Abstract
Aim
To estimate the floor of retinal nerve fibre layer (RNFL) thickness measurements and the corresponding retinal sensitivity loss in glaucoma.
Methods
Visual field (VF), Spectralis RNFL (83 patients and 37 healthy subjects) and RTVue RNFL data obtained separately (56 patients and 36 healthy subjects) were reviewed. Global and quadrant residual layer thicknesses and corresponding VF losses were estimated using two Bayesian change point models.
Results
The respective residual thicknesses from change point model 1 (CPM1) on Spectralis and RTVue (respectively) were 49.9 and 70.6 μm globally, 57.1 and 83.7 μm superiorly, 55.2 and 79.0 μm inferiorly, 43.1 and 60.5 μm nasally, and 40.1 and 59.5 μm temporally. Corresponding VF losses ranged between −25.1 and −21.7 dB (Spectralis) and between −21.8 and −3.4 dB (RTVue). From CPM2, RNFL thinning reached horizontal asymptotes at VF losses between −18.0 and −10.7 dB (Spectralis) and between −12.1 and −2.5 dB (RTVue). There were no significant differences between postchange point residual layer thicknesses from CPM1 and CPM2 on Spectralis (37.0–50.8 μm vs 38.3–56.0 μm) and RTVue (60.6–80.5 μm vs 58.4–88.8 μm).
Conclusions
Global RNFL thinning reaches the floor at a smaller VF loss level with Spectralis than with RTVue. The nasal and temporal quadrants retain thinner residual layers than superior and inferior quadrant RNFL. Measuring RNFL below their minimums will not yield useful clinical information.
INTRODUCTION
Monitoring glaucoma progression is critical once the diagnosis is made. From a structural standpoint, spectral domain optical coherence tomography (SDOCT) allows quantitative assessment of peripapillary retinal nerve fibre layer (RNFL) thickness, optic disc topography and ganglion cell layer thickness with excellent reproducibility.1–4 Deterioration of these structural parameters over time may provide a reliable indication of glaucoma progression.5,6 Postmortem histopathological studies have shown residual retinal ganglion cells (RGCs) and their axons even after a long-standing advanced glaucoma or glaucoma-induced blindness.7 Similarly, linear models of the relationship between RNFL thickness and visual field (VF) deficit from glaucoma and anterior ischaemic optic neuropathy have predicted the persistence of a residual layer following complete loss of RNFL.8–10 In contrast, an experimental model predicted a complete loss of RNFL without residual.11
The advent of OCT has renewed the interest in investigating the in vivo residual RNFL thickness in eyes with advanced glaucoma or glaucoma-induced blindness. It has been determined using time domain (TD) OCT that blind eyes from glaucomatous and non-glaucomatous optic neuropathies retain a residual RNFL thickness of about 45 μm.12,13 Clinically, this would signify that if interindividual variation is discounted, one cannot use average RNFL thickness to monitor glaucoma progression once it has reached 45 μm. This has been regarded as the floor of the RNFL thickness or bottom value beyond which no further average RNFL thinning can be detected. It is important to know the floor of RNFL measurements because it helps define the dynamic range of the instrument’s ability to measure RNFL thickness, from completely normal RNFL to the minimum measurable thickness. Because SDOCT is currently more widely used than TDOCT, it is important to investigate the thickness of the residual layer in glaucoma using SDOCT since the algorithms for segmenting retinal layers differ between TDOCT and SDOCT. Two SDOCT-based studies reported average residual thicknesses of 45.59 and 55.5 μm,14 after averaging superior and inferior hemifield values. However, the corresponding VF losses at the time RNFL thickness reached the floor were not reported. This is important from a clinical perspective because it provides information on the extent of the remaining function once the corresponding structure has completely thinned out. This study was designed to estimate the floor of global and sectoral RNFL thicknesses and the VF loss at which global and sectoral RNFL thinning reach their floor using two different SDOCT devices.
