Abstract
The serologic hallmark of primary biliary cirrhosis (PBC) is the presence of high titer, and specific anti-mitochondrial antibodies (AMA). Interestingly, although there is no global immune defect in patients with PBC, there is widespread dysregulated B cell function, including increased sera levels of IgM and enhanced B cell responses to CpG stimulation. The mechanisms involved in this B cell dysfunction have remained unknown. To address this issue, we focused on identifying the frequencies of B cell subsets in patients with PBC and the mechanisms that lead to B cell dysregulation, including the relationships with CXCR5+CD4+T cells. Herein we report that elevations of both serum and intrahepatic IL-21 were found in patients with PBC and, in particular, promoted B cell proliferation, STAT3 phosphorylation and AMA production in vitro. More importantly, upon stimulation with rPDC-E2, CXCR5+CD4+T cells in PBC produced higher levels of IL-21 than healthy controls. Additionally, sorted CXCR5+CD4+T cells increased production of AMA by autologous CD19+B cells. Indeed, elevated expression of intrahepatic CXCL13, a key chemokine of CXCR5+ cells, was uniquely found within the portal tracts in PBC, accompanied by infiltrates of CD4+, CXCR5+, CD19+, and CD38+ cells. In conclusion, CXCL13 promotes aggregation of CD19+B cells and CXCR5+CD4+T cells, which directs the aberrant AMA response via IL-21. These data have implications for potential immunotherapy and also reflect the unique lymphoid biology in the liver of PBC.
Keywords: Primary biliary cirrhosis, anti-mitochondrial antibodies, B cells, follicular helper T cells, interleukin-21, CXCL13
Introduction
Although there is a selective failure of immune tolerance to specific mitochondrial autoantigens in primary biliary cirrhosis (PBC), and systemic evidence of B cell dysregulation,1,2 there is a paucity of data that provide a mechanistic explanation of these abnormalities of B cell immune responses. Clearly, there are multiple factors that regulate B cell homeostasis, including activation of B cell receptors, interaction with multiple co-receptors, and interactions with the local cytokine microenvironment.3 The lymphoid microenvironment is particularly critical for B cell proliferation and differentiation as well as fulfilling a requirement for establishment of an enduring immune response. Interleukin-21 (IL-21) in particular is predominantly produced by follicular T helper cells (Tfh), a critical subset of CD4+T cells that arises from hot spots within the lymph node germinal centers (GCs), and is characterized by the presence of B cell lymphoma 6 (Bcl-6), molecular surface expression of chemokine (C-X-C motif) receptor 5 (CXCR5), inducible co-stimulator (ICOS), and programmed cell death 1 (PD-1).4 Identifying the mechanisms that lead to the disordered but polarized immune responses in PBC has significant potential for understanding breach of tolerance and potentially its restoration.
The immune effector mechanisms in PBC that lead to selective destruction of intrahepatic bile ducts are composed of a multi-orchestrated lymphoid response which includes both adaptive and innate immunity.1,2,5,6 Indeed, at various stages of disease, different lymphoid elements may play specific roles. However, in any case, chemokines will direct the homing of such cells. As an example, by acting through its cognate receptor CXCR5, chemokine (C-X-C motif) ligand 13 (CXCL13) is known to dictate homing and motility of both B cells and Tfh cells.7 However, little is known about the contribution of CXCL13 in lymphocytes homing and in sustaining dysregulated B cell responses in PBC. To address these issues, we took advantage of a large cohort of patients with PBC to characterize not only their B cell subsets, but also potential regulatory factors that are associated with aberrant B cell expansion and anti-mitochondrial antibodies (AMA) production. We report herein that over expression of intrahepatic CXCL13 serves as a key regulator for CXCR5+ cells and is critically involved in trafficking of B cells and CXCR5+CD4+T cells. We submit that these key elements not only provide a mechanism able to explain B cell dysregulation, but also provide a platform for potential immunotherapy of PBC.
Materials and Methods
Patients and Samples
Sixty-six patients with PBC, including 25 patients who participated in a clinical trial of ursodeoxycholic acid (UDCA, 13–15mg/kg/day) therapy were studied here. In addition, 52 healthy individuals (HC) and 41 patients with chronic hepatitis B (CHB) with high levels of total bilirubin (TB >20 μmol/L) were enrolled as controls. Ultrasound-guided needle biopsy specimens of liver from 5 treatment-naïve PBC patients and liver tissues from 3 end-stage PBC patients were collected. As controls, 14 portions of healthy liver were obtained coincidentally following surgery for hepatic hemangioma or benign hepatocellular adenoma. Liver tissues were also obtained from 10 patients with CHB. The diagnosis of PBC were based on established criteria.8 All subjects were recruited at Nanfang Hospital (Guangzhou, China) or Xijing Hospital (Xi’an, China) (Supplementary Table 1). PBC patients using corticosteroids, immunosuppressive agents, and those suffering from either extrahepatic biliary obstruction, co-infection with hepatitis B virus or hepatitis C virus, hepatocellular carcinoma, or an unrelated autoimmune disease were excluded. Informed consent was obtained from all subjects. The study protocol was approved by the ethical committee of Nanfang Hospital and Xijing Hospital. Twenty-five PBC patients from the clinical trial of UDCA therapy were studied longitudinally from entry through week 24. Subjects were classified based on their biochemical response and were considered to be UDCA responsive, coined UR, if they achieved all of the following criteria: alkaline phosphatase (ALP) level <3 × ULN (the upper limit of normal), aspartate aminotransferase (AST) level <2 × ULN, and normal TB level. Patients were considered to be non-UDCA responsive, coined NUR, if they were unable to achieve all of the above criteria9 (Supplementary Table 2).
Phenotype Analysis and Intracellular Cytokine Staining (ICS)
Peripheral blood mononuclear cells (PBMCs) and liver infiltrating lymphocytes were isolated as described.10 PBMCs and liver-derived lymphocytes were stained with fluorescence mAbs at 4°C for 30 minutes. For ICS of CXCR5+CD4+T cells, PBMCs were stimulated with rPDC-E2 (5μg/mL; PeproTech) or control for 72 hours at 37°C. BFA was added for the last 5 hours of culture. Phenotyping was performed with CD4-PECy7 and CXCR5-PerCP-Cy5.5 mAbs, fixed, permeabilized, and stained intracellularly with IL-21-PE, IL-17-FITC, and IFN-γ-APC mAbs. For ICS of CD19+B cells, PBMCs were incubated with CD40L (1μg/mL; PeproTech) and CpG (10μg/mL; Invivogen) for 48 hours at 37°C. BFA was added for the last 5 hours along with phorbol-12-myristate-13-acetate/ionomycin. After CD19-PerCP mAb, intracellular cytokines were stained with IL-6-PE, IL-10-FITC, IFN-γ-APC, and TNF-α-PECy7 mAbs. Data were analyzed on a BD FACSCanto II flow cytometer.
Enzyme-Linked Immunosorbent Assay (ELISA)
Titers of AMA in culture supernatants, serum concentrations of IL-4, IL-6, IL-10, IL-21 (eBioscience), and CXCL13 (R&D Systems) were quantitated by ELISA in duplicate according to the manufacturer’s instructions.
Proliferation Assay
Purified CD19+Bcells were labeled with carboxyfluorescein succinimidyl ester (CFSE, 1.5μM; Molecular Probes) and resuspended at 106 cells/mL. Labeled cells were cultured with rIL-4, rIL-6, rIL-10 or rIL-21 (50ng/mL) respectively for 7 days, or with medium only as a control. The proliferation rate of CD19+B cells was expressed as the percentage of cells that diluted the CFSE intensity at least once at the time of harvest.
In Vitro AMA Production Assay
T cells (CXCR5+CD4+, CXCR5−CD4+) and CD19+B cells were sorted by BD influx cell sorter. Purified CD19+B cells (1×105 cells/well) were co-cultured with CXCR5+CD4+T cells or CXCR5−CD4+T cells (1×105 cells/well) respectively following stimulation with either rPDC-E2 or rHCV protein in 96-well U bottom plates for 12 days. Additionally, purified CD19+B cells alone (2×105 cells/well) were incubated with rIL-4, rIL-6, rIL-10 or rIL-21 (50ng/mL) respectively for 12 days, or with medium only as a control. Supernatants were collected for detection of AMA.
Expression of Phosphorylated STAT3
Purified CD19+B cells were stimulated with medium alone or with rIL-4, rIL-6, rIL-10 or rIL-21 (50ng/mL, respectively) for 30 minutes at 37°C, then fixed by Lyse/Fix Buffer (BD Phosflow™) for 20 minutes at 4°C. Cells were permeabilized using ice-cold Perm Buffer III (BD Phosflow™) for 30 minutes, and stained intracellularly with mAb against phospho-STAT3 (pY705) and expression determined by flow cytometry.
Quantitative PCR Analysis
Total liver RNA was isolated using an RNA Extraction Kit (Macherey-Nagel). Complementary DNA synthesis was performed using a Transcriptor cDNA Kit (Roche). Expression of CXCL13 and IL-21 were determined using predesigned QuantiTect Primer Assays (Qiagen). Quantitative PCR was conducted with the Roche LightCycler®480 system and relative mRNA expression was normalized to GAPDH and calculated using the 2−ΔΔCT method.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed and paraffin-embedded 4-μM tissue. Sections were incubated overnight at 4°C with appropriate concentrations of primary antibodies, including rabbit anti-CD4, mouse anti-CD19, rabbit anti-CD38, mouse anti-CD68, mouse anti-CXCR5, rabbit anti-IL-21, and goat anti-CXCL13, and then incubated with the Dako Chemate Envision Kit. The reaction was visualized by CheMate™ DAB plus chromogen and the number of CXCL13 positive cells in portal tracts was independently counted by two blinded observers based on four high-power microscopic fields (400×).
Statistical Analysis
Data were expressed as median (interquartile range) or median (range). The Mann-Whitney U test and the Wilcoxin signed-rank test were used when two groups were compared. The Kruskal-Wallis H test was used when more than two groups were compared. Correlations between variables were assessed with Spearman’s rank-order correlation coefficient. All analyses were two-tailed and values with P<0.05 were considered statistically significant.
