Abstract
Background
Secondhand smoke (SHS) exposure increases cardiovascular disease risk. The objective of this study was to examine the association of SHS exposure in childhood and adulthood with adult arterial thickness.
Methods
The study cohort consisted of 415 nonsmoking adults (301 whites and 114 blacks; ages 26.2-48.0 years) enrolled in 2004-2010. The arterial wall thickness was measured as common, bulb and internal carotid artery intima-media thickness (IMT). SHS exposure data in childhood and adulthood were obtained by a questionnaire survey.
Results
Increased adult composite carotid IMT was significantly associated with SHS exposure (regression coefficient, β=53.1 μm, p<0.001) after adjusting for race, age, gender, education, income, body mass index, systolic blood pressure, LDL cholesterol and triglycerides/HDL cholesterol ratio, with blacks (β=81.2 μm, p=0.005) and whites (β=38.9 μm, p=0.017) showing the same direction of the association. Furthermore, the SHS exposure in childhood showed a relatively stronger association with increased carotid IMT than the exposure in adulthood based on standardized βs (0.180 vs 0.106); the same trend in the difference between childhood and adulthood exposure was noted for duration of SHS exposure (0.186 vs 0.145). The covariates-adjusted composite carotid IMT showed a significant increasing trend by the order of exposure status of none, adulthood only, childhood only and both (p for trend<0.001).
Conclusions
If the relationship is causal, the associations observed in this study suggest that more awareness should be raised on the dangers of SHS exposure during childhood so that its effect may be mitigated and controlled early in the cardiovascular disease process.
Introduction
Secondhand smoke (SHS) is associated with cardiovascular (C-V) disease by ∼30% increase in the risk among nonsmokers (1-5). Epidemiologic studies have shown that SHS exposure accelerates atherosclerotic lesions and causes C-V disease through multiple mechanisms, including endothelial dysfunction, alterations in arterial wall elasticity and thickness, traditional C-V risk factors, oxidative stress and inflammation (5-11). In addition to active smoking, the passive smoking resulting from environmental tobacco exposure represents an important public health concern and a major threat for C-V health of nonsmokers (1-5,12).
C-V disease is now well recognized to begin early in life (13). The concept of “childhood origins” of C-V disease is supported by numerous publications from four large-scale population-based cohorts followed since childhood (Bogalusa, Muscatine, Finland and Australia) which are now collaborating as the International Childhood Cardiovascular Cohort consortium (13). Furthermore, the C-V, endocrine, and immune systems in children may be more vulnerable to damage as a result of SHS exposure (14-16). Studies have shown that SHS exposure is associated with multiple C-V risk factors and subclinical changes in structure and function of the C-V system in the pediatric population (16-19). Whether the influence of SHS exposure of the critical periods in childhood persists into adulthood in relation to C-V risk is not clear. The objective of the present study was to examine the association of SHS exposure from childhood to adulthood with adult arterial wall thickness utilizing a cohort from the Bogalusa Heart Study.
Methods
Study Cohort
The Bogalusa Heart Study is a biracial (65% white and 35% black) community-based long-term investigation of the early natural history of C-V disease beginning in childhood since 1973. During the 2004-2010 survey in the Bogalusa Heart Study, 1016 adult subjects were examined for carotid artery intima-media thickness (IMT) and C-V risk factors. By excluding 380 current and former smokers, SHS exposure questionnaires were mailed out to 636 nonsmokers in 2014, and 415 questionnaires were received with a response rate of 65.3%. The current study cohort consisted of 415 nonsmoking adults (301 whites and 114 blacks; ages 26.2-48.0 years) who responded to the SHS questionnaire survey. Characteristics of the study variables were compared between 415 responders and 221 non-responders of the SHS questionnaire survey. Adult subjects included in the current analysis vs those who were not included had more females (65.3% vs 51.0%, p<0.001), higher education levels (p=0.001) and higher income (p=0.024). After adjusting for race, sex, age education and income, C-V risk variables and carotid IMT did not differ significantly between the two groups.
All subjects in this study gave informed consent for the surveys. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
General Examination
Replicate measurements of height and weight were made, and the mean values were used for analysis. Body mass index (BMI, weight in kilograms divided by the square of the height in meters) was used as a measure of obesity. Blood pressure (BP) was measured using a mercury sphygmomanometer between 8:00 AM and 10:00 AM on the right arm of subjects in a relaxed, sitting position by two trained observers (three replicates each). The mean values of the six readings were used for analysis.