SUBJECTS AND METHODS
Subjects
The medical records of patients with moderate to severe open-angle glaucoma (OAG) were reviewed between January 2011 and March 2013 in the Departments of Ophthalmology of the University of North Carolina (UNC) and University of California at San Francisco (UCSF). OAG was defined based on characteristic glaucomatous optic disc damage with accompanying typical glaucomatous VF loss in eyes with open angles on gonioscopy. Moderate and severe glaucomas were defined based on VF mean deviation (MD) <−6 dB but ≤−12 dB and <−12 dB, respectively.15 In addition, a set of Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) from age-matched normal subjects who participated in an earlier study16 and such RTVue OCT (Optovue, Fremont, California, USA) data from the RTVue RNFL normative database along with their VFs were also used in this study.
Exclusion criteria for both glaucoma patients and normal subjects were (1) age below 18 years; (2) history of or current ocular pathology such as retinal diseases; (3) a history of non-glaucomatous optic neuropathy, neurological disease or treatment that may lead to optic neuropathy; and (4) poor quality scans defined as having quality score <20 (Spectralis) or signal strength index <35 (RTVue), algorithm segmentation malfunction and/or motion or blinking artefacts. Patients with glaucoma were also excluded if they had a history of intraocular surgery within the 3 months preceding OCT scanning. Unreliable VFs (>33% fixation losses and false negatives, and >15% fixation losses) were also excluded.
RNFL thickness measurement
Peripapillary RNFL thickness was measured with Spectralis (software V.5.4.6.0, Eye Explorer Software V.1.6.1.0) in UNC patients and RTVue (software V.6.1.0.4) in UCSF patients using the 3.4 mm scan circle around the optic disc. Only global and quadrant RNFL thicknesses were analysed.
Visual field data management
All 52 values from the total deviation numerical plot were used for analysis. To allow for assessment of sectoral correlation between VF loss and RNFL thickness, we slightly modified the Kanamori and colleagues’ structure–function map17 to obtain only four RNFL sectors (figure 1) and four corresponding VF sectors (superior and inferior: 21 data points each; temporal: 3 data points; and central: 7 data points) (figure 1). The VF loss of each sector was obtained by averaging values of all its data points; the global VF loss was the average of all 52 data point total deviations.
Figure 1.
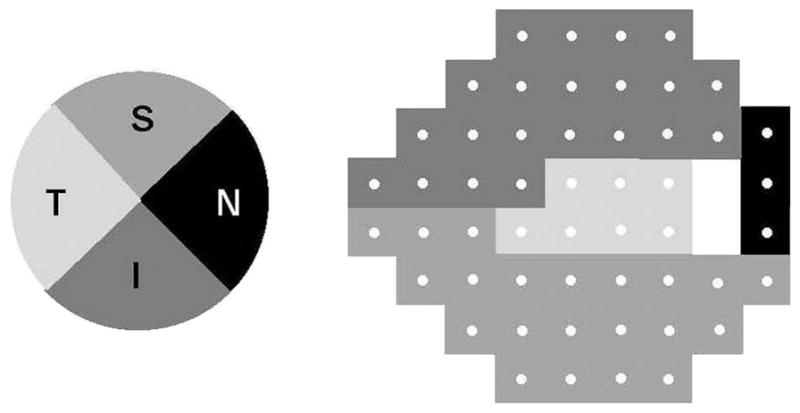
Structure–function map between retinal nerve fibre layer sectors (left) and corresponding visual field sectors (right).
Data analysis
Spectralis and RTVue data were analysed separately, and no comparisons were performed between data from the two devices because they were obtained in two different groups of subjects. The structure–function correlation was assessed by plotting global and sectoral RNFL thicknesses against their corresponding total deviation sensitivities. We introduce two change point models (CPMs) in the Bayesian setting that attempt to estimate the point of VF loss at which the RNFL thinning ends (change point) and the corresponding RNFL thickness value. We formally compare the two models globally and sectorally using deviance information criterion (DIC),18 a tool used in the Bayesian setting with smaller values being preferred and differences ≥7 taken to be significant.
Change point model 1
To estimate the magnitude of VF loss at which the RNFL thinning ends, we modified Hood and Kardon’s19 original model and modelled the data using a change point regression analysis in the Bayesian setting20 to allow the RNFL thickness to level off after a certain value of VF loss, no longer thinning continuously with ever-decreasing sensitivity. Additional details on the model and Bayesian statistics in general are provided in the online supplementary file.
