling show non-autonomous defects in MCC orientation [112]. In the mouse airway, this tissue-level polarity seems to involve ciliary orientation via specialized microtubules linking the apical cytoskeleton of MCCs to the asymmetric protein domains delimited by PCP proteins [101]. Finally, the PCP proteins Vangl2, Dvl1/2/3, Celsr2, and Celsr3 all have a demonstrated requirement in MCC polarization in murine ependymal cells, suggesting a conservation of this polarization paradigm across tissues and vertebrate species [21, 100, 113–115].
The polarity motifs discussed above seem largely independent of MCC context, however brain ependymal cells additionally exhibit a unique unipolar clustering of their basal bodies, resulting in a polarized axonemal tuft (Fig. 1a). This property, known as translational polarity, has been recently reviewed [116], and so we discuss it only briefly. Translational clustering of basal bodies depends on the primary cilia of the radial glia precursors of multiciliated ependymal cells, as conditional ablation of the cilia of these cells results in a failure of unipolar basal bodies clustering after these cells adopt an ependymal fate [117]. Tissue level coordination of translational polarity requires the function of the PCP pathway in the radial glial precursors, specifically this requires Celsr1 and appears to be independent of Celsr2/3 (which are required for later rotational polarity) [114]. The actual translational migration of basal bodies depends upon the activity of Myosin II and appears to be largely independent of PCP [113]. Many questions remain about how this specialized translational polarity is accomplished, especially as it appears to be independent of the rotational polarity that is a more general property of MCCs.
Concluding remarks
Here we have attempted to provide an overview of multiciliation, a fascinating biological problem. Spatial constraints prevented us from thoroughly discussing of all the topics broached here, especially questions of unicellular physiology and the evolution of multiciliation. As recourse, we have provided references to in-depth reviews of the individual aspects of MCC biology where available.
While many aspects of multiciliogenesis are becoming clearer thanks to decades of study, there are still many open questions: Did multiciliation arise completely independently in different lineages, or is there some common thread? Does deuterosomal basal body biogenesis occur in metazoans other than vertebrates? Is there some evolutionary constant in the molecular mechanism of de novo centriole biogenesis, or is it a case of convergent evolution? These questions will require careful molecular and phylogenetic analyses, but should help answer an important evolutionary question about a cell type found across the entire eukaryotic lineage.
Even on the scale of more thoroughly studied vertebrate MCCs, we still understand little about the molecular biology of multiciliation. Do all vertebrate MCCs use equivalent transcriptional cascades beginning with Multicilin, or is there some variability before they converge on downstream factors? More generally, how is the transcriptional cascade of multiciliogenesis initiated? What proteins comprise the deuterosome, and how is this structure regulated? What aspects of actin regulation are important for basal body docking and migration? These and many other questions provide exciting avenues into understanding the biology of MCCs, key players in neurogenesis, respiration and fertility [2–4].
Acknowledgements
We thank S. Ohata and A. Alvarz-Buylla for providing images of ependymal MCCs and E. Vladar and J. Axelrod for SEM images of mouse tracheal MCCs. We also thank J. Tabler and our anonymous reviewers for critical reading and insight. This work was supported by grants from the NIGMS and the NHLBI to JBW. JBW is an Early Career Scientist of the Howard Hughes Medical Institute.