Information on indoor SHS exposure and duration was retrospectively collected in a mailing questionnaire survey on adult nonsmokers (excluding current and former smokers) in 2014. The questionnaire was modified from the one that was developed by WHO International Agency for Research on Cancer (20), including status and years of SHS exposure in childhood (0-18 years) and adulthood (19 years-date). The indoor SHS exposure during childhood was defined as father, mother and/or others smoking indoor and the number of years exposed; the indoor SHS exposure during adulthood was defined as any persons who lived together smoking indoor and the number of years exposed. Smokers were defined as smoking at least one cigarette per day during the past 12 months.
Laboratory Analysis
Serum lipoprotein cholesterols and triglycerides were analyzed by using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN). The laboratory is monitored for precision and accuracy by the Lipid Standardization Program of the Centers for Disease Control and Prevention, Atlanta, GA.
Carotid Ultrasonography
Carotid ultrasound measurements were done on a Toshiba digital ultrasound instrument (Xario, SSA-660A, Toshiba America Medical Systems, Tustin CA), using a 7.5 MHz linear array transducer. Images were recorded at the common carotid, carotid bulb (bifurcation), and internal carotid arteries bilaterally according to previously developed protocols for the Atherosclerosis Risk in Communities study (21). The maximum carotid IMT readings of left and right far walls were averaged for each segment. Then the averaged carotid IMT of common, bulb, and internal segments was calculated as composite carotid IMT. Common carotid IMT, carotid bulb IMT, internal carotid IMT and the composite carotid IMT were used for analyses. Re-screenees (n=46) were re-measured for reproducibility analysis. The correlation coefficients for repeat scans were 0.72 for the common carotid, 0.69 for the carotid bulb, and 0.80 for the internal carotid.
Statistical Methods
Analyses of covariance were performed using generalized linear models (GLM) to test differences in study variables between SHS-exposed and not exposed groups and calculate covariate-adjusted least square means of carotid IMT. There were 24 hypertensives and 9 dyslipidemia subjects who took medications at the time of examination. Their BP and lipid variable values were replaced with the corresponding mean values of the remaining hypertensives and dyslipidemia subjects who did not take medications.
The association analyses of SHS exposure status and duration with carotid IMT were performed using multiple linear regression models, adjusted for race, age, gender, BMI, systolic BP, LDL cholesterol and triglycerides/HDL cholesterol ratio. The SHS exposure status was included in separate regression models as “yes/no” or in subgroups of “exposed in childhood only”, “exposed in adulthood only” and “exposed in both” with “not exposed” as a reference; years of SHS exposure in childhood, adulthood and both was included as a continuous variable in the analysis models. The differences in the association parameters of SHS exposure with carotid IMT between race groups were examined in interaction regression models (homogeneity-of-slopes models), adjusted for covariates. All data analyses were performed using Statistical Analysis System (SAS) version 9.1.
Results
Table 1 summarizes the study variables by race and SHS exposure status. There were significantly more males in the “not exposed” group among whites and in the total sample. All other C-V risk variables did not differ significantly between SHS exposure groups in whites, blacks and in the total sample, except a higher BMI in the SHS exposure group than in the non-exposure group among whites (p=0.034). Nonsmokers exposed to SHS had significantly greater values of segmental and composite carotid IMT than those not exposed in blacks, whites and the total sample except common carotid IMT in whites.
Table 1. Means (SD) of study variables by race and SHS exposure status.