Results
Expanded Circulating CD19+B Cells and Dysregulated B Cell Subsets in Patients with PBC
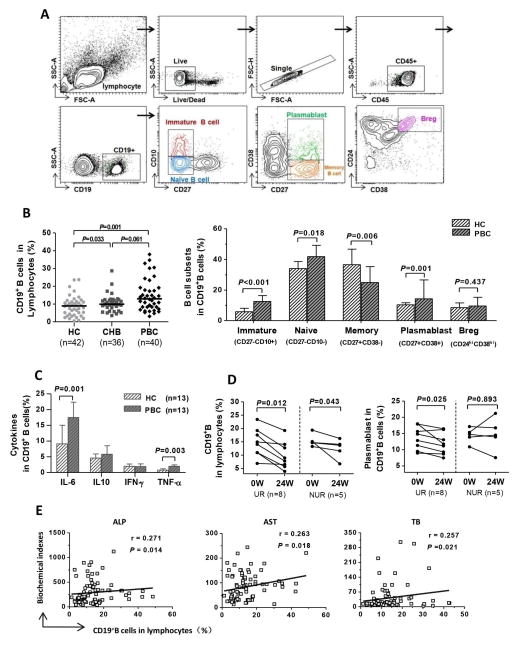
There was a significantly higher frequency of CD19+B cells in treatment-naïve PBC patients compared to HC, while no significant difference was found between PBC and CHB (P=0.001 and P=0.061; Figure 1A,B). Additionally, there was a significant increase in the percentage of immature and naïve B cells (P<0.001 and P=0.018, respectively) as well as plasmablasts (P=0.001) in PBC. In contrast, there was a significant decrease of memory B cells in PBC compared to HC (P=0.006; Figure 1B) and no significant differences in the percentage of regulatory B cells identified as the CD19+CD24highCD38high subset (Figure 1B). Compared with HC, PBC patients exhibited a significantly higher frequency of IL-6 and TNF-α-producing CD19+B cells under co-stimulation conditions with CD40L and CpG (P=0.001 and P=0.003, respectively; Figure 1C). Furthermore, in the longitudinal study, the percentage of CD19+B cells significantly decreased in both the UR (P=0.012) and the NUR group (P=0.043) during UDCA therapy (Figure 1D). There was also a strikingly significant decrease in the percentage of plasmablasts observed but this was limited to patients with UR (P=0.025; Figure 1D). Finally, we noted that the frequency of CD19+B cells positively correlated with serum biochemical indexes, including ALP, AST, and TB in PBC (P=0.014, P=0.018, and P=0.021, respectively; Figure 1E).
Figure 1. B cell phenotype in PBC.
(A) Gating strategies. (B) Comparison of the frequency of circulating total CD19+B cells and subsets of CD19+B cell between HC, CHB, and PBC. (C) Comparison of the frequencies of IL-6, IL-10, IFN-γ, and TNF-α-producing CD19+B cells following co-stimulation with CD40L and CpG in PBC and HC. (D) Serial changes of circulating total CD19+B cells and the plasmablast subset in the UR and NUR groups following UDCA treatment. (E) Correlation of biochemical indexes (ALP, AST, and TB) and frequency of CD19+B cells.
High Levels of IL-21 Enhance CD19+B Cell Proliferation and AMA Production in Patients with PBC
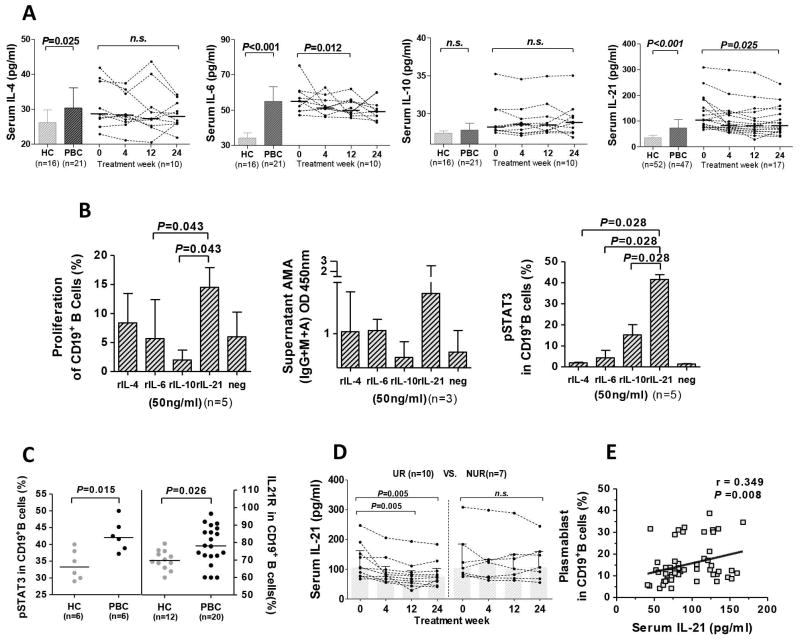
In the cross-sectional cohort, serum levels of IL-4, IL-6, and IL-21 were significantly higher in treatment-naïve PBC patients compared to HC (P=0.025, P<0.001, and P<0.001, respectively; Figure 2A). Following UDCA therapy, the levels of serum IL-6 and IL-21 gradually decreased from baseline (Figure 2A). Upon stimulation of PBC B cells with rIL-21, there was a significant enhancement of B cell proliferation and AMA production (Figure 2B). Notably, compared to control cytokines, IL-21 induced the highest levels of phosphorylated STAT3 (Figure 2B). Additionally, PBC patients manifested higher levels of phosphorylated STAT3 than HC (P=0.015; Figure 2C). Interestingly, there was a significant increase of IL-21R expression in CD19+B cells in PBC compared to HC (P=0.026; Figure 2C). In the longitudinal cohort, serum IL-21 levels significantly decreased in the UR group, but not the NUR group (P=0.005; Figure 2D). Furthermore, serum IL-21 levels significantly correlated with the percentage of plasmablasts in PBC (r=0.349, P=0.008; Figure 2E). Collectively, these data suggest that IL-21 acts as a critical element in the humoral immune response dysregulation in PBC.
Figure 2. IL-21 and CD19+B cell proliferation and AMA production.
(A) Serum levels of IL-4, IL-6, IL-10, and IL-21 in cross-sectional cohort and serial changes following UDCA treatment. (B) Proliferation, AMA production and STAT3 phosphorylation (pSTAT3) of purified CD19+B cells following stimulation with rIL-4, rIL-6, rIL-10, or rIL-21. (C) Comparison of pSTAT3 and IL-21Rexpression of CD19+B cells in HC and PBC. (D) Serial changes of serum IL-21 levels in the UR and NUR groups following UDCA treatment. (E) Correlation of serum IL-21 and frequency of the plasmablast subset.
Expanded Circulating CXCR5+CD4+T cells Secrete High Levels of IL-21 and Promote CD19+B Cells to Produce AMA
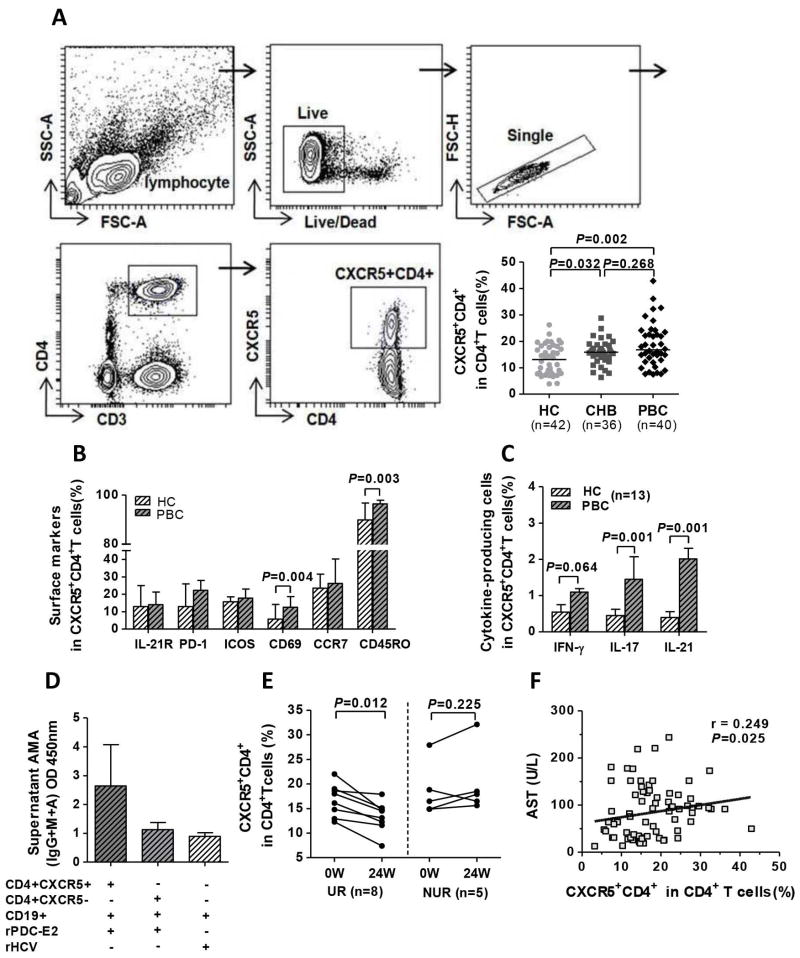
There was a significant increase of CXCR5+CD4+T cells in treatment-naïve PBC compared to HC (P=0.002; Figure 3A), but the difference between PBC and CHB was not significant (P=0.268; Figure 3A); CXCR5+CD4+T cells in PBC had a significantly higher expression of CD69 and CD45RO compared to HC (P=0.004 and P=0.003, respectively; Figure 3B). Additionally, following stimulation with rPDC-E2, CXCR5+CD4+T cells from PBC produced higher levels of IL-21 (P=0.001) and IL-17 (P=0.001) compared to HC (Figure 3C). Notably, sorted CXCR5+CD4+T cells from PBC promoted autologous CD19+B cells to produce higher titers of AMA compared with CXCR5−CD4+T cells (Figure 3D). In the longitudinal study, the frequency of CXCR5+CD4+T cells significantly decreased in the UR (P=0.012) but not in the NUR group (P=0.225; Figure 3E). Interestingly, similar data were also observed in quantifying the plasmablast subset and serum IL-21. Moreover, the frequency of CXCR5+CD4+T cells positively correlated with level of serum AST in PBC (r=0.249, P=0.025; Figure 3F).These data suggest that the expanded and activated CXCR5+CD4+T cells, via IL-21, promote B cells to produce anti-PDC-E2 autoantibodies.