Change point model 2
In CPM2, we once again work in the Bayesian setting but specify a model for the RNFL thicknesses and corresponding VF loss values on the linear scale. This model consists of piecing together two simple linear regression lines at the appropriate point (change point). The postchange point line has a slope of zero corresponding to no further reduction in the RNFL values. The point at which the two lines connect is the change point. We treat this as an unknown parameter in the model and estimate it accordingly. The prechange point line has an unknown slope that we also estimate using the data. Details regarding this model are also presented in the online supplementary file.
To determine whether the measured residual layer thickness differed between the two models, we compared (Student t test) CPM1-related and CPM2-related thicknesses obtained by averaging all thicknesses with VF losses equal to or worse than the change point. Values of p<0.05 indicated statistically significant differences.
RESULTS
Participant characteristics
Participant characteristics are shown in table 1. Patients (n=83, 41 moderate and 42 severe in the Spectralis group; and n=56, 36 moderate and 20 severe in the RTVue group) and normal subjects (n=37 in the Spectralis group and 36 in the RTVue group) were comparable in age (p>0.05), but differed significantly regarding VF MD, all RNFL thicknesses, total deviation values (all p<0.001).
Table 1.
Study participants demographic and clinical characteristics
| Spectralis OCT
|
RTVue OCT
|
|||||
|---|---|---|---|---|---|---|
| Normal | Glaucoma | p Value | Normal | Glaucoma | p Value | |
| Age (years) | 64.9±7.0 | 69.1±14.7 | 0.099 | 71.0±10.3 | 72.3±11.7 | 0.55 |
| Visual field MD (dB) | 0.59±1.21 | −14.27±7.20 | <0.001 | −0.04±1.07 | −11.84±5.59 | <0.001 |
| Total deviation loss (dB) | ||||||
| Global | 0.52±0.93 | −14.05±7.37 | <0.001 | 0.00±1.14 | −11.86±5.43 | <0.001 |
| Superior field | 0.67±0.97 | −17.04±8.88 | <0.001 | −0.01±1.39 | −15.19±8.48 | <0.001 |
| Inferior field | 0.51±1.14 | −14.47±8.81 | <0.001 | −0.07±1.19 | −9.39±7.57 | <0.001 |
| Central field | 0.45±1.01 | −14.37±9.56 | <0.001 | 0.12±1.12 | −9.81±7.62 | <0.001 |
| Temporal field | 0.47±1.39 | −10.33±8.88 | <0.001 | 0.36±1.38 | −8.68±8.22 | <0.001 |
| RNFL thickness (μm) | ||||||
| Global | 93.64±8.10 | 54.34±10.14 | <0.001 | 100.2±10.3 | 73.2±9.9 | <0.001 |
| Superior quadrant | 117.87±14.59 | 63.81±14.46 | <0.001 | 123.6±15.8 | 89.0±14.6 | <0.001 |
| Inferior quadrant | 119.71±14.53 | 60.86±14.69 | <0.001 | 123.3±15.0 | 83.7±15.2 | <0.001 |
| Central quadrant | 72.15±10.39 | 44.99±12.46 | <0.001 | 77.4±8.9 | 59.7±13.4 | <0.001 |
| Temporal quadrant | 64.86±9.29 | 47.71±12.39 | <0.001 | 76.4±9.7 | 60.2±10.4 | <0.001 |
MD, mean deviation; OCT, optical coherence tomography; RNFL, retinal nerve fibre layer.
Residual thickness and field loss change points
The Bayesian CPM1 analysis estimates of residual layer thickness on Spectralis and RTVue were 49.9 and 70.6 μm globally (figure 2), 57.1 and 83.7 μm superiorly, 55.2 and 79.0 μm inferiorly, 43.1 and 60.5 μm nasally, and 40.1 and 59.5 μm for temporally (see online supplementary figure S2), respectively. The corresponding change points ranged between −25.1 and −21.7 dB for residual layers measured with Spectralis and between −21.8 and −3.4 dB for residual layers measured with RTVue (table 2).
Figure 2.