| Whites | Blacks | Total | ||||
|---|---|---|---|---|---|---|
| Not Exposed (n=96) | Exposed (n=205) | Not Exposed (n=42) | Exposed (n=72) | Not Exposed (n=138) | Exposed (n=277) | |
| Males (n, %) | 49 (51.0%) | 70 (34.2%)b | 10 (23.8%) | 15 (20.8%) | 59 (42.8%) | 85 (30.7%)a |
| Age (year) | 36.6 (4.5) | 36.2 (4.2) | 35.6 (4.6) | 36.3 (4.7) | 36.3 (4.5) | 36.2 (4.3) |
| Education (n, %) | ||||||
| Elementary school | 0 (0.0%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (1.4%) |
| Middle school | 1 (1.0%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | 2 (0.7%) |
| High school | 32 (33.3%) | 77 (37.6%) | 32 (76.2%) | 42 (58.3%) | 64 (46.4%) | 119 (43.0%) |
| College and above | 63 (65.6%) | 122 (59.5%) | 10 (23.8%) | 30 (41.7%) | 73 (52.9%) | 152 (54.9%) |
| Income/year (n, %) | ||||||
| <=$15000 | 6 (6.3%) | 12 (5.9%) | 21 (50.0%) | 33 (45.8%) | 27 (19.6%) | 45 (16.3%) |
| $15001-$30000 | 8 (8.3%) | 37 (18.1%) | 10 (23.8%) | 20 (27.8%) | 18 (13.0%) | 57 (20.6%) |
| $30001-$45000 | 18 (18.8%) | 28 (13.7%) | 3 (7.1%) | 8 (11.1%) | 21 (15.2%) | 36 (13.0%) |
| >$45000 | 64 (66.7%) | 128 (62.4%) | 8 (19.1%) | 11 (15.3%) | 72 (52.2%) | 139 (50.2%) |
| BMI (kg/m2) | 27.6 (5.6) | 29.2 (6.5)* | 30.5 (6.8) | 30.7 (7.7) | 28.5 (6.1) | 29.6 (6.9) |
| Systolic BP (mmHg) | 114.5 (12.7) | 112.4 (10.4) | 119.5 (19.3) | 123 (17.5) | 116 (15.1) | 115.1 (13.5) |
| LDL-C (mg/dL) | 124.2 (32.7) | 128.3 (34.6) | 116.8 (34.8) | 125.1 (25.7) | 121.9 (33.4) | 127.5 (32.5) |
| TG/HDL-C | 3.4 (3.4) | 3.1 (2.7) | 2.0 (1.4) | 2.2 (3.2) | 3.0 (3.0) | 2.8 (2.8) |
| Common IMT (μm) | 726 (112) | 727 (122) | 754 (130) | 813 (118)* | 735 (118) | 749 (127)* |
| Bulb IMT (μm) | 895 (160) | 922 (201)* | 914 (193) | 998 (421)* | 901 (170) | 955 (280)** |
| Internal IMT (μm) | 677 (171) | 726 (296)* | 675 (144) | 776 (230)* | 677 (163) | 739 (281)** |
| Composite IMT (μm) | 766 (106) | 792 (158)* | 781 (122) | 879 (195)** | 771 (111) | 814 (172)** |
SHS=secondhand smoke; BMI=body mass index; BP=blood pressure; L(H)DL-C=low(high)-density lipoprotein cholesterol; TG=triglycerides; IMT=intima-media thickness
p<0.05;
p<0.01 by Chi-square tests
Difference in continuous variables between SHS exposure and non-exposure groups was tested in GLM models, adjusted for sex, age, education, income and/or race;
p<0.05;
p<0.01
Among the 415 study subjects, there were 277 (66.7%) subjects who were exposed to SHS; the prevalence of the exposure in these 277 individuals was 48.7% (n=135) in childhood only, 16.2% (n=45) in adulthood only and 35.0% (n=97) in both childhood and adulthood. The average duration of the SHS exposure in these 277 individuals was 13.9 years (range=2-18 years) in childhood only, 6.0 years (range=1-26 years) in adulthood only and 19.9 years (range=5-46 years) in both childhood and adulthood. The prevalence and duration of SHS exposure did not differ significantly between blacks and whites.
Table 2 shows study variables by SHS exposure status in the total sample. There were more males (p=0.033) and more subjects who had higher family income (p<0.001) in the “not exposed” group. The “exposed in adulthood only” group had significantly high BMI than the “not exposed” group. Compared with the “not exposed” group, the “exposed in childhood only” and “exposed in both” groups had significantly high common, bulb, internal and composite carotid IMT except for common carotid IMT in the “exposed in childhood only” group. Segmental and composite carotid IMT did not differ significantly between the “not exposed” and “exposed in adulthood only” groups.
Table 2. Means (SD) of study variables by SHS exposure status.