References
- 1.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature Reviews Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JLR, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 3.Wanner A, Salathé M, O'Riordan TG. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 4.Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat. Rev. Mol. Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 6.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 7.Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu. Rev. Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- 8.Golestanian R, Yeomans JM, Uchida N. Hydrodynamic synchronization at low Reynolds number. Soft Matter. 2011;7:3074–3082. [Google Scholar]
- 9.Brokaw CJ. Thinking about flagellar oscillation. Cell Motil. Cytoskeleton. 2009;66:425–436. doi: 10.1002/cm.20313. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DR. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv. Exp. Med. Biol. 2007;607:130–140. doi: 10.1007/978-0-387-74021-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jékely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 12.Nikolaev SI, Berney C, Petrov NB, Mylnikov AP, Fahrni JF, Pawlowski J. Phylogenetic position of Multicilia marina and the evolution of Amoebozoa. Int. J. Syst. Evol. Microbiol. 2006;56:1449–1458. doi: 10.1099/ijs.0.63763-0. [DOI] [PubMed] [Google Scholar]
- 13.Allen RD. The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J. Cell Biol. 1969;40:716–733. doi: 10.1083/jcb.40.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machemer H. Ciliary activity and the origin of metachrony in Paramecium: effects of increased viscosity. J. Exp. Biol. 1972;57:239–259. doi: 10.1242/jeb.57.1.239. [DOI] [PubMed] [Google Scholar]
- 15.Riesgo A, Taylor C, Leys SP. Reproduction in a carnivorous sponge: the significance of the absence of an aquiferous system to the sponge body plan. Evolution & development. 2007;9:618–631. doi: 10.1111/j.1525-142X.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen C. Structure and function of metazoan ciliary bands and their phylogenetic significance. Acta zoologica. 1987;68:205–262. [Google Scholar]
- 17.Mizukami I, Gall J. Centriole replication II. Sperm formation in the fern, Marsilea, and the cycad, Zamia. J. Cell Biol. 1966;29:97–111. doi: 10.1083/jcb.29.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges ME, Wickstead B, Gull K, Langdale JA. The evolution of land plant cilia. New Phytologist. 2012;195:526–540. doi: 10.1111/j.1469-8137.2012.04197.x. [DOI] [PubMed] [Google Scholar]
- 19.Worthington WC, Cathcart RS. Ependymal Cilia: Distribution and Activity in the Adult Human Brain. Science. 1963;139:221–222. doi: 10.1126/science.139.3551.221. [DOI] [PubMed] [Google Scholar]
- 20.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 21.Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi J-M, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han Y-G, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 22.Assheton R. Notes on the ciliation of the ectoderm of the amphibian embryo. Quarterly Journal of Microscopical science. 1895;38:465–484. [Google Scholar]
- 23.Nokhbatolfoghahai M, Downie JR, Clelland AK, Rennison K. The surface ciliation of anuran amphibian embryos and early larvae: patterns, timing differences and functions. Journal of Natural history. 2005;39:887–929. [Google Scholar]
- 24.Werner ME, Mitchell BJ. Understanding ciliated epithelia: the power of Xenopus. Genesis. 2012;50:176–185. doi: 10.1002/dvg.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 26.Ma M, Jiang Y-J. Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 2007;3:e18. doi: 10.1371/journal.pgen.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rompolas P, Patel-King RS, King SM. Schmidtea mediterranea: a model system for analysis of motile cilia. Methods Cell Biol. 2009;93:81–98. doi: 10.1016/S0091-679X(08)93004-1. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz F, Beisson J, Rossier J, Dupuis-Williams P. Basal body duplication in Paramecium requires gamma-tubulin. Curr. Biol. 1999;9:43–46. doi: 10.1016/s0960-9822(99)80045-1. [DOI] [PubMed] [Google Scholar]
- 29.Gogendeau D, Hurbain I, Raposo G, Cohen J, Koll F, Basto R. Sas-4 proteins are required during basal body duplication in Paramecium. Mol. Biol. Cell. 2011;22:1035–1044. doi: 10.1091/mbc.E10-11-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stemm-Wolf AJ, Morgan G, Giddings TH, White EA, Marchione R, McDonald HB, Winey M. Basal body duplication and maintenance require one member of the Tetrahymena thermophila centrin gene family. Molecular Biology of the Cell. 2005;16:3606–3619. doi: 10.1091/mbc.E04-10-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lidow MS, Menco BPM. Observations on axonemes and membranes of olfactory and respiratory cilia in frogs and rats using tannic acid-supplemented fixation and photographic rotation. Journal of ultrastructure research. 1984;86:18–30. doi: 10.1016/s0022-5320(84)90092-3. [DOI] [PubMed] [Google Scholar]
