Abstract
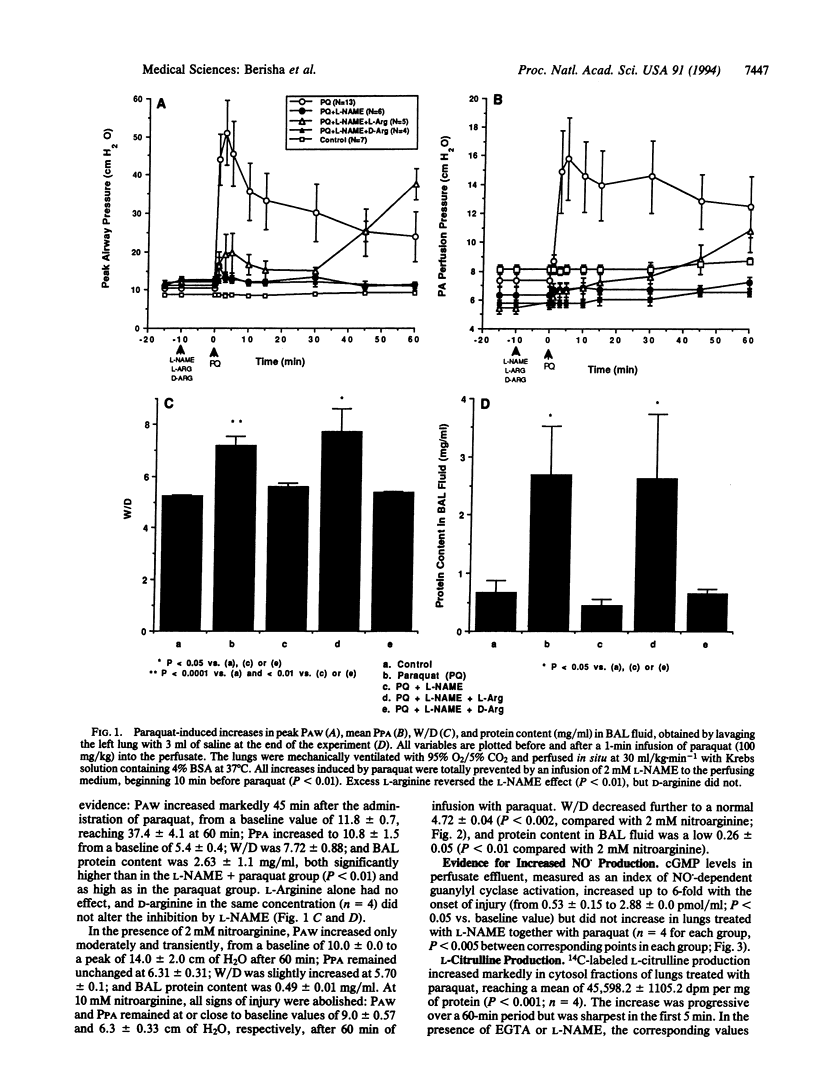
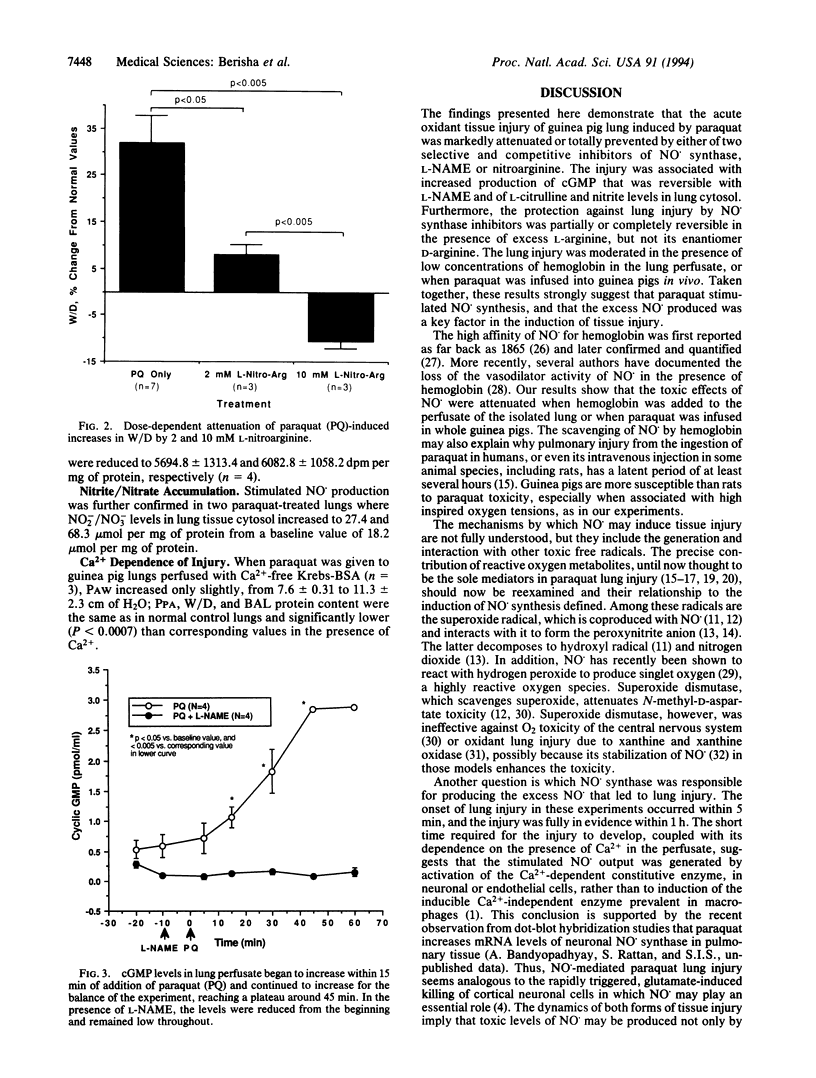
At low concentrations, nitric oxide is a physiological transmitter, but in excessive concentrations it may cause cell and tissue injury. We report that in acute oxidant injury induced by the herbicide paraquat in isolated guinea pig lungs, nitric oxide synthesis was markedly stimulated, as evidenced by increased levels of cyclic GMP in lung perfusate and of nitrite and L-citrulline production in lung tissue. All signs of injury, including increased airway and perfusion pressures, pulmonary edema, and protein leakage into the airspaces, were dose-dependently attenuated or totally prevented by either NG-nitro-L-arginine methyl ester or N omega-nitro-L-arginine, selective and competitive inhibitors of nitric oxide synthase. Protection was reversed by excess L-arginine but not by its enantiomer D-arginine. When blood was added to the lung perfusate, the paraquat injury was moderated or delayed as it was when paraquat was given to anesthetized guinea pigs. The rapid onset of injury and its failure to occur in the absence of Ca2+ suggest that constitutive rather than inducible nitric oxide synthase was responsible for the stimulated nitric oxide synthesis. The findings indicate that nitric oxide plays a critical role in the production of lung tissue injury due to paraquat, and it may be a pathogenetic factor in other forms of oxidant tissue injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisha H., Foda H., Sakakibara H., Trotz M., Pakbaz H., Said S. I. Vasoactive intestinal peptide prevents lung injury due to xanthine/xanthine oxidase. Am J Physiol. 1990 Aug;259(2 Pt 1):L151–L155. doi: 10.1152/ajplung.1990.259.2.L151. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Bush P. A., Gonzalez N. E., Ignarro L. J. Biosynthesis of nitric oxide and citrulline from L-arginine by constitutive nitric oxide synthase present in rabbit corpus cavernosum. Biochem Biophys Res Commun. 1992 Jul 15;186(1):308–314. doi: 10.1016/s0006-291x(05)80808-3. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L., Snyder S. H. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992 Sep;32(3):297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957 May 23;136(3):507–524. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E., Feinstein D. L., Reis D. J. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992 May;13(5):157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H., Moreno J. J., Pryor W. A., Ischiropoulos H., Beckman J. S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992 Nov-Dec;5(6):834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987 May 15;46(7):2402–2406. [PubMed] [Google Scholar]