| Not Exposed (n=138) | Exposed in Adulthood only (n=45) | Exposed in childhood only (n=135) | Exposed in Both (n=97) | |
|---|---|---|---|---|
| Whites (n, %) | 96 (69.6) | 31 (68.9) | 106 (78.5) | 68 (70.1) |
| Males (n, %)a | 59 (42.8) | 11 (24.4) | 48 (35.6) | 26 (26.8) |
| Age (year) | 36.3 (4.5) | 36.9 (4.6) | 36.7 (4.0) | 35.2 (4.3) |
| Education (n, %) | ||||
| Elementary school | 0 | 1 (2.2) | 0 | 3 (3.1) |
| Middle school | 1 (0.7) | 0 | 2 (1.5) | 0 |
| High school | 64 (46.4) | 15 (33.3) | 54 (40.0) | 50 (51.5) |
| College and above | 73 (52.9) | 29 (64.4) | 79 (58.5) | 44 (45.4) |
| Income/year (n, %)b | ||||
| <=$15000 | 27 (19.6) | 2 (4.4) | 16 (11.9) | 27 (27.8) |
| $15001-$30000 | 18 (13.0) | 17 (37.8) | 18 (13.3) | 22 (22.7) |
| $30001-$45000 | 21 (15.2) | 5 (11.1) | 24 (17.8) | 7 (7.2) |
| >$45000 | 72 (52.2) | 21 (46. 7) | 77 (57.0) | 41 (42.3) |
| BMI (kg/m2) | 28.5 (6.1) | 31.3 (9.0)** | 28.8 (6.1) | 29.8 (6.7) |
| Systolic BP (mmHg) | 116.0 (15.1) | 115.7 (13.2) | 113.4 (11.9) | 117.3 (15.4) |
| LDL-C (mg/dL) | 121.9 (33.4) | 121.8 (35.2) | 128.6 (31.3) | 128.5 (32.9) |
| TG/HDL-C | 3.0 (3.0) | 2.8 (2.0) | 2.9 (2.9) | 2.7 (3.1) |
| Common IMT (μm) | 735 (118) | 754 (122) | 749 (128) | 747 (129)* |
| Bulb IMT (μm) | 901 (170) | 916 (204) | 964 (275)** | 960 (318)** |
| Internal IMT (μm) | 677 (163) | 703 (135.2) | 728 (220)** | 772 (386)** |
| Composite IMT (μm) | 771 (111) | 791 (128) | 814 (161)** | 826 (202)** |
SHS=secondhand smoke; BMI=body mass index; BP=blood pressure; L(H)DL-C=low(high)-density lipoprotein cholesterol; TG=triglycerides; IMT=intima-media thickness
p<0.05;
p<0.01 by Chi-square tests
Difference in continuous variables between three SHS exposure groups and the non-exposure group was tested in GLM models, adjusted for sex, age, education, income and/or race;
P<0.05;
P<0.01
Table 3 presents regression coefficients (β) of SHS exposure for composite carotid IMT with adjustment for age, gender, education, income, BMI, systolic BP, LDL-C, triglycerides/HDL-C ratio and/or race. While the covariates showed different association patterns with carotid IMT between race groups, SHS exposure (yes/no) had a consistent, positive and significant association with carotid IMT in blacks and whites. Blacks and whites had the same direction in the association parameters (p=0.168 for difference in regression coefficients between races). In the total sample, all the covariates except for education, income and BMI were associated with carotid IMT. SHS exposure (yes/no) showed significant associations with increased carotid IMT. In separate regression models with SHS exposure duration included as a predictor variable, adjusting for covariates mentioned above, the regression coefficients of years of SHS exposure in blacks (β=0.9 μm, p=0.402) and whites (β=1.6 μm, p=0.011) did not differ significantly (p=0.311 for race-SHS exposure duration interaction). One year SHS exposure was associated with 1.5 μm increase in carotid IMT (p=0.009) in the total sample.
Table 3. Linear regression coefficients of SHS exposure (independent variable) and covariates for composite carotid artery IMT (dependent variable).