- 32.Salathe M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 33.Gueron S, Levit-Gurevich K. Energetic considerations of ciliary beating and the advantage of metachronal coordination. PNAS. 1999;96:12240–12245. doi: 10.1073/pnas.96.22.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brumley DR, Polin M, Pedley TJ, Goldstein RE. Hydrodynamic synchronization and metachronal waves on the surface of the colonial alga Volvox carteri. Physical review letters. 2012;109:268102. doi: 10.1103/PhysRevLett.109.268102. [DOI] [PubMed] [Google Scholar]
- 35.Gueron S, Levit-Gurevich K, Liron N, Blum JJ. Cilia internal mechanism and metachronal coordination as the result of hydrodynamical coupling. PNAS. 1997;94:6001–6006. doi: 10.1073/pnas.94.12.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elgeti J, Gompper G. Emergence of metachronal waves in cilia arrays. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4470–4475. doi: 10.1073/pnas.1218869110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guirao B, Joanny J-F. Spontaneous creation of macroscopic flow and metachronal waves in an array of cilia. Biophysical Journal. 2007;92:1900–1917. doi: 10.1529/biophysj.106.084897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rompolas P, Patel-King RS, King SM. An outer arm Dynein conformational switch is required for metachronal synchrony of motile cilia in planaria. Mol. Biol. Cell. 2010;21:3669–3679. doi: 10.1091/mbc.E10-04-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, Mitchell BJ. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sleigh MA. Primary Ciliary Dyskinesia. The Lancet. 1981;318:476. doi: 10.1016/s0140-6736(81)90811-4. [DOI] [PubMed] [Google Scholar]
- 41.Sleigh MA. Adaptations of ciliary systems for the propulsion of water and mucus. Comparative Biochemistry and Physiology Part A: Physiology. 1989;94:359–364. doi: 10.1016/0300-9629(89)90559-8. [DOI] [PubMed] [Google Scholar]
- 42.Schrøder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J. Cell. Sci. 2011;124:2539–2551. doi: 10.1242/jcs.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubo A, Yuba-Kubo A, Tsukita S, Tsukita S, Amagai M. Sentan: a novel specific component of the apical structure of vertebrate motile cilia. Mol. Biol. Cell. 2008;19:5338–5346. doi: 10.1091/mbc.E08-07-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks ER, Wallingford JB. Control of vertebrate intraflagellar transport by the planar cell polarity effector Fuz. J. Cell Biol. 2012;198:37–45. doi: 10.1083/jcb.201204072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 46.Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omoto CK, Kung C. Rotation and twist of the central-pair microtubules in the cilia of Paramecium. J. Cell Biol. 1980;87:33–46. doi: 10.1083/jcb.87.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choksi SP, Lauter G, Swoboda P, Roy S. Switching on cilia: transcriptional networks regulating ciliogenesis. Development. 2014;141:1427–1441. doi: 10.1242/dev.074666. [DOI] [PubMed] [Google Scholar]
- 49.Deblandre GA, Wettstein DA, Koyano-Nakagawa N, Kintner C. A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development. 1999;126:4715–4728. doi: 10.1242/dev.126.21.4715. [DOI] [PubMed] [Google Scholar]
- 50.Tsao P-N, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stubbs JL, Davidson L, Keller R, Kintner C. Radial intercalation of ciliated cells during Xenopus skin development. Development. 2006;133:2507–2515. doi: 10.1242/dev.02417. [DOI] [PubMed] [Google Scholar]
- 52.Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J. Cell. Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi L-E, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Genet. 2011;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 54.Gerovac BJ, Valencia M, Baumlin N, Salathe M, Conner GE, Fregien NL. Submersion and Hypoxia Inhibit Ciliated Cell Differentiation in a Notch Dependent Manner. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0237OC. 140422132500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 2012;14:140–147. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma L, Quigley I, Omran H, Kintner C. Multicilin drives centriole biogenesis via E2f proteins. Genes Dev. 2014;28:1461–1471. doi: 10.1101/gad.243832.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol. Cell. 2000;5:411–421. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- 58.Piasecki BP, Burghoorn J, Swoboda P. Regulatory Factor X (RFX)-mediated transcriptional rewiring of ciliary genes in animals. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12969–12974. doi: 10.1073/pnas.0914241107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung M-I, Peyrot SM, LeBoeuf S, Park TJ, McGary KL, Marcotte EM, Wallingford JB. RFX2 is broadly required for ciliogenesis during vertebrate development. Dev. Biol. 2012;363:155–165. doi: 10.1016/j.ydbio.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bisgrove BW, Makova S, Yost HJ, Brueckner M. RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow. Dev. Biol. 2012;363:166–178. doi: 10.1016/j.ydbio.