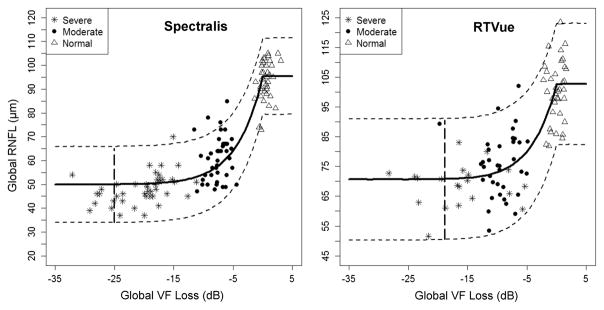
Plots of the Bayesian change point analyses (model 1) of the relationship of global retinal nerve fibre layer (RNFL) thickness from Spectralis and RTVue with global retinal sensitivity loss. The plain thick curve represents the course of the predicted RNFL thinning as a function of retinal sensitivity loss; the dotted lines are the upper and lower 95% credible intervals. VF, visual field.
Table 2.
Predicted residual layer thickness and corresponding sensitivity loss (change point) from change point model 1
| Residual layer (μm)* | VF loss (dB) (change point) | |
|---|---|---|
| Spectralis OCT | ||
| Global | 49.9 (48.0 to 51.9) | −25.1 (−31.8 to −16.1) |
| Superior quadrant | 57.1 (53.8 to 60.5) | −24.8 (−32.4 to −14.8) |
| Inferior quadrant | 55.2 (52.3 to 58.2) | −24.9 (−31.5 to −15.8) |
| Temporal quadrant | 40.1 (37.3 to 42.8) | −24.4 (−33.5 to −13.0) |
| Nasal quadrant | 43.1 (39.8 to 46.4) | −21.7 (−30.8 to −10.0) |
| RTVue OCT | ||
| Global | 70.6 (67.5 to 74.1) | −18.9 (−27.9 to −8.1) |
| Superior quadrant | 83.7 (77.1 to 91.0) | −18.1 (−29.2 to −3.9) |
| Inferior quadrant | 79.0 (75.0 to 83.0) | −21.8 (−29.9 to −11.4) |
| Temporal quadrant | 59.5 (53.8 to 64.1) | −6.7 (−29.4 to −1.3) |
| Nasal quadrant | 60.5 (55.8 to 64.1) | −3.4 (−17.8 to −1.1) |
Values represent posterior means from the Bayesian change point analyses; values in parentheses indicate lower and upper 95% credible intervals.
OCT, optical coherence tomography; VF, visual field.
The Bayesian CPM2 analysis revealed that global and all quadrant RNFL thicknesses reached the change point at VF losses ranging from −18.0 to −10.7 dB for RNFL thickness measured with Spectralis and from −12.1 to −2.5 dB when RTVue was used (Table 3). Plots for global measures are shown in figure 3. Plots for sectoral measures are provided in online supplementary figure S3.
Table 3.
Predicted residual layer thickness and corresponding sensitivity loss (change point) from change point model 2
| Residual layer (μm)* | VF loss (dB) (change point) | |
|---|---|---|
| Spectralis OCT | ||
| Global | 48.8 (46.1 to 51.4) | −10.7 (−12.3 to −9.4) |
| Superior quadrant | 56.0 (50.7 to 60.9) | −11.4 (−14.3 to −8.6) |
| Inferior quadrant | 54.3 (50.1 to 58.4) | −11.5 (−14.0 to −8.8) |
| Temporal quadrant | 38.2 (34.1 to 41.7) | −12.8 (−18.2 to −9.2) |
| Nasal quadrant | 38.1 (30.2 to 44.2) | −18.0 (−29.4 to −9.7) |
| RTVue OCT | ||
| Global | 71.5 (67.6 to 75.0) | −8.5 (−11.8 to −5.2) |
| Superior quadrant | 87.4 (76.6 to 93.7) | −5.6 (−12.7 to −2.8) |
| Inferior quadrant | 77.3 (71.8 to 82.6) | −12.1 (−16.3 to −8.4) |
| Temporal quadrant | 58.6 (45.9 to 64.2) | −8.4 (−29.3 to −2.2) |
| Nasal quadrant | 61.0 (57.4 to 64.3) | −2.5 (−5.9 to −0.2) |
Values represent posterior means from the Bayesian change point analyses; values in parentheses indicate lower and upper 95% credible intervals.
OCT, optical coherence tomography; VF, visual field.
Figure 3.