| Independent variable | Whites | Blacks | Total | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| Black race | --- | --- | --- | --- | 69.5 (32.7, 106.3) | <0.001 |
| Female gender | -52.1 (-84.4, -19.8) | 0.002 | -4.8 (-74.2, 64.6) | 0.892 | -46.5 (-76.9, -16.1) | 0.003 |
| Age | 8.2 (4.7, 11.7) | <0.001 | 11.5 (5.6, 17.4) | <0.001 | 8.8 (5.7, 11.9) | <0.001 |
| Education | -14.5 (-43.7, 14.7) | 0.331 | -5.2 (-65.4, 55.0) | 0.865 | -10.2 (-36.9, 16.5) | 0.453 |
| Income | 8.9 (-9.5, 27.3) | 0.348 | -10.4 (-36.9, 16.1) | 0.444 | 0.8 (-14.3, 15.9) | 0.921 |
| BMI | 1.1 (-1.6, 3.8) | 0.411 | -1.3 (-5.0, 2.4) | 0.492 | 0.2 (-2.0, 2.4) | 0.827 |
| Systolic BP | 2.5 (0.9, 4.1) | 0.002 | 0.4 (-1.2, 2.0) | 0.587 | 1.7 (0.5, 2.9) | 0.002 |
| LDL-C | 0.6 (0.2, 1.0) | 0.008 | 0.1 (-0.9, 1.1) | 0.853 | 0.4 (0.01, 0.8) | 0.048 |
| TG/HDL-C | 1.3, (-4.2, 6.8) | 0.638 | 33.7 (23.1, 44.3) | <0.001 | 9.5 (4.6, 14.4) | <0.001 |
| SHS exposurea | 38.9 (7.0, 70.8) | 0.017 | 81.2 (25.7, 136.7) | 0.005 | 53.1 (25.1, 81.1) | <0.001 |
SHS exposure was coded as: 0=no exposure, 1=exposure in childhood, adulthood or both
SHS=secondhand smoke; IMT=intima-media thickness (μm); BMI=body mass index; BP=blood pressure; L(H)DL-C=low- (high-) density lipoprotein cholesterol; TG=triglycerides
β (95% CI) = unstandardized regression coefficients (95% confidence interval)
Table 4 shows unstandardized (β) and standardized regression coefficients (β′) of SHS exposure (yes/no and years) for composite carotid IMT in the total sample, with adjustment for race, age, gender, education, income, BMI, systolic BP, LDL-C and triglycerides/HDL-C ratio. The regression analyses were performed in three separate models with SHS exposure in childhood only, adulthood only or both as independent variables. SHS exposure (yes/no) in childhood only and the exposure in both childhood and adulthood showed significant associations with increased carotid IMT; however, the SHS exposure (yes/no) in adulthood only was not significantly associated with carotid IMT. The duration of SHS exposure was significantly associated with increased carotid IMT in all three models with SHS exposure during childhood only, adulthood only and both. Of note, the SHS exposure (yes/no) in childhood was relatively more strongly associated with increased carotid IMT than the exposure in adulthood based on standardized regression coefficients (β′=0.180 vs 0.106); the same trend in the difference between childhood and adulthood was noted for duration of SHS exposure (β′=0.186 vs 0.145).
Table 4. Linear regression coefficients of SHS exposure in childhood, adulthood and both (independent variable) for composite carotid artery IMT (dependent variable) in the total sample, adjusting for covariatesa.
| Independent Variable | Yes/No | Duration in Years | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | β′ | R2 | β (95% CI) | β′ | R2 | |
| Model 1: SHS exposure in childhood only | 25.0** (10.1, 39.9) | 0.180** | 2.9% | 3.2** (1.4, 5.0) | 0.186** | 2.9% |
| Model 2: SHS exposure in adulthood only | 28.3 (-6.8, 63.4) | 0.106 | 1.0% | 1.5* (0.1, 2.9) | 0.145* | 1.9% |
| Model 3: SHS exposure in both childhood and adulthood | 24.9** (12.6, 37.2) | 0.235** | 3.1% | 2.1** (0.7, 3.5) | 0.181** | 1.9% |
Race, sex, age, education, income, BMI, systolic BP, LDL-C and TG/HDL-C ratio were included in the models as covariates for adjustment.
SHS=secondhand smoke; IMT= intima-media thickness (μm); BMI=body mass index; BP= blood pressure; L(H)DL-C=low-(high-) density lipoprotein cholesterol; TG=triglycerides
β (95% CI) = unstandardized regression coefficients (95% confidence interval); β′ = standardized regression coefficients; R2 =variance of IMT explained by the independent variable
Regression coefficients different from 0:
p<0.05;
p<0.01
The associations between SHS exposure (yes/no) and segmental carotid IMT at individual sites were examined in separate regression models by race as well as in the total sample with adjustment for the covariates mentioned above. SHS exposure showed positive associations with common carotid IMT in whites (β=12.4, p=0.370), blacks (β=49.3, p=0.023) and the total sample (β=21.7, p=0.055), with bulb carotid IMT in whites (β=44.7, p=0.042), blacks (β=110.2, p=0.044) and the total sample (β=67.8, p=0.005), with internal carotid IMT in whites (β=59.8, p=0.068), blacks (β=84.0, p=0.043) and the total sample (β=69.7, p=0.007).