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung M-I, Kwon T, Tu F, Brooks ER, Gupta R, Meyer M, Baker JC, Marcotte EM, Wallingford JB. Coordinated genomic control of ciliogenesis and cell movement by RFX2. Elife. 2014;3:e01439. doi: 10.7554/eLife.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Didon L, Zwick RK, Chao IW, Walters MS, Wang R, Hackett NR, Crystal RG. RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium. Respir. Res. 2013;14:70. doi: 10.1186/1465-9921-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zein El L, Ait-Lounis A, Morlé L, Thomas J, Chhin B, Spassky N, Reith W, Durand B. RFX3 governs growth and beating efficiency of motile cilia in mouse and controls the expression of genes involved in human ciliopathies. Journal of Cell Science. 2009;122:3180–3189. doi: 10.1242/jcs.048348. [DOI] [PubMed] [Google Scholar]
- 64.Baas D, Meiniel A, Benadiba C, Bonnafe E, Meiniel O, Reith W, Durand B. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur. J. Neurosci. 2006;24:1020–1030. doi: 10.1111/j.1460-9568.2006.05002.x. [DOI] [PubMed] [Google Scholar]
- 65.Tan FE, Vladar EK, Ma L, Fuentealba LC, Hoh R, Espinoza FH, Axelrod JD, Alvarez-Buylla A, Stearns T, Kintner C, et al. Myb promotes centriole amplification and later steps of the multiciliogenesis program. Development. 2013;140:4277–4286. doi: 10.1242/dev.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Fu C, Fan H, Du T, Dong M, Chen Y, Jin Y, Zhou Y, Deng M, Gu A, et al. miR-34b regulates multiciliogenesis during organ formation in zebrafish. Development. 2013;140:2755–2764. doi: 10.1242/dev.092825. [DOI] [PubMed] [Google Scholar]
- 67.Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- 68.Hagenlocher C, Walentek P, Müller C, Thumberger T, Feistel K. Ciliogenesis and cerebrospinal fluid flow in the developing Xenopus brain are regulated by foxj1. Cilia. 2013;2:12. doi: 10.1186/2046-2530-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J. Cell. Sci. 2007;120:1868–1876. doi: 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- 70.Vij S, Rink JC, Ho HK, Babu D, Eitel M, Narasimhan V, Tiku V, Westbrook J, Schierwater B, Roy S. Evolutionarily ancient association of the FoxJ1 transcription factor with the motile ciliogenic program. PLoS Genet. 2012;8:e1003019. doi: 10.1371/journal.pgen.1003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomperts BN, Gong-Cooper X, Hackett BP. Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J. Cell. Sci. 2004;117:1329–1337. doi: 10.1242/jcs.00978. [DOI] [PubMed] [Google Scholar]
- 72.Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- 73.Stubbs JL, Oishi I, Izpisúa Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drysdale TA, Elinson RP. Cell migration and induction in the development of the surface ectodermal pattern of the Xenopus laevis tadpole. Development, growth & differentiation. 1992;34:51–59. doi: 10.1111/j.1440-169X.1992.00051.x. [DOI] [PubMed] [Google Scholar]
- 75.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sorokin S. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell. Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 78.Steinman RM. An electron microscopic study of ciliogenesis in developing epidermis and trachea in the embryo of Xenopus laevis. Am. J. Anat. 1968;122:19–55. doi: 10.1002/aja.1001220103. [DOI] [PubMed] [Google Scholar]
- 79.Kalnins VI, Porter KR. Centriole replication during ciliogenesis in the chick tracheal epithelium. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1969;100:1–30. doi: 10.1007/BF00343818. [DOI] [PubMed] [Google Scholar]
- 80.Dirksen ER. Centriole morphogenesis in developing ciliated epithelium of the mouse oviduct. J. Cell Biol. 1971;51:286–302. doi: 10.1083/jcb.51.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klos Dehring DA, Vladar EK, Werner ME, Mitchell JW, Hwang P, Mitchell BJ. Deuterosome-Mediated Centriole Biogenesis. Dev. Cell. 2013;27:103–112. doi: 10.1016/j.devcel.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao H, Zhu L, Zhu Y, Cao J, Li S, Huang Q, Xu T, Huang X, Yan X, Zhu X. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat. Cell Biol. 2013;15:1434–1444. doi: 10.1038/ncb2880. [DOI] [PubMed] [Google Scholar]
- 84.Wallmeier J, Al-Mutairi DA, Chen C-T, Loges NT, Pennekamp P, Menchen T, Ma L, Shamseldin HE, Olbrich H, Dougherty GW, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014 doi: 10.1038/ng.2961. [DOI] [PubMed] [Google Scholar]
- 85.Boisvieux-Ulrich E, Lainé M-C, Sandoz D. Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell and tissue research. 1990;259:443–454. doi: 10.1007/BF01740770. [DOI] [PubMed] [Google Scholar]
- 86.Azimzadeh J, Wong ML, Downhour DM, Sánchez Alvarado A, Marshall WF. Centrosome loss in the evolution of planarians. Science. 2012;335:461–463. doi: 10.1126/science.1214457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iftode F, Cohen J, Ruiz F, Rueda AT, Chen-Shan L, Adoutte A, Beisson J. Development of surface pattern during division in Paramecium. I. Mapping of duplication and reorganization of cortical cytoskeletal structures in the wild type. Development. 1989;105:191–211. [Google Scholar]