Figure 3. Circulating CXCR5+CD4+T cells facilitate AMA production.
(A) Gating strategies and comparison of the frequencies of circulating CXCR5+CD4+T cells between HC, CHB, and PBC. (B) Phenotyping of CXCR5+CD4+T cells in PBC and HC. (C) Cytokine production of CXCR5+CD4+T cells following stimulation with rPDC-E2. (D) Supernatant AMA titers in the co-culture assay of T cells (CXCR5+CD4+ or CXCR5−CD4+) and autologous CD19+B cells in PBC (n=3) in the presence of rPDC-E2 or rHCV. (E) Serial changes in the frequency of CXCR5+CD4+T cells in the UR and NUR groups. (F) Correlation of the frequency of CXCR5+CD4+T cells and AST in PBC.
CXCR5+CD4+T cells and CD19+B Cells are Enriched in Livers of PBC and Accompanied by an Increase of Intrahepatic IL-21 Expression
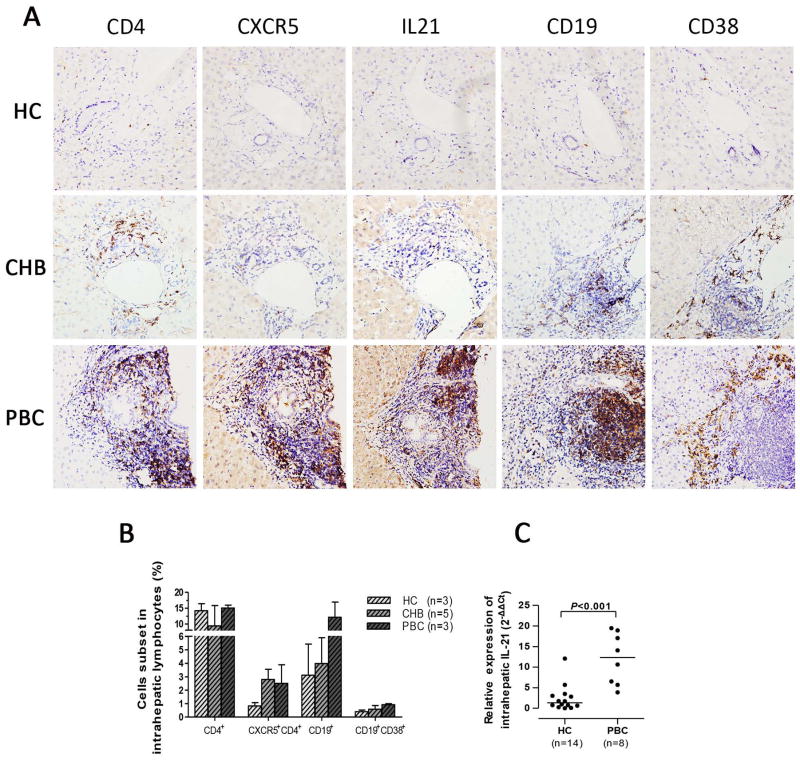
Immunohistochemical staining reflected an accumulation of CD4+, CXCR5+, CD19+, and CD38+ cells in portal tracts of PBC but not in HC and CHB (Figure 4A). FACS analysis of isolated liver lymphocytes further demonstrated an increased frequency of CXCR5+CD4+T cells, CD19+B cells, and CD19+CD38+ B cells in end-stage PBC relative to HC (Figure 4B). Since IL-21 is a critical component of T cell-directed B cell activation, proliferation, and plasma cell differentiation, the transcriptional level of intrahepatic IL-21 was measured. As anticipated, the mRNA levels of IL-21 were significantly higher in PBC compared with HC (P<0.001; Figure 4C). Moreover, enhanced expression of IL-21 was observed in portal tracts of PBC (Figure 4A).
Figure 4. Enrichment of CXCR5+CD4+T cell and CD19+B cells in PBC liver.
(A) Immunohistochemical staining of CD4+, CXCR5+, IL-21+, CD19+, and CD38+ cells in liver from HC, CHB, and PBC. Magnification ×400. (B) Frequency of intrahepatic CD4+T, CXCR5+CD4+T, CD19+B, and CD19+CD38+B cells in end-stage PBC following liver transplantation compared to healthy donors and CHB. (C) Quantitative PCR of intrahepatic IL-21 in HC and PBC. Expression levels are based relative to GAPDH. Horizontal bars reflect the median.