- Meyer J., Traber L. D., Nelson S., Lentz C. W., Nakazawa H., Herndon D. N., Noda H., Traber D. L. Reversal of hyperdynamic response to continuous endotoxin administration by inhibition of NO synthesis. J Appl Physiol (1985) 1992 Jul;73(1):324–328. doi: 10.1152/jappl.1992.73.1.324. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha-Dutra A. A., Epperlein M. M., Woolf N. Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett. 1993 Apr 19;321(1):59–62. doi: 10.1016/0014-5793(93)80621-z. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993 Nov;16(11):439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Oury T. D., Ho Y. S., Piantadosi C. A., Crapo J. D. Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9715–9719. doi: 10.1073/pnas.89.20.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakbaz H., Foda H. D., Berisha H. I., Trotz M., Said S. I. Paraquat-induced lung injury: prevention by vasoactive intestinal peptide and related peptide helodermin. Am J Physiol. 1993 Oct;265(4 Pt 1):L369–L373. doi: 10.1152/ajplung.1993.265.4.L369. [DOI] [PubMed] [Google Scholar]
- Pou S., Pou W. S., Bredt D. S., Snyder S. H., Rosen G. M. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992 Dec 5;267(34):24173–24176. [PubMed] [Google Scholar]
- Rimar S., Gillis C. N. Selective pulmonary vasodilation by inhaled nitric oxide is due to hemoglobin inactivation. Circulation. 1993 Dec;88(6):2884–2887. doi: 10.1161/01.cir.88.6.2884. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Korbut R., Anggård E., Vane J. R. Lipopolysaccharide increases release of a nitric oxide-like factor from endothelial cells. Eur J Pharmacol. 1989 Nov 14;171(1):135–136. doi: 10.1016/0014-2999(89)90437-8. [DOI] [PubMed] [Google Scholar]
- Sata T., Kubota E., Misra H. P., Mojarad M., Pakbaz H., Said S. I. Paraquat-induced lung injury: prevention by N-tert-butyl-alpha-phenylnitrone, a free-radical spin-trapping agent. Am J Physiol. 1992 Feb;262(2 Pt 1):L147–L152. doi: 10.1152/ajplung.1992.262.2.L147. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Nakane M., Förstermann U., Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992 Apr;41(4):615–624. [PubMed] [Google Scholar]
- Smith R. E., Radomski M. W., Moncada S. Nitric oxide mediates the vascular actions of cytokines in septic shock. Eur J Clin Invest. 1992 Jun;22(6):438–439. doi: 10.1111/j.1365-2362.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Gross S. S., Thiel B. A., Levi R., Nathan C. F. Synthesis of nitrogen oxides from L-arginine by macrophage cytosol: requirement for inducible and constitutive components. Biochem Biophys Res Commun. 1989 Jun 15;161(2):420–426. doi: 10.1016/0006-291x(89)92615-6. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Kasprzak K. S., Maragos C. M., Elespuru R. K., Misra M., Dunams T. M., Cebula T. A., Koch W. H., Andrews A. W., Allen J. S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991 Nov 15;254(5034):1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Vane J. R. Induction of nitric oxide synthase activity by toxic shock syndrome toxin 1 in a macrophage-monocyte cell line. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2051–2055. doi: 10.1073/pnas.89.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]