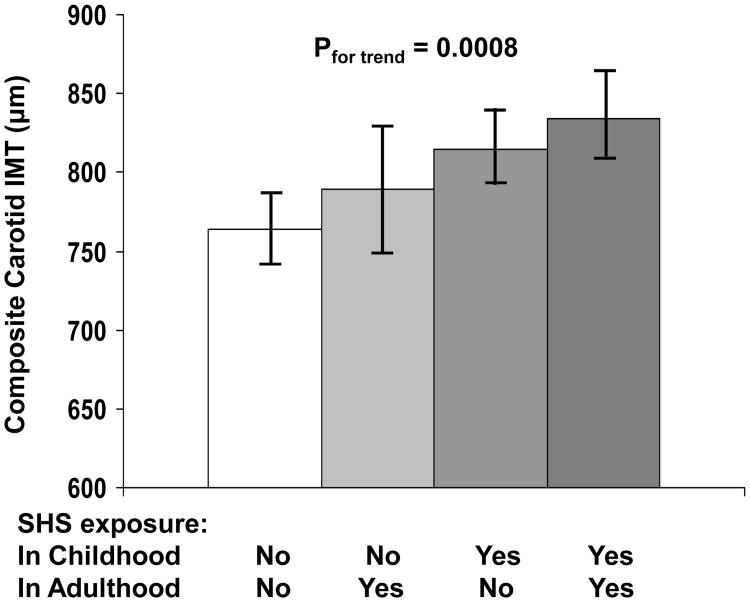
Figure 1 illustrates race-, age-, gender-, education-, income-, BMI-, systolic BP-, LDL cholesterol- and HDL cholesterol/triglycerides ratio-adjusted means (95% CIs) of composite carotid IMT in nonsmoking adults by SHS exposure status in childhood and adulthood. The covariates-adjusted composite carotid IMT showed a significant increasing trend by the order of SHS exposure status of none, adulthood only, childhood only and both (p<0.001 for trend).
Figure 1.
Race-, age-, gender-, education-, income-, body mass index-, systolic blood pressure-, LDL cholesterol- and HDL cholesterol/triglycerides ratio-adjusted means (95% confidence intervals) of composite carotid IMT in nonsmoking adults by SHS exposure status in childhood and adulthood
L(H)DL-C=low(high)-density lipoprotein cholesterol; IMT=intima-media thickness; SHS=secondhand smoke
As a sensitivity analysis, estimated glomerular filtration rate (eGFR), uric acid, insulin resistance index were included in the regression models as shown in Table 3 as additional covariates in a subset of 326 subjects of the study cohort. These three variables were not associated with carotid IMT in either whites (p=0.338-0.963), blacks (p=0.156-0.604) or the total sample (p=0.160-0.476). The SHS exposure-IMT association parameters did not change substantially in whites (β=37.9, p=0.025), blacks (β=85.9, p=0.004) and the total sample (β=53.0, p<0.001).
Discussion
There has been almost uniform agreement that both active and passive smoking is an established risk factor for C-V morbidity and mortality (1-5). Epidemiologic studies have shown that SHS exposure accelerates atherosclerotic lesions and causes C-V disease through multiple mechanisms, including detrimental effects on traditional C-V risk factors, oxidative stress, inflammation, endothelial dysfunction, structural and functional alterations of the C-V system (5-10). Longitudinal data from the Atherosclerosis Risk in Communities (ARIC) study have shown a significant relationship between SHS exposure and greater progression of atherosclerosis measured as carotid IMT among 10,914 adults. After adjustment for demographic characteristics, C-V risk factors and lifestyle variables, an increase of 5.9 μm in the progression rates over a 3-year period due to exposure to SHS was observed among nonsmokers (9). A small sample of 570 nonsmoking adults from the ARIC study cohort has shown that both early and current exposure to SHS was associated with increased carotid IMT (22). Carotid IMT was 24 μm higher in the group of 77 adults who were exposed to SHS 12-14 years earlier than the non-exposure group of 211 adults (709 vs 685 μm). The difference in carotid IMT was 46 μm between the group of 282 adults who were currently exposed to SHS and the non-exposure group of 211 adults (731 vs 685 μm). The current study found that carotid IMT was 53 μm higher in nonsmoking adults who were exposed to SHS than in those who were not exposed after controlling for covariates. Furthermore, the duration of the exposure was also associated with greater carotid IMT, and childhood exposure showed a relatively stronger association in this cohort. The findings from this study provide suggestive evidence for the detrimental influence of SHS exposure on subclinical atherosclerosis.
