Abstract
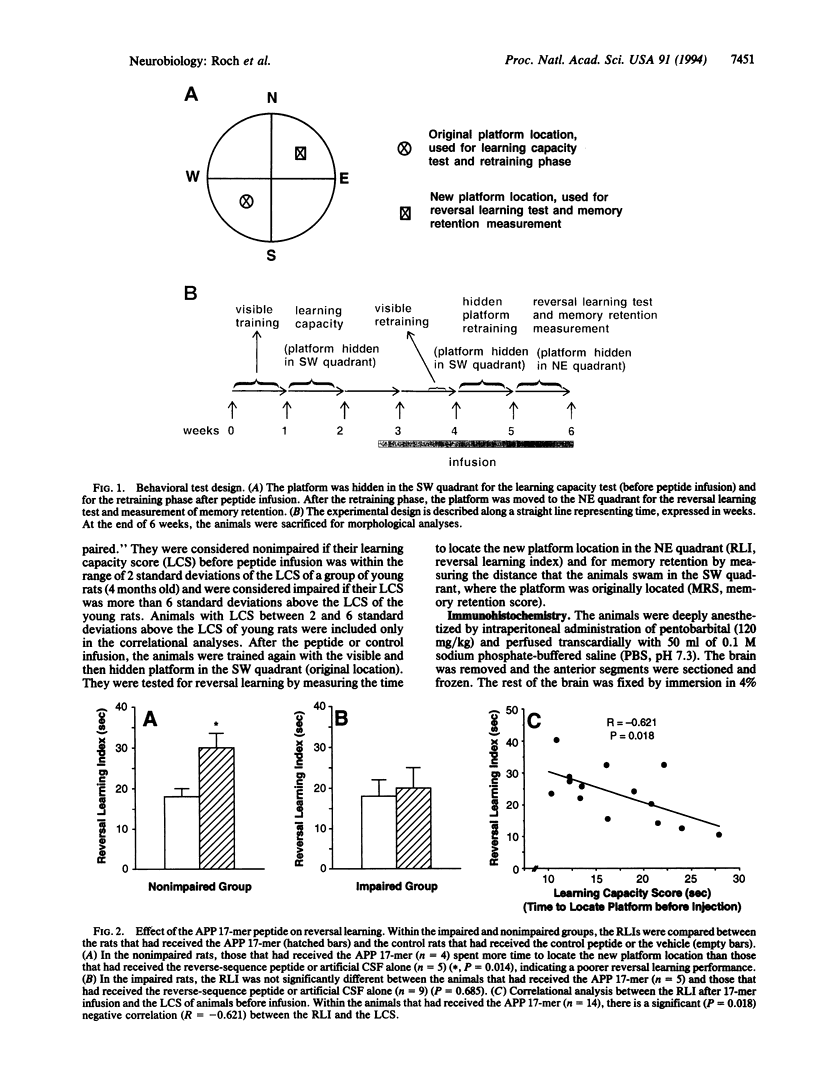
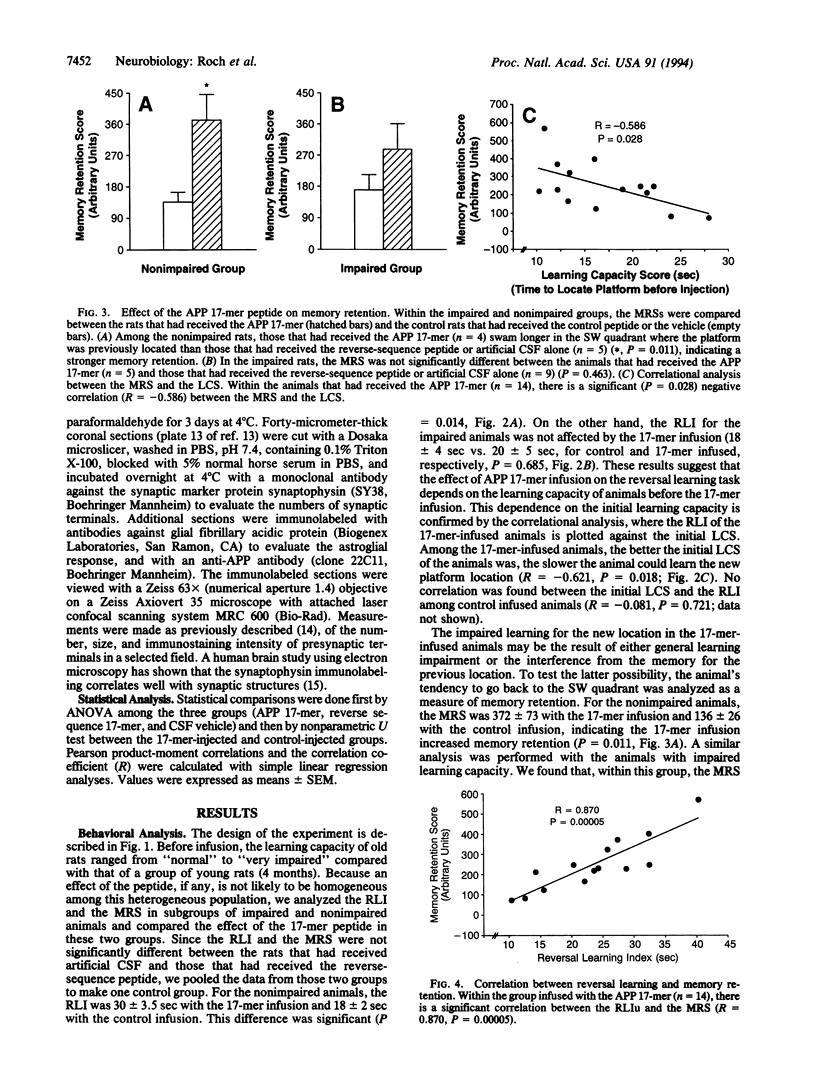
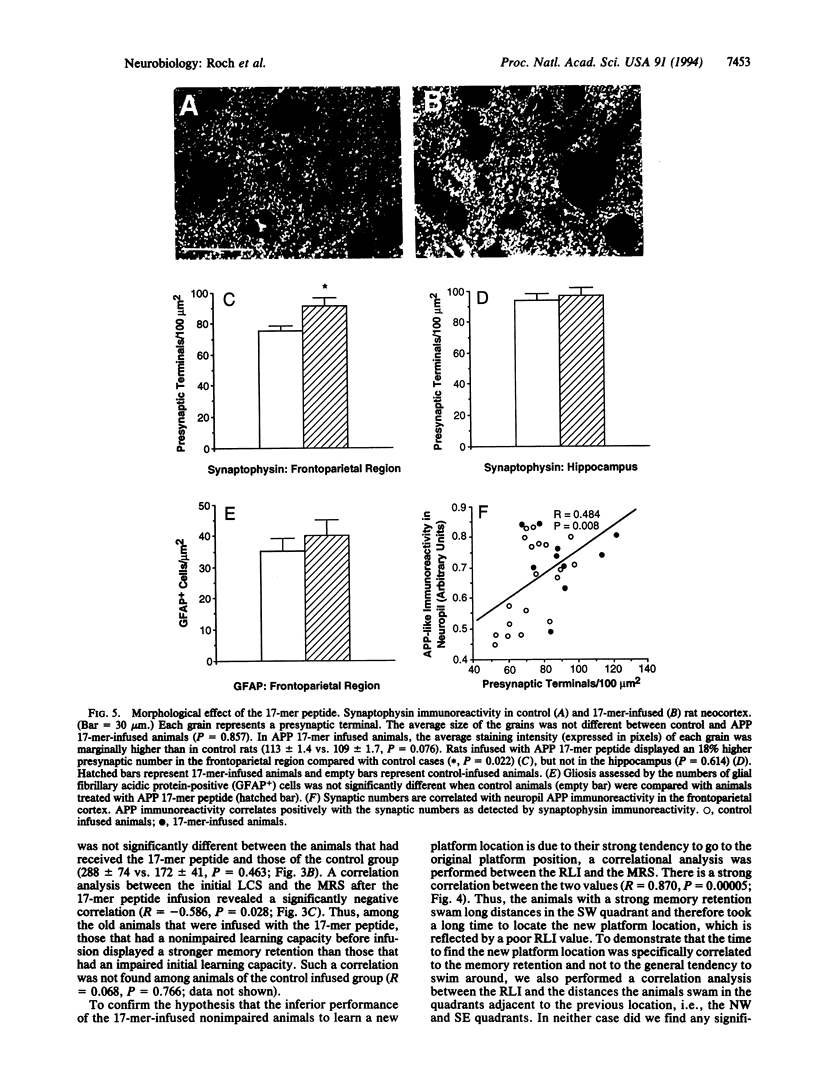
The secreted form (sAPP) of the Alzheimer amyloid beta/A4 protein precursor (APP) has been shown to be involved in the in vitro regulation of fibroblast growth and neurite extension from neuronal cells. The active site of sAPP responsible for these functions is within a small domain just C-terminal to the Kunitz-type protease inhibitor (KPI) insertion site. We report here that a 17-mer peptide, containing this active domain of sAPP, can induce cellular and behavioral changes when infused into rat brains. After 2 weeks of APP 17-mer peptide infusion, the animals were tested for reversal learning and memory retention and were sacrificed for morphological examination of brains. We found that administration of the APP 17-mer peptide resulted in an 18% increase in the number of presynaptic terminals in the frontoparietal cortex. At the behavioral level, 17-mer-infused animals with nonimpaired learning capability showed an increased memory retention that seemed to interfere with reversal learning performance. This APP 17-mer effect on memory retention was not observed in animals with impaired initial learning capacity. These results suggest that APP is involved in memory retention through its effect on synaptic structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki W., Kitaguchi N., Tokushima Y., Ishii K., Aratake H., Shimohama S., Nakamura S., Kimura J. Trophic effect of beta-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991 Nov 27;181(1):265–271. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- Cole G. M., Masliah E., Shelton E. R., Chan H. W., Terry R. D., Saitoh T. Accumulation of amyloid precursor fragment in Alzheimer plaques. Neurobiol Aging. 1991 Mar-Apr;12(2):85–91. doi: 10.1016/0197-4580(91)90046-m. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Rostagno A., Gardella J. E., Liem L., Gorevic P. D., Frangione B. A 109-amino-acid C-terminal fragment of Alzheimer's-disease amyloid precursor protein contains a sequence, -RHDS-, that promotes cell adhesion. Biochem J. 1992 Dec 15;288(Pt 3):1053–1059. doi: 10.1042/bj2881053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo E. H., Park L., Selkoe D. J. Amyloid beta-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S. Alzheimer's disease: a cell biological perspective. Science. 1992 May 8;256(5058):780–783. doi: 10.1126/science.1589757. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Majocha R. E., Tate B. Molecular and cellular biology of Alzheimer amyloid. J Mol Neurosci. 1992;3(3):111–125. doi: 10.1007/BF02919403. [DOI] [PubMed] [Google Scholar]
- Masliah E., Fagan A. M., Terry R. D., DeTeresa R., Mallory M., Gage F. H. Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with GAP-43 in the dentate gyrus of the adult rat. Exp Neurol. 1991 Aug;113(2):131–142. doi: 10.1016/0014-4886(91)90169-d. [DOI] [PubMed] [Google Scholar]
- Masliah E., Hansen L., Albright T., Mallory M., Terry R. D. Immunoelectron microscopic study of synaptic pathology in Alzheimer's disease. Acta Neuropathol. 1991;81(4):428–433. doi: 10.1007/BF00293464. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Culwell A. R., Esch F. S., Lieberburg I., Rydel R. E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993 Feb;10(2):243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Milward E. A., Papadopoulos R., Fuller S. J., Moir R. D., Small D., Beyreuther K., Masters C. L. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992 Jul;9(1):129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984 May;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya H., Roch J. M., Sundsmo M. P., Otero D. A., Saitoh T. Amino acid sequence RERMS represents the active domain of amyloid beta/A4 protein precursor that promotes fibroblast growth. J Cell Biol. 1993 May;121(4):879–886. doi: 10.1083/jcb.121.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch J. M., Shapiro I. P., Sundsmo M. P., Otero D. A., Refolo L. M., Robakis N. K., Saitoh T. Bacterial expression, purification, and functional mapping of the amyloid beta/A4 protein precursor. J Biol Chem. 1992 Feb 5;267(4):2214–2221. [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]