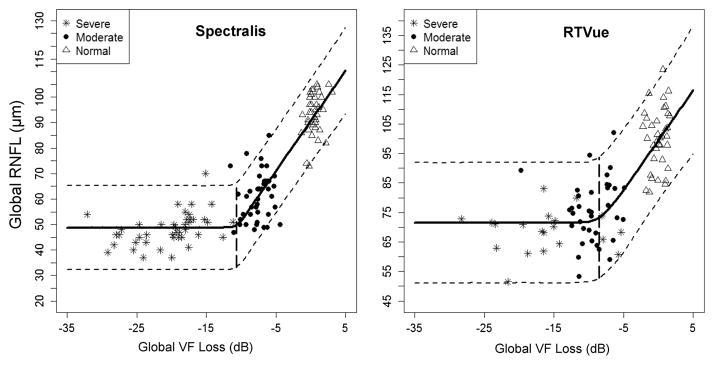
Plots of the Bayesian change point analyses (model 2) of the relationship of global retinal nerve fibre layer (RNFL) thickness from Spectralis and RTVue with global retinal sensitivity loss. The point at which the two plain thick lines connect is the change point. The dotted lines are the upper and lower 95% credible intervals. VF, visual field.
The results from formally comparing the model fits from CPM1 and CPM2 are displayed in table 4. For Spectralis, CPM1 outperforms CPM2 for the global, superior and nasal sectors. In the other sectors, there is no real difference observed in the model fits. For RTVue, the model fits are somewhat comparable with neither model being overwhelmingly preferred.
Table 4.
Model comparison using deviance information criterion
| Change point model 1 | Change point model 2 | |
|---|---|---|
| Spectralis OCT | ||
| Global | 843.7 | 851.2 |
| Superior quadrant | 963.4 | 970.2 |
| Inferior quadrant | 926.8 | 925.9 |
| Temporal quadrant | 913.1 | 914.5 |
| Nasal quadrant | 945.4 | 960.6 |
| RTVue OCT | ||
| Global | 690.8 | 692.0 |
| Superior quadrant | 788.2 | 787.7 |
| Inferior quadrant | 703.3 | 701.2 |
| Temporal quadrant | 724.5 | 728.2 |
| Nasal quadrant | 741.6 | 747.1 |
OCT, optical coherence tomography.
The residual layer thicknesses measured with Spectralis and RTVue obtained by averaging all thickness values with VF losses equal to or worse than change points from CPM1 were 44.8 and 70.0 μm globally, 50.8 and 80.5 μm superiorly, 50.8 and 77.4 μm inferiorly, 37.0 and 60.9 μm nasally, and 37.2 and 60.6 μm temporally, respectively (table 5). On both Spectralis and RTVue, the superior and inferior residual layers were thicker than the nasal and temporal layers (all p<0.001). The corresponding postchange point average VF losses ranged between −29.7 and −27.2 dB (Spectralis, all p>0.05) and between −26.5 and −10.9 dB (RTVue), with greater sensitivity losses globally, superiorly and inferiorly than centrally, and temporally for RTVue (table 5). Averaging thickness values with corresponding VF losses equal to or greater than the change points from CPM2 resulted in residual thicknesses of 48.7 and 71.3 μm globally, 56.0 and 88.8 μm superiorly, 54.2 and 76.8 μm inferiorly, 38.4 and 58.4 μm temporally, and 38.3 and 60.7 μm nasally, respectively. These values were not statistically significantly different from those obtained from CPM1 (all p>0.05, table 5). However, differences between corresponding mean VF losses from the two models were statistically significant (all p<0.05), except in the temporal field sector (Spectralis and RTVue) and the central field sector (RTVue).
Table 5.
Measured residual layer thickness and functional loss in corresponding field sectors
| Post-CPM1 residual layer (μm) | Post-CPM1 visual field loss (dB) | Post-CPM2 residual layer (μm) | Post-CPM2 visual field loss (dB) | p1 | p2 | |
|---|---|---|---|---|---|---|
| Spectralis OCT | ||||||
| Global | 44.8 (41.4 to 48.1) | −27.9 (−31.3 to −24.5) | 48.7 (46.6 to 50.9) | −20.0 (−22.1 to −17.8) | 0.15 | <0.001 |
| Superior quadrant | 50.8 (46.5 to 55.1) | −29.7 (−34.0 to −25.4) | 56.0 (52.9 to 59.2) | −20.4 (−23.5 to −17.3) | 0.09 | <0.001 |
| Inferior quadrant | 50.8 (48.0 to 53.6) | −28.0 (−30.8 to −25.2) | 54.2 (51.5 to 56.9) | −22.4 (−25.2 to −19.7) | 0.14 | <0.001 |
| Temporal quadrant | 37.0 (34.8 to 39.2) | −28.6 (−30.8 to −26.5) | 38.4 (36.5 to 40.4) | −22.3 (−24.3 to −20.3) | 0.42 | <0.001 |
| Nasal quadrant | 37.2 (31.6 to 42.9) | −27.2 (−32.9 to −21.6) | 38.3 (33.0 to 43.6) | −25.9 (−31.3 to −20.6) | 0.79 | 0.37 |
| RTVue OCT | ||||||
| Global | 70.0 (61.6 to 78.4) | −22.8 (−31.2 to −14.4) | 71.3 (68.5 to 74.1) | −14.1 (−16.9 to −11.4) | 0.73 | <0.001 |
| Superior quadrant | 80.5 (75.5 to 85.5) | −24.7 (−29.6 to −19.7) | 88.8 (83.6 to 93.9) | −12.9 (−18.1 to −7.8) | 0.16 | <0.001 |
| Inferior quadrant | 77.4 (72.2 to 82.7) | −26.5 (−31.8 to −21.3) | 76.8 (73.4 to 80.2) | −21.0 (−24.4 to −17.6) | 0.85 | 0.01 |
| Temporal quadrant | 60.9 (55.9 to 65.8) | −15.0 (−20.0 to −10.1) | 58.4 (53.5 to 63.4) | −16.6 (−21.5 to −11.6) | 0.50 | 0.39 |
| Nasal quadrant | 60.6 (57.4 to 63.7) | −10.9 (−14.0 to −7.7) | 60.7 (57.6 to 63.8) | −10.2 (−13.3 to −7.1) | 0.96 | 0.70 |
Values are expressed as mean±SD; values in parentheses indicate lower and upper 95% CIs.
p1 indicates significance level of the difference between postchange point residual layer thickness from CPM1 and CPM2.
p2 indicates significance level of the difference between postchange point visual residual losses from CPM1 and CPM2.
CPM1, change point model 1; CPM2, change point model 2; OCT, optical coherence tomography.
DISCUSSION
The change point analyses of the relationship of RNFL as a function of VF loss revealed that global and sectoral RNFL thicknesses decrease exponentially before reaching the floor. The same trend has been reported both in glaucomatous and non-glaucomatous optic neuropathies.8–10,14,19 The structure–function relationship plots from both CPM1 and CPM2 showed increasing variability of the data with increasing disease severity, so that the correlation disappears in the late stage of the disease as reported by others.21 The predicted thickness of global residual layer of 49.9 μm (CPM1 analysis on Spectralis data) is comparable to 50.5 μm reported by Hood et al,10 but slightly lower than 55.5 μm found by Kim et al.14 However, the values in the former study were obtained with TDOCT and were only based on the residual layers in the superior and inferior arcuate sectors. Unlike earlier studies that only determined the global residual layer thickness, we report for the first time global and quadrant residual layer thicknesses.
To the best of our knowledge, the first report on residual layer thickness measured with OCT resulted from two analyses performed by Hood.8 In the first analysis, the residual thickness of the superior hemifield RNFL was approximately 35 μm with a corresponding functional loss of about −10 dB. The second analysis estimated the residual thickness of the 7 o’clock RNFL sector at 41.6 μm, corresponding to approximately −12 dB. However, these estimates were obtained by averaging thicknesses with corresponding VF losses greater than the presumed asymptotic point.
Overall our findings agree with the hypothesis that loss of RGC axons leaves a residual layer whose thickness is measureable with OCT10 and suggest in addition that the thickness of the residual layer varies from sector to sector. This view is at odds with the hypothesis of complete loss of axons without residual layer.11 Interestingly, histological studies in primate normal eyes have also shown that the proportion of non-axonal content of RNFL varies from location to location.22 Another plausible explanation for a residual layer persistence is the proliferation of glial content in human retina and optic nerve head after glaucoma-induced axonal degeneration.23,24
The global residual layer thickness of 44.8 μm, from averaging all Spectralis postchange point RNFL thicknesses from CPM1, is consistent with 44.9 μm measured with TDOCT in eyes with glaucoma-induced blindness,13 though none of our patients had complete blindness. Similarly, studies with TDOCT in people with non-glaucomatous optic neuropathies and vision ranging from 20/200 to no light perception reported residual thicknesses between 45.4 and 48.4 μm.12,25 In a histological study of 21 eyes enucleated for absolute angle-closure glaucoma, the global residual thickness was 40 μm, and the residual in the temporal and nasal quadrants (36 μm) was significantly thinner than in the superior and inferior quadrants (45 μm).26 The difference in residual thickness among quadrants reflects differences in RNFL thickness among sectors in normal subjects. Our interpretation is that sectors with thicker RNFL prior to the disease retain a thicker postdisease residual layer compared with sectors with thinner RNFL.
Unique to this study is the use of a Bayesian change point analysis, which predicted when the RNFL thinning process ends. While statistically CPM1 may be favoured over CPM2 for some sectors, CPM2 may be clinically more relevant because of lower VF losses for similar residual thicknesses globally and in the superior and inferior quadrants. Interestingly, the post-change point residual thicknesses from CPM1 and CPM2 were also similar, with lower VF losses in CPM2. The CPM2 results signify, clinically, that in severe glaucoma function may continue to deteriorate and to be monitored beyond when RNFL thickness reaches the floor. This remaining function suggests the existence of functional axons in the residual layer. However, the overall number of these axons does not contribute significantly to the thickness of the non-axonal component of the residual layer. This argument aligns with evidence of persisting RGCs and their axons in blind eyes enucleated for glaucoma.7
Although Spectralis and RTVue data were obtained in different groups of subjects, it was surprising that the RTVue data predicted thicker residuals than Spectralis. This may be related to the difference in segmentation algorithms on the two devices, RTVue being known to compute thicker measurements than Spectralis.27 Additionally, in both models the change point 95% CI were generally wider for RTVue, likely resulting from (1) misalignment of RTVue scan circles due to the lack of eye tracking during image acquisition compared with Spectralis28 and (2) the narrower VF data dynamic range in RTVue patients. This may have reduced the change point prediction accuracy for RTVue.
In summary, RNFL thinning in glaucoma reaches the floor earlier than functional loss. The nasal and temporal quadrants retain thinner residual layers than superior and inferior quadrants. Caution is needed before applying the RNFL floor values reported herein to individual subjects because the actual floor may be different due to interindividual variability of RNFL thickness prior to the disease.
Supplementary Material
Acknowledgments
The authors thank Ms Sarah A Morton for helping with visual field data entry.
Funding Supported by an unrestricted grant from Research to Prevent Blindness (RPB), New York, NY, USA.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/bjophthalmol-2014-305745).
Competing interests None.
Patient consent Obtained.
Ethics approval IRB at UNC and UCSF.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Garvin MK, Lee K, Burns TL, et al. Reproducibility of SD-OCT-based ganglion cell-layer thickness in glaucoma using two different segmentation algorithms. Invest Ophthalmol Vis Sci. 2013;54:6998–7004. doi: 10.1167/iovs.13-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51:5724–30. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwanza JC, Oakley JD, Budenz DL, et al. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8323–9. doi: 10.1167/iovs.11-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierro L, Gagliardi M, Iuliano L, et al. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012;53:5912–20. doi: 10.1167/iovs.11-8644. [DOI] [PubMed] [Google Scholar]
