Abstract
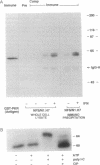
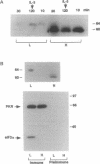
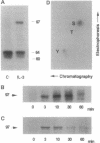
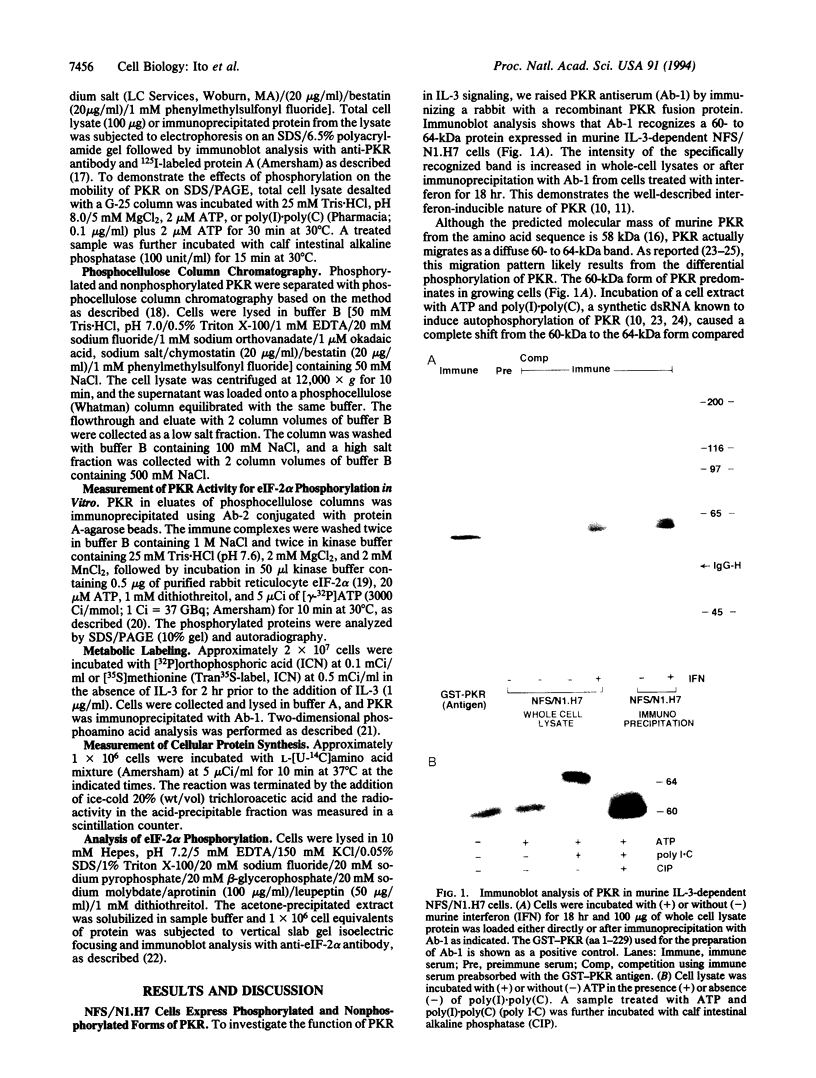
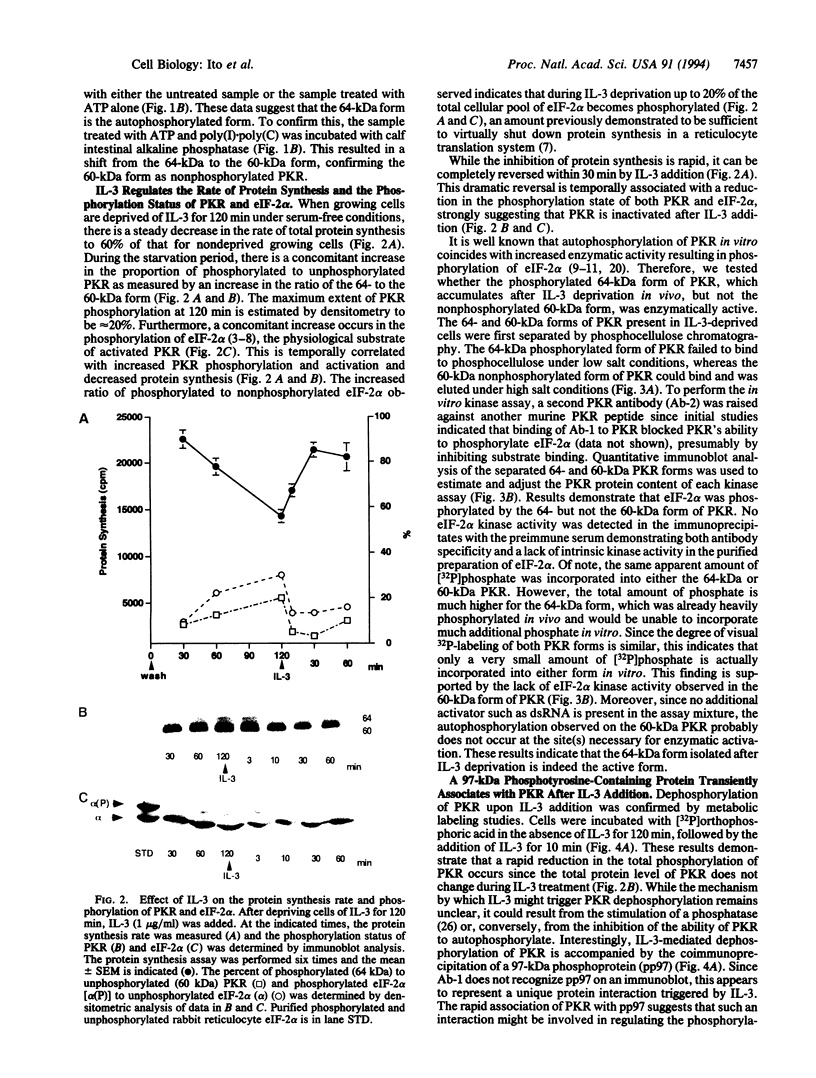
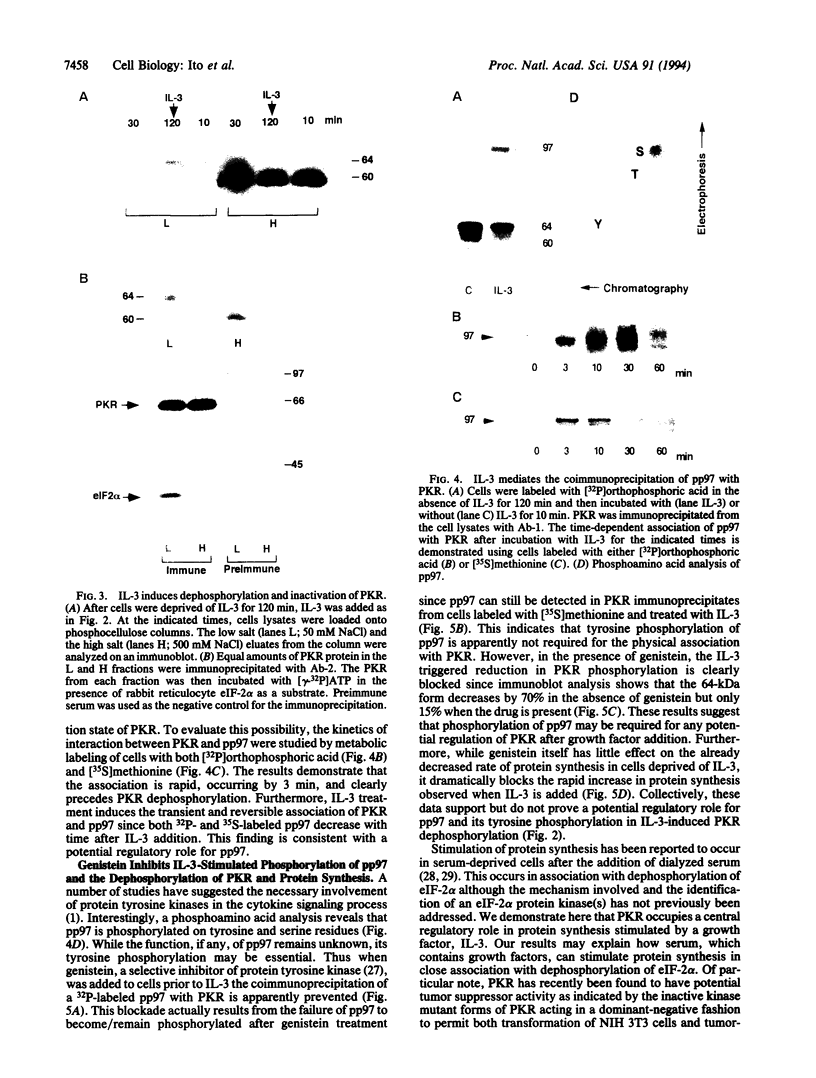
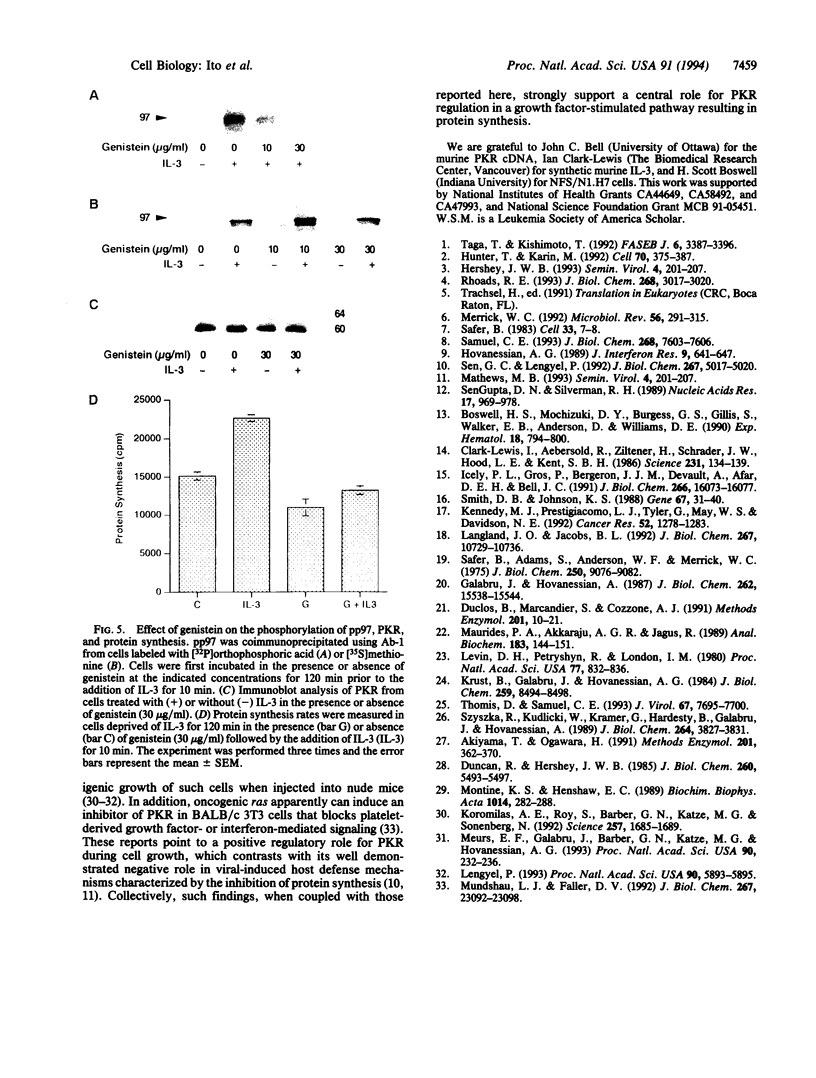
In a murine interleukin 3 (IL-3)-dependent cell line, IL-3 deprivation resulted in increased autophosphorylation of double-stranded RNA-dependent protein kinase (PKR) that has been reported to inhibit protein synthesis by phosphorylating the alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha). Autophosphorylation was characterized by a shift up in mobility of PKR on SDS/PAGE gels from a 60- to a 64-kDa form. In vitro kinase studies