There is now substantial evidence to confirm that the roots of C-V disease extend back into childhood (13). Obesity and other C-V risk factors beginning as early as in childhood are related to carotid IMT in adulthood as shown in our previous studies (23) and the International Childhood Cardiovascular Cohort consortium (13,24). Questions have been raised, however, about whether exposure to SHS in childhood has relevance to adult C-V disease risk. This research area is important because the toxicokinetic parameters of exposure, absorption, metabolism, distribution, and target organ susceptibility are different in children and adults (14). Children are vulnerable to the effects of SHS due to smaller airways and higher respiration rates and may have greater levels of SHS inhalation exposure per unit of body weight than adults (15). Furthermore, physiologically immature children may be more vulnerable to damage as a result of SHS exposure because of their partially developed or compromised C-V, endocrine, and immune systems (16). Previous epidemiologic studies have shown that SHS exposure is associated with high BP, dyslipidemia, oxidative stress, endothelial dysfunction and arterial wall elasticity and thickness in the pediatric population (16-19). Whether the influence of SHS exposure of this critical period in childhood persists into adulthood is not clear. In the current study, the SHS exposure in childhood only showed a relatively stronger association with increased adult carotid IMT than the exposure in adulthood only based on standardized regression coefficients (0.180 vs 0.106); the same trend in the difference was noted for duration of SHS exposure in childhood versus adulthood (0.186 vs 0.145). The findings in this regard suggest that the SHS exposure might have a long-term harmful influence of on subclinical atherosclerosis.
Smoking bans in public places have been increasing in the United States during the past decade. A CDC report on state laws restricting smoking in effect as of December 31, 2010 showed that the number of states with laws that prohibit smoking in indoor areas of worksites, restaurants, and bars increased from zero in 2000 to 26 in 2010 (25). Data from a series of National Health and Nutrition Examination Surveys (NHANES) measuring serum cotinine as an index of SHS exposure over a period of 14 years from 1988 through 2002 documented a substantial decline of approximately 70% in serum cotinine concentrations in nonsmokers during this period (26). In the present study, the percentage of SHS exposure in childhood (32.5%) during the 1970s-1990s was three times the percentage in adulthood (10.8%), indicating that the awareness and attitudes towards the harmful effect of SHS have been substantially improved during recent years. Given that the heavier exposure to SHS occurred 3-4 decades ago and the exposure exerted a significant and adverse influence on adult carotid IMT, how to mitigate this long-term detrimental influence is a important research topic in preventing C-V disease among individuals who were exposed to SHS in early life during the past few decades.
This community-based epidemiologic study has certain limitations. First, the relatively small sample size, especially blacks, had a limited statistical power to detect weak-to-moderate association between SHS exposure and subclinical atherosclerosis measures. Second, childhood socioeconomic data were not available, and adulthood eGFR, uric acid and insulin resistance data were available only in a subset of subjects in the current study. Further investigation is needed to confirm the results in this regard. Third, the retrospectively collected information on SHS exposure and the cross sectional nature of carotid IMT data have a limitation in causal inference in this regard.
In summary, the current study demonstrates an association of SHS exposure status and the length of exposure from childhood to adulthood with carotid artery IMT in middle-aged black and white nonsmoking adults. If the relationship is causal, the associations observed in this study suggest that more awareness should be raised on the dangers of SHS exposure during childhood so that its effect may be mitigated and controlled early in the cardiovascular disease process. These findings underscore the importance of a comprehensive health promotion program to mitigate the adverse impact of SHS exposure on the C-V system. Extensive research on the underlying mechanisms and how to reduce the long-term detrimental influence of the SHS exposure has implications in undertaking preventive strategies for C-V disease among individuals who were already exposed to SHS in early life during the past few decades.
Secondhand smoke (SHS) exposure and adult arterial wall thickness were examined.
Lifetime SHS exposure was associated with increased carotid artery wall thickness.
The SHS exposure in childhood showed a relatively stronger association.
Acknowledgments
This study was supported by grants 5R01ES021724 from National Institute of Environmental Health Science, 2R01AG016592 from the National Institute on Aging and 13SDG14650068 from American Heart Association. Shengxu Li is a scholar of the Building Interdisciplinary Research in Women's Health program, supported by award number K12HD043451 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Abbreviations
- C-V
cardiovascular
- SHS
secondhand smoke
- IMT
intima-media thickness
- BMI
body mass index
- BP
blood pressure
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- TG
triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine. Secondhand smoke exposure and cardiovascular effects: making sense of the evidence. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 2.Glantz SA, Parmley WW. Passive smoking and heart disease: epidemiology, physiology, and biochemistry. Circulation. 1991;83:1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]
- 3.He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease - a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340:920–926. doi: 10.1056/NEJM199903253401204. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. Available at http://www.surgeongeneral.gov/library/secondhandsmoke/report. [Google Scholar]
- 5.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 6.Glantz S, Parmley W. Passive smoking and heart disease: mechanisms and risk. JAMA. 1995;273:1047–1053. [PubMed] [Google Scholar]
- 7.Bonetti PO, Lardi E, Geissmann C, Kuhn MU, Brüesch H, Reinhart WH. Effect of brief secondhand smoke exposure on endothelial function and circulating markers of inflammation. Atherosclerosis. 2011;215:218–222. doi: 10.1016/j.atherosclerosis.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Peinemann F, Moebus S, Dragano N, Möhlenkamp S, Lehmann N, Zeeb H, Erbel R, Jöckel KH, Hoffmann B Heinz Nixdorf Recall Study Investigative Group. Secondhand smoke exposure and coronary artery calcification among nonsmoking participants of a population-based cohort. Environ Health Perspect. 2011;119:1556–1561. doi: 10.1289/ehp.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5:276–292. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- 11.Mack WJ, Islam T, Lee Z, Selzer RH, Hodis HN. Environmental tobacco smoke and carotid arterial stiffness. Prev Med. 2003;37:148–154. doi: 10.1016/s0091-7435(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 12.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988-2002. Environ Health Perspect. 2006;114:853–8. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer T, Magnussen CG, Sun C, Raitakari OT, Schork NJ, Venn A, Burns TL, Juonala M, Steinberger J, Sinaiko AR, Prineas RJ, Davis PH, Woo JG, Morrison JA, Daniels SR, Chen W, Xu J, Srinivasan SR, Viikari JSA, Berenson GS. Cohort Profile: The International Childhood Cardiovascular Cohort (i3C) Consortium. Int J Epidemiol. 2013;42:86–96. doi: 10.1093/ije/dys004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bearer CF. How Are Children Different from Adults? Environ Health Perspect. 1995;103(Suppl 6):7–12. doi: 10.1289/ehp.95103s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook DG, Strachan DP. Parental smoking, bronchial reactivity and peak flow variability in children. Thorax. 1998;53:295–301. doi: 10.1136/thx.53.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metsios GS, Flouris AD, Angioi M, Koutedakis Y. Passive smoking and the development of cardiovascular disease in children: a systematic review. Cardiol Res Pract. 2010;2011:1–6. doi: 10.4061/2011/587650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzman M, Cook S, Auinger P, Florin TA, Daniels S, Nguyen M, Winickoff JP. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112:862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 18.Kallio K, Jokinen E, Saarinen M, Hämäläinen M, Volanen I, Kaitosaari T, Rönnemaa T, Viikari J, Raitakari OT, Simell O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010;3:196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
- 19.Kallio K, Jokinen E, Raitakari OT, Hämäläinen M, Siltala M, Volanen I, Kaitosaari T, Viikari J, Rönnemaa T, Simell O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–3212. doi: 10.1161/CIRCULATIONAHA.106.674804. [DOI] [PubMed] [Google Scholar]
- 20.WHO International Agency for Research on Cancer (IARC) European multicenter case-control study of lung cancer in non-smoker: Detailed results on exposure to environmental tobacco smoke, IARC Technical Report No 33. Lyon: 1998. [Google Scholar]
- 21.Bond MG, Barnes RW, Wiley WA, Wilmoth SK, Chambless LE, Howard G. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. The ARIC Study Group. J Neuroimmaging. 1991;1:168–172. [PubMed] [Google Scholar]
- 22.Diez-Roux AV, Nieto FJ, Comstock GW, Howard G, Szklo M. The relationship of active and passive smoking to carotid atherosclerosis 12-14 years later. Prev Med. 1995;24:48–55. doi: 10.1006/pmed.1995.1007. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in Adulthood: The Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 24.Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kähönen M, Laitinen T, Taittonen L, Berenson GS, Viikari JS, Raitakari OT. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122:2514–2520. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 25.Tynan M, Babb S, MacNeil A, Griffin M. State smoke-free laws for worksites, restaurants, and bars - United States, 2000-2010. MMWR. 2011;60:472–475. [PubMed] [Google Scholar]
- 26.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988-2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]