- 5.Leung CK, Chiu V, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118:1558–62. doi: 10.1016/j.ophtha.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Na JH, Sung KR, Lee JR, et al. Detection of glaucomatous progression by spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1388–95. doi: 10.1016/j.ophtha.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Pavlidis M, Stupp T, Naskar R, et al. Retinal ganglion cells resistant to advanced glaucoma: a postmortem study of human retinas with the carbocyanine dye DiI. Invest Ophthalmol Vis Sci. 2003;44:5196–205. doi: 10.1167/iovs.03-0614. [DOI] [PubMed] [Google Scholar]
- 8.Hood DC. Relating retinal nerve fiber thickness to behavioral sensitivity in patients with glaucoma: application of a linear model. J Opt Soc Am A Opt Image Sci Vis. 2007;24:1426–30. doi: 10.1364/josaa.24.001426. [DOI] [PubMed] [Google Scholar]
- 9.Hood DC, Anderson S, Rouleau J, et al. Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy. A test of a linear model Ophthalmology. 2008;115:904–10. doi: 10.1016/j.ophtha.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood DC, Anderson SC, Wall M, et al. Structure versus function in glaucoma: an application of a linear model. Invest Ophthalmol Vis Sci. 2007;48:3662–8. doi: 10.1167/iovs.06-1401. [DOI] [PubMed] [Google Scholar]
- 11.Harwerth RS, Vilupuru AS, Rangaswamy NV, et al. The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–73. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 12.Chan CK, Miller NR. Peripapillary nerve fiber layer thickness measured by optical coherence tomography in patients with no light perception from long-standing nonglaucomatous optic neuropathies. J Neuroophthalmol. 2007;27:176–9. doi: 10.1097/WNO.0b013e31814b1ac4. [DOI] [PubMed] [Google Scholar]
- 13.Sihota R, Sony P, Gupta V, et al. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47:2006–10. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Jeoung JW, Park KH, et al. Correlation between retinal nerve fiber layer thickness and visual field sensitivity: diffuse atrophy imaging study. Ophthalmic Surg Lasers Imaging. 2012;43:S75–82. doi: 10.3928/15428877-20121001-01. [DOI] [PubMed] [Google Scholar]
- 15.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. St Louis: Mosby-Year Book; 1993. [Google Scholar]
- 16.Mwanza JC, Sayyad FE, Budenz DL. Choroidal thickness in unilateral advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53:6695–701. doi: 10.1167/iovs.12-10388. [DOI] [PubMed] [Google Scholar]
- 17.Kanamori A, Naka M, Nagai-Kusuhara A, et al. Regional relationship between retinal nerve fiber layer thickness and corresponding visual field sensitivity in glaucomatous eyes. Arch Ophthalmol. 2008;126:1500–6. doi: 10.1001/archopht.126.11.1500. [DOI] [PubMed] [Google Scholar]
- 18.Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J R Stat Soc Series B (Statistical Methodol) 2002;64:583–639. [Google Scholar]
- 19.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlin BP, Gelfand AE, Smith AFM. Hierarchical Bayesian analysis of changepoint problems. Appl Statist. 1992;41:389–405. [Google Scholar]
- 21.Blumenthal EZ, Horani A, Sasikumar R, et al. Correlating structure with function in end-stage glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:218–23. doi: 10.3928/15428877-20060501-06. [DOI] [PubMed] [Google Scholar]
- 22.Ogden TE. Nerve fiber layer of the primate retina: thickness and glial content. Vision Res. 1983;23:581–7. doi: 10.1016/0042-6989(83)90063-9. [DOI] [PubMed] [Google Scholar]
- 23.Varela HJ, Hernandez MR. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J Glaucoma. 1997;6:303–13. [PubMed] [Google Scholar]
- 24.Wang L, Cioffi GA, Cull G, et al. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002;43:1088–94. [PubMed] [Google Scholar]
- 25.Cunha LP, Costa-Cunha LV, Malta RF, et al. Comparison between retinal nerve fiber layer and macular thickness measured with OCT detecting progressive axonal loss following traumatic optic neuropathy. Arq Bras Oftalmol. 2009;72:622–5. doi: 10.1590/s0004-27492009000500004. [DOI] [PubMed] [Google Scholar]
- 26.Dichtl A, Jonas JB, Naumann GO. Retinal nerve fiber layer thickness in human eyes. Graefes Arch Clin Exp Ophthalmol. 1999;237:474–9. doi: 10.1007/s004170050264. [DOI] [PubMed] [Google Scholar]
- 27.Leite MT, Rao HL, Weinreb RN, et al. Agreement among spectral-domain optical coherence tomography instruments for assessing retinal nerve fiber layer thickness. Am J Ophthalmol. 2011;151:85–92. doi: 10.1016/j.ajo.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Crabb DP, Schlottmann PG, et al. Aligning scan acquision circles in optical coherence tomography images of the retinal nerve fiber layer. IEEE Trans Med Imaging. 2011;30:1228–38. doi: 10.1109/TMI.2011.2109962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