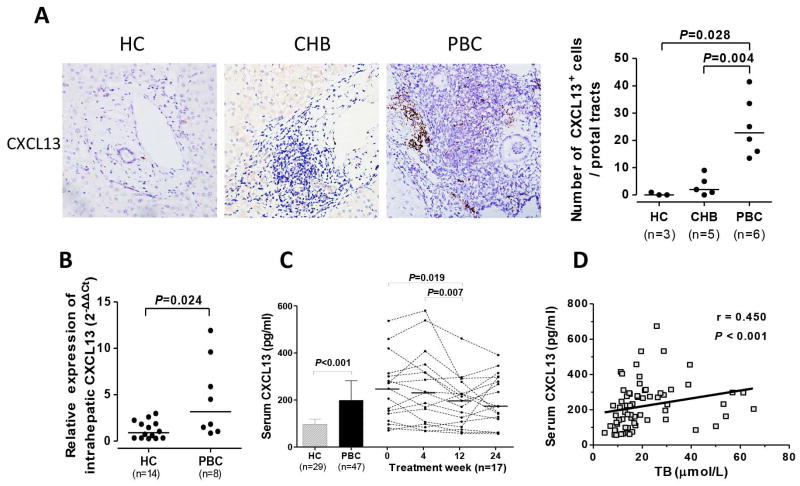
High-level Expression of Intrahepatic CXCL13 Promotes the Recruitment of CXCR5+ Lymphocytes to the Liver in Patients with PBC
CXCL13 is known to dictate homing and motility of CXCR5+ lymphocytes, including CD19+B cells and CD4+T cells (Supplementary Figure 1A). In situ staining revealed significantly higher expression of CXCL13 within the portal tracts in PBC than HC and CHB (P=0.028 and P=0.004; Figure 5A). Notably, there was a significantly higher mRNA expression of intrahepatic CXCL13 in PBC relative to HC (P=0.024; Figure 5B). We further noted that the levels of serum CXCL13 were significantly higher in treatment-naïve PBC compared to HC (P<0.001), but gradually decreased during the longitudinal phase of UDCA treatment (Figure 5C). Moreover, level of serum CXCL13 positively correlated with the TB level (r=0.450, P<0.001; Figure 5D). These data suggest that CXCL13 serves as a critical regulator for the recruitment of CXCR5+ lymphocytes.
Figure 5. Over-expression of intrahepatic CXCL13 in PBC.
(A) Immunohistochemical staining of CXCL13 in liver of HC, CHB, and PBC, including comparison of CXCL13+ cells in portal tracts. Magnification ×400. (B) Quantitative PCR of intrahepatic CXCL13 in HC and PBC. Expression levels are based relative to GAPDH. Horizontal bars reflect median. (C) Serum levels of CXCL13 in HC and PBC and serial changes following treatment with UDCA. (D) Correlation of serum levels of CXCL13 and TB in PBC.
Discussion
The goals of this study were to perform a qualitative and quantitative assessment of B cell subsets in PBC and the immunological network involved in its regulation by utilization of both cross-sectional and longitudinal cohorts of patients. Our data reveal an expanded population of CD19+B cells and an increased frequency of circulating plasmablasts. More importantly, we demonstrate that the over-expression of intrahepatic CXCL13 meditates infiltration of B cells and CXCR5+CD4+T cells surrounding the targeted intrahepatic biliary ductules of PBC. Furthermore, increased production of IL-21 appears to be a major mechanism through which CXCR5+CD4+T cells mediates differentiation of B cells into antibody-secreting plasmablasts, aberrant B cell expansion and AMA production.
Our data suggest that CXCL13 is a key regulator for the dedicated homing of CXCR5+ lymphocytes, with implications for the unique B cell and CXCR5+CD4+T cell biology of PBC. Further, our data on IL-21 provide additional support for the requirement of an ongoing adaptive immune response for the perpetuation of abnormal humoral responses in PBC. PBC has been theorized as a multi-hit disease with different key immune pathways at different stages of disease, including both the requirement of innate immunity for initiation, and both innate and adaptive immunity for the continued inflammatory responses.5,11,12 Our data on IL-21 would suggest that there is potential for modulating the adaptive response by altering production and/or reducing IL-21.
The presence of AMAs in PBC are not only a serologic marker, but recent data have implicated that AMAs as a contributing factor for the destruction of cholangiocytes.12,13 Hence the presence of continuously activated B cells is of particular importance. We note that the data herein reflect an expanded total CD19+B cell population is different from earlier work which reported no significant differences in total B cells between PBC and HC.2,14 We suggest that these differences may be related to stage and duration of disease. Nonetheless, our data are similar in demonstrating a striking increase in frequency of circulating PDC-E2-specific plasmablasts in PBC.2 Furthermore, we also report an increased frequency of a circulating plasmablast subset which has the potential for autoantibody production. Interestingly, our data demonstrate a decreased frequency of plasmablasts in UDCA-treated patients and suggest that differences in earlier work on B cell populations might be related to the duration of UDCA treatment. Previous work has also noted that the titers of serum AMA significantly correlate with the distinct coronal arrangement of CD38+B cells surrounding the intrahepatic bile ducts in PBC.1 Our work notes the enrichment of intrahepatic CD19+CD38+B cells and the accumulation of CD38+cells in the portal tract. Unlike the intrahepatic findings of others,1 we did not find a correlation between the frequency of circulating plasmablasts and titers of AMA and so we propose that these differences are secondary to duration of UDCA treatment in the various patient population groups. Clearly, however, there is a close relationship between intrahepatic infiltrating CD38+ plasmablasts and titers of AMA.
GCs within lymphoid follicles are the critical site for the generation of T cell-dependent antibody responses mediated by the interaction between resident B cells and Tfh cells. Human CXCR5+CD4+T cells share functional properties with GC Tfh cells.15 Similar to recent data on such CXCR5+CD4+T cells in PBC,6 our data reflect an enhanced potential of this subset in supporting production of AMA when cultured with autologous B cells. In other words, our data imply the existence of PDC-E2-specific CXCR5+CD4+T cells. In addition to the multiple cytokines involved in inflammation, IL-21 is critical for regulation of B cell expansion and differentiation into plasmablasts. A general feature of cytokines is their ability to activate intermediate signaling pathways, including the JAK-STAT pathway in B cells,16 and it is established that STAT3-dependent signaling is critical for generating high-affinity antigen-specific antibody.17 Based on known IL-21 biology and its ability to promote STAT3 phosphorylation, and our own data on AMA production, we propose that CXCR5+CD4+T cell-derived IL-21 is a major contributor that mediates aberrant B cell responses through STAT3 signaling in PBC.
Historically B cells were thought to be involved only in autoantibody production, but the role of B cells in inflammation in both producing and responding to cytokines, and serving as antigen presenting cells, is becoming increasingly important in autoimmunity.18,19 Notably, the proinflammatory cytokine IL-6 has pivotal roles in generation of IL-21-secreting Tfh cells and formation of GCs in vivo,20,21 and recent data support the necessity and sufficiency of B cell-derived IL-6 in Tfh cell development or expansion.22 In PBC, selective down-regulation of B cell dysregulatory responses may have an importance far beyond that of suppressing autoantibodies. To accomplish this, one would need therapeutic agents that are more selective than drugs like anti-CD20. Interestingly, there is a novel subset of regulatory B cells known as B10 cells.23 We suggest that focus on this subset and the ability to more rigorously define B cell subsets will be important if one is to develop a selective treatment for PBC.
Our data also indicate a significant increase in mRNA levels of intrahepatic CXCL13 with intense portal tract staining, explaining partly the specific recruitment and trafficking of CXCR5+ lymphocytes in PBC. Additionally, CXCL13 has a unique role in GC organization during T cell-dependent responses.24 Increased expression of CXCL13/CXCR5 participates in ectopic lymphoid follicle formation in both autoimmune and chronic inflammatory diseases.25,26 Histologic examination of damaged bile ducts in PBC frequently reveals lymphoid follicle-like structures,1,6 and this feature may be secondary to CXCL13-induced recruitment of CXCR5+ lymphocytes. CXCL13 is known to be primarily produced in secondary lymphoid tissues. However, monocytes/macrophages also produce CXCL13, particularly in inflammatory sites wherein lymphoid neogenesis occurs.27 These observations are interesting based on our own data that reflect diffuse staining of intrahepatic CD68+ cells in patients with PBC (Supplementary Figure 1B). We also note that intrahepatic CD68+ macrophages from healthy donors are capable of producing CXCL13 ex vivo when stimulated with poly I:C, a toll-like receptor 3 agonist (Zhang XY. et al, unpublished data). Indeed, previous data has demonstrated beneficial effect of neutralization of CXCL13 in collagen-induced arthritis.28 Thus we propose that immunotherapeutic approaches that target pathogenic chemokine, cytokine or their cognate receptors may potentially treat PBC. Clearly, however, further studies are necessary to focus and identify the relationships of our data to patients with PBC at different stages of disease activity and, in particular, to determine whether such abnormalities play a primary versus a secondary role in pathogenesis.
The effector mechanisms of PBC are mutlifaceted and dependent on genetic background, disease duration and the kinetics of progression. It is possible that more than one therapy will be required for a given patient and that such therapy may be individually determined based on the clinical characteristics of a given patient. We submit that data such as that presented herein will help focus on these issues, including the identification of not only potential therapies, but also the therapeutic windows.
Supplementary Material
(A) Schema reflecting the distribution of CXCR5+cell subsets in PBMC. Note that CXCR5 is primarily expressed on CD4+ and CD19+cells. (B) Immunohistochemical staining of CD68+cells in liver from HC, CHB, and PBC. Magnification ×400.
Acknowledgments
Financial Support: This work was supported by the Major Science and Technology Special Project of China (2012ZX10002-003), the National Natural Science Foundation of China (81470836), and the National Institutes of Health (DK39588).
We thank Prof. Yu Zhang from Peking University Health Science Center for helpful comments on this manuscript.
List of Abbreviations
- ALP
alkaline phosphatase
- AMA
anti-mitochondrial antibodies
- AST
aspartate aminotransferase
- Bcl-6
B-cell lymphoma 6
- BFA
brefeldin A
- CFSE
carboxyfluorescein succinimidyl ester
- CHB
chronic hepatitis B
- CXCL13
chemokine (C-X-C motif) ligand 13
- CXCR5
chemokine (C-X-C motif) receptor 5
- ELISA
enzyme-linked immunosorbent assay
- GC
germinal center
- HC
healthy controls
- ICOS
inducible co-stimulator
- ICS
intracellular cytokine staining
- IL-21
interleukin-21
- NUR
non-UDCA biochemical response
- PBC
primary biliary cirrhosis
- PBMC
peripheral blood mononuclear cells
- PDC-E2
E2 subunit of the pyruvate dehydrogenase complex
- PD-1
programmed cell death 1
- rHCV
recombinant HCV core protein
- STAT
signal transducers and activator of transcription
- TB
total bilirubin
- Tfh
follicular helper T cells
- UDCA
ursodeoxycholic acid
- ULN
upper limit of normal
- UR
UDCA biochemical response
Footnotes
Conflict of Interest: All authors have no conflicts of interest to declare.
Author names in bold designate shared co-first authorship.
Contributor Information
Yongyin Li, Email: yongyinli@foxmail.com.
Weibin Wang, Email: weibinwang1988@foxmail.com.
Libo Tang, Email: tanglibosmu@foxmail.com.
Xuanqiu He, Email: lmsh815@163.com.
Xin Yan, Email: 948345899@qq.com.
Xiaoyong Zhang, Email: xiaoyong.zhang@msn.com.
Youfu Zhu, Email: zhuyf0118@gmail.com.
Jian Sun, Email: doctorsunjian@qq.com.
Yongquan Shi, Email: shiyquan@fmmu.edu.cn.
Xiong Ma, Email: maxiongmd@hotmail.com.
Ian R. Mackay, Email: ian.mackay@monash.edu.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
Ying Han, Email: hanying@fmmu.edu.cn.
Jinlin Hou, Email: jlhousmu@163.com.
References
- 1.Takahashi T, Miura T, Nakamura J, Yamada S, Miura T, Yanagi M, et al. Plasma cells and the chronic nonsuppurative destructive cholangitis of primary biliary cirrhosis. Hepatology. 2012;55:846–855. doi: 10.1002/hep.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Zhang W, Leung PS, Bowlus CL, Dhaliwal S, Coppel RL, et al. Ongoing activation of autoantigen-specific B cells in primary biliary cirrhosis. Hepatology. 2014;60:1708–1716. doi: 10.1002/hep.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desjardins M, Mazer BD. B-cell memory and primary immune deficiencies: interleukin-21 related defects. Curr Opin Allergy Clin Immunol. 2013;13:639–645. doi: 10.1097/ACI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 4.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, et al. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Sun Y, Zhang Z, Jia Y, Zou Z, Ding J, et al. CXCR5 CD4 T follicular helper cells participate in the pathogenesis of primary biliary cirrhosis. Hepatology. 2014 doi: 10.1002/hep.27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 8.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Tu Z, Bozorgzadeh A, Crispe IN, Orloff MS. The activation state of human intrahepatic lymphocytes. Clin Exp Immunol. 2007;149:186–193. doi: 10.1111/j.1365-2249.2007.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishibashi H, Nakamura M, Shimoda S, Gershwin ME. T cell immunity and primary biliary cirrhosis. Autoimmun Rev. 2003;2:19–24. doi: 10.1016/s1568-9972(02)00122-2. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura S, Van De Water J, Leung P, Odin JA, Yamamoto K, Gores GJ, et al. Caspase induction by IgA antimitochondrial antibody: IgA-mediated biliary injury in primary biliary cirrhosis. Hepatology. 2004;39:1415–1422. doi: 10.1002/hep.20175. [DOI] [PubMed] [Google Scholar]
- 13.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Bayona B, Ramos-Amaya A, Lopez-Blanco R, Campos-Caro A, Brieva JA. STAT-3 activation by differential cytokines is critical for human in vivo-generated plasma cell survival and Ig secretion. J Immunol. 2013;191:4996–5004. doi: 10.4049/jimmunol.1301559. [DOI] [PubMed] [Google Scholar]
- 18.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luu VP, Vazquez MI, Zlotnik A. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity. 2014;47:1–12. doi: 10.3109/08916934.2013.856006. [DOI] [PubMed] [Google Scholar]
- 20.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 21.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 25.Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–371. doi: 10.1136/gut.51.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corsiero E, Bombardieri M, Manzo A, Bugatti S, Uguccioni M, Pitzalis C. Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol Lett. 2012;145:62–67. doi: 10.1016/j.imlet.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 28.Zheng B, Ozen Z, Zhang X, De Silva S, Marinova E, Guo L, et al. CXCL13 neutralization reduces the severity of collagen-induced arthritis. Arthritis Rheum. 2005;52:620–626. doi: 10.1002/art.20768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schema reflecting the distribution of CXCR5+cell subsets in PBMC. Note that CXCR5 is primarily expressed on CD4+ and CD19+cells. (B) Immunohistochemical staining of CD68+cells in liver from HC, CHB, and PBC. Magnification ×400.