comparing the autophosphorylated 64-kDa PKR with the nonphosphorylated 60-kDa PKR confirmed that only the 64-kDa form was active for eIF-2 alpha phosphorylation. PKR activation in vivo was associated with phosphorylation of eIF-2 alpha and inhibition of protein synthesis. Addition of IL-3 to deprived cells elicited a reciprocal response characterized by the rapid dephosphorylation of PKR and eIF-2 alpha, indicating inactivation of PKR. This was rapidly followed by the full recovery of protein synthesis. Furthermore, upon IL-3 addition, a 97-kDa phosphotyrosine-containing protein becomes rapidly and transiently associated with PKR prior to dephosphorylation of PKR and eIF-2 alpha. Genistein, a tyrosine kinase inhibitor, blocks both phosphorylation of the 97-kDa phosphoprotein and protein synthesis after IL-3 addition, suggesting a role for the 97-kDa phosphoprotein in the mechanism of inactivation of PKR and stimulation of protein synthesis. Thus, IL-3 appears to positively regulate protein synthesis by inducing the inactivation of PKR in a growth factor signaling pathway.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Boswell H. S., Mochizuki D. Y., Burgess G. S., Gillis S., Walker E. B., Anderson D., Williams D. E. A novel mast cell growth factor (MCGF-3) produced by marrow-adherent cells that synergizes with interleukin 3 and interleukin 4. Exp Hematol. 1990 Aug;18(7):794–800. [PubMed] [Google Scholar]
- Clark-Lewis I., Aebersold R., Ziltener H., Schrader J. