Abstract
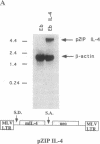
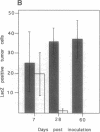
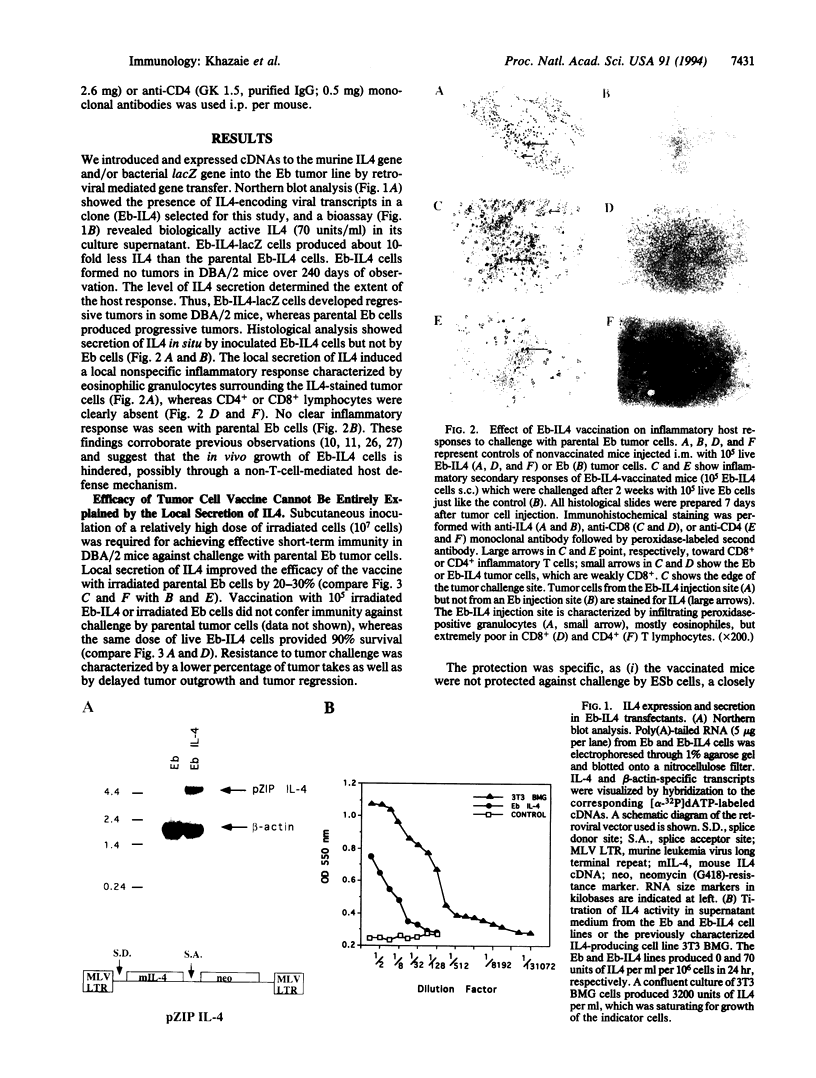
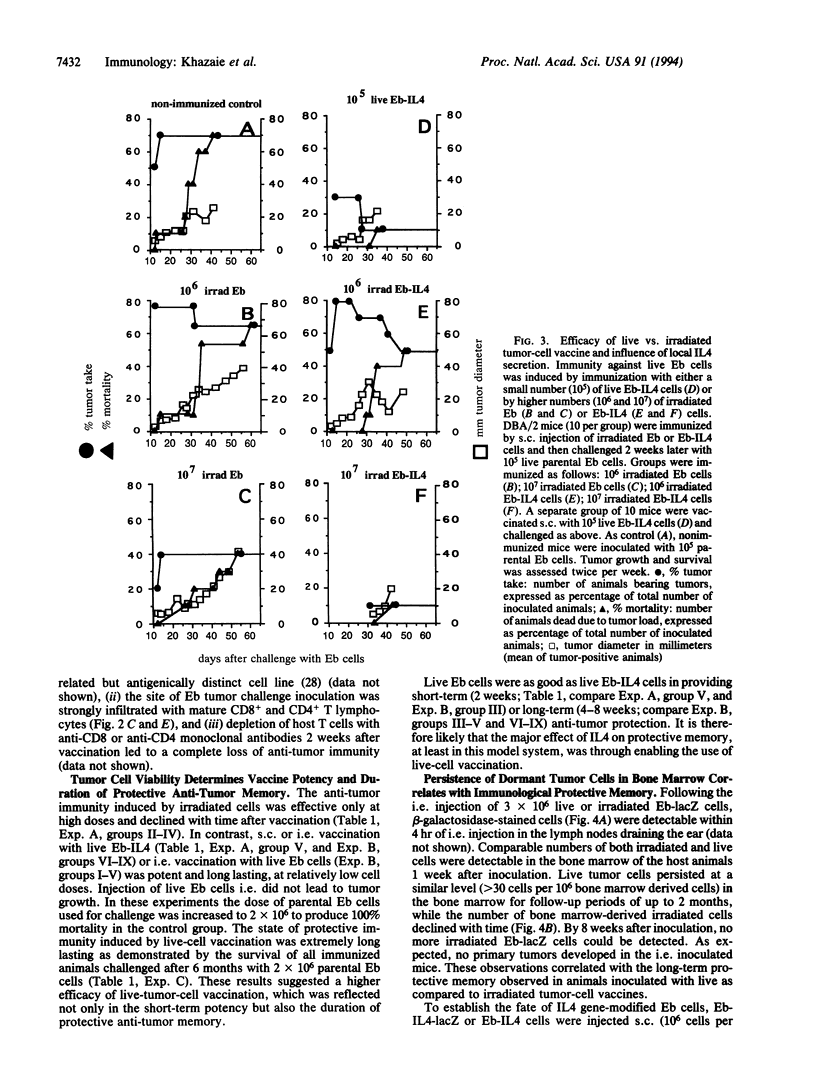
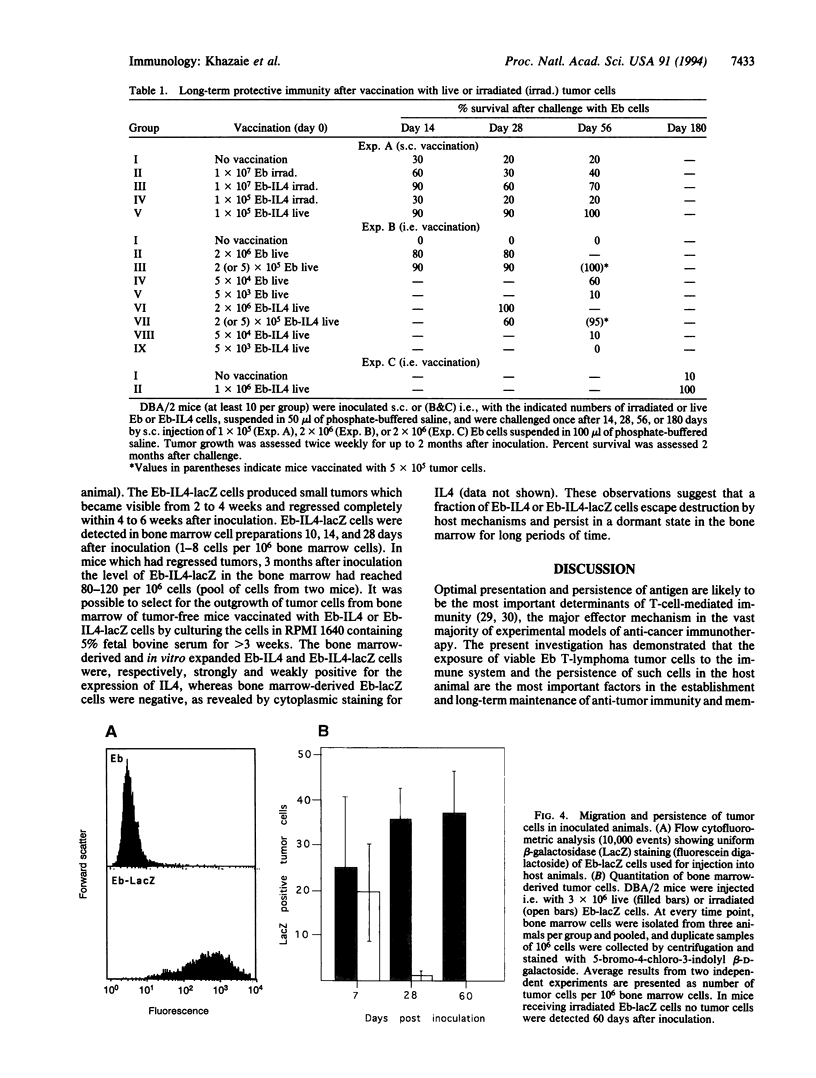
Live proliferation-competent and irradiated proliferation-incompetent L5178 murine lymphoma cells (Eb cell line) were compared for their potency to induce systemic anti-tumor immunity in syngeneic DBA/2 mice. The tumorigenic potential in vivo of live Eb cells was suppressed through local secretion of interleukin 4 (IL4) or alternatively by injection of parental cells at a site refractory to tumor growth. Inoculation of nontumorigenic doses of live Eb or Eb-IL4 cells led to long-lasting specific and systemic T-cell-mediated antitumor response requiring both CD4+ and CD8+ T lymphocytes. Irradiated cells offered only limited short-term protection, which could be marginally improved by IL4. The more effective protection offered by vaccination with live tumor cells correlated with rapid migration and persistence of tumor cells in the bone marrow of host animals after tumor cell inoculation. In contrast, irradiated Eb-lacZ cells had a short persistence. Tumor cells recovered from the bone marrow of host animals injected with live Eb-IL4 cells still expressed IL4. These observations indicate that in the course of vaccination with live Eb or Eb-IL4 cells, a fraction of these cells escaped destruction by host mechanisms and persisted in a dormant state in the bone marrow for long periods of time. Persistence of dormant tumor in the bone marrow correlated with the duration of anti-tumor immunity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugger W., Bross K. J., Glatt M., Weber F., Mertelsmann R., Kanz L. Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors. Blood. 1994 Feb 1;83(3):636–640. [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiering S. N., Roederer M., Nolan G. P., Micklem D. R., Parks D. R., Herzenberg L. A. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12(4):291–301. doi: 10.1002/cyto.990120402. [DOI] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Gray D., Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991 Nov 1;174(5):969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H., Dorsch M., Kunzendorf U., Qin Z., Diamantstein T., Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H., Dorsch M., Kunzendorf U., Uberla K., Qin Z., Diamantstein T., Blankenstein T. Vaccinations with tumor cells genetically engineered to produce different cytokines: effectivity not superior to a classical adjuvant. Cancer Res. 1993 Feb 15;53(4):714–716. [PubMed] [Google Scholar]
- Hültner L., Moeller J., Schmitt E., Jäger G., Reisbach G., Ring J., Dörmer P. Thiol-sensitive mast cell lines derived from mouse bone marrow respond to a mast cell growth-enhancing activity different from both IL-3 and IL-4. J Immunol. 1989 May 15;142(10):3440–3446. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lindemann F., Schlimok G., Dirschedl P., Witte J., Riethmüller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992 Sep 19;340(8821):685–689. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oehen S., Waldner H., Kündig T. M., Hengartner H., Zinkernagel R. M. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J Exp Med. 1992 Nov 1;176(5):1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Porgador A., Bannerji R., Watanabe Y., Feldman M., Gilboa E., Eisenbach L. Antimetastatic vaccination of tumor-bearing mice with two types of IFN-gamma gene-inserted tumor cells. J Immunol. 1993 Feb 15;150(4):1458–1470. [PubMed] [Google Scholar]
- Porgador A., Gansbacher B., Bannerji R., Tzehoval E., Gilboa E., Feldman M., Eisenbach L. Anti-metastatic vaccination of tumor-bearing mice with IL-2-gene-inserted tumor cells. Int J Cancer. 1993 Feb 1;53(3):471–477. doi: 10.1002/ijc.2910530320. [DOI] [PubMed] [Google Scholar]
- Russell S. J. Lymphokine gene therapy for cancer. Immunol Today. 1990 Jun;11(6):196–200. doi: 10.1016/0167-5699(90)90081-j. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H. J., Kyewski B., Von Hoegen P., Schirrmacher V. CD4+ helper T cells are required for resistance to a highly metastatic murine tumor. Eur J Immunol. 1987 Dec;17(12):1863–1866. doi: 10.1002/eji.1830171231. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Leidig S., Griesbach A. In situ activation of syngeneic tumour-specific cytotoxic T lymphocytes: intra-pinna immunization followed by restimulation in the peritoneal cavity. Cancer Immunol Immunother. 1991;33(5):299–306. doi: 10.1007/BF01756594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmacher V., Shantz G., Clauer K., Komitowski D., Zimmermann H. P., Lohmann-Matthes M. L. Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. I. Tumor invasiveness in vitro and metastasis formation in vivo. Int J Cancer. 1979 Feb;23(2):233–244. doi: 10.1002/ijc.2910230215. [DOI] [PubMed] [Google Scholar]
- Schlimok G., Funke I., Pantel K., Strobel F., Lindemann F., Witte J., Riethmüller G. Micrometastatic tumour cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer. 1991;27(11):1461–1465. doi: 10.1016/0277-5379(91)90032-9. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Coffman R. L., Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992 Jul 24;257(5069):548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Picker L. J., Scheuermann R. H., Tucker T. F., Vitetta E. S., Uhr J. W. Cancer dormancy: isolation and characterization of dormant lymphoma cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1829–1833. doi: 10.1073/pnas.90.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]