- 88.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell. Sci. 2007;120:7–15. doi: 10.1242/jcs.03305. [DOI] [PubMed] [Google Scholar]
- 90.Hoyer-Fender S. Centriole maturation and transformation to basal body. Semin. Cell Dev. Biol. 2010;21:142–147. doi: 10.1016/j.semcdb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J. Cell Biol. 2011;193:435–444. doi: 10.1083/jcb.201101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lemullois M, Boisvieux-Ulrich E, Laine MC, Chailley B, Sandoz D. Development and functions of the cytoskeleton during ciliogenesis in metazoa. Biol. Cell. 1988;63:195–208. doi: 10.1016/0248-4900(88)90058-5. [DOI] [PubMed] [Google Scholar]
- 93.Sandoz D, Chailley B, Boisvieux-Ulrich E, Lemullois M, Laine MC, Bautista-Harris G. Organization and functions of cytoskeleton in metazoan ciliated cells. Biol. Cell. 1988;63:183–193. doi: 10.1016/0248-4900(88)90057-3. [DOI] [PubMed] [Google Scholar]
- 94.Huang T, You Y, Spoor MS, Richer EJ, Kudva VV, Paige RC, Seiler MP, Liebler JM, Zabner J, Plopper CG, et al. Foxj1 is required for apical localization of ezrin in airway epithelial cells. J. Cell. Sci. 2003;116:4935–4945. doi: 10.1242/jcs.00830. [DOI] [PubMed] [Google Scholar]
- 95.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 97.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat. Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brooks ER, Wallingford JB. The Small GTPase Rsg1 is important for the cytoplasmic localization and axonemal dynamics of intraflagellar transport proteins. Cilia. 2013;2:13. doi: 10.1186/2046-2530-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat. Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 101.Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Antoniades I, Stylianou P, Skourides PA. Making the connection: ciliary adhesion complexes anchor Basal bodies to the actin cytoskeleton. Dev. Cell. 2014;28:70–80. doi: 10.1016/j.devcel.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Ioannou A, Santama N, Skourides PA. Xenopus laevis nucleotide binding protein 1 (xNubp1) is important for convergent extension movements and controls ciliogenesis via regulation of the actin cytoskeleton. Dev. Biol. 2013;380:243–258. doi: 10.1016/j.ydbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Marshall WF, Kintner C. Cilia orientation and the fluid mechanics of development. Curr. Opin. Cell Biol. 2008;20:48–52. doi: 10.1016/j.ceb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallingford JB. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr. Opin. Cell Biol. 2010;22:597–604. doi: 10.1016/j.ceb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 107.Boisvieux-Ulrich E, Laine MC, Sandoz D. The orientation of ciliary basal bodies in quail oviduct is related to the ciliary beating cycle commencement. Biology of the Cell. 1985;55:147–150. doi: 10.1111/j.1768-322x.1985.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 108.Matsuo M, Shimada A, Koshida S, Saga Y, Takeda H. The establishment of rotational polarity in the airway and ependymal cilia: analysis with a novel cilium motility mutant mouse. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;304:L736–L45. doi: 10.1152/ajplung.00425.2012. [DOI] [PubMed] [Google Scholar]
- 109.Kunimoto K, Yamazaki Y, Nishida T, Shinohara K, Ishikawa H, Hasegawa T, Okanoue T, Hamada H, Noda T, Tamura A, et al. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 2012;148:189–200. doi: 10.1016/j.cell.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 110.Chien Y-H, Werner ME, Stubbs J, Joens MS, Li J, Chien S, Fitzpatrick JAJ, Mitchell BJ, Kintner C. Bbof1 is required to maintain cilia orientation. Development. 2013;140:3468–3477. doi: 10.1242/dev.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr. Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirota Y, Meunier A, Huang S, Shimozawa T, Yamada O, Kida YS, Inoue M, Ito T, Kato H, Sakaguchi M, et al. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137:3037–3046. doi: 10.1242/dev.050120. [DOI] [PubMed] [Google Scholar]
- 114.Boutin C, Labedan P, Dimidschstein J, Richard F, Cremer H, André P, Yang Y, Montcouquiol M, Goffinet AM, Tissir F. A dual role for planar cell polarity genes in ciliated cells. Proc. Natl. Acad. Sci. U.S.A. 2014 doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohata S, Nakatani J, Herranz-Pérez V, Cheng J, Belinson H, Inubushi T, Snider WD, Garcia-Verdugo JM, Wynshaw-Boris A, Alvarez-Buylla A. Loss of Dishevelleds Disrupts Planar Polarity in Ependymal Motile Cilia and Results in Hydrocephalus. Neuron. 2014 doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kishimoto N, Sawamoto K. Planar polarity of ependymal cilia. Differentiation. 2012;83:S86–S90. doi: 10.1016/j.diff.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 117.Mirzadeh Z, Han Y-G, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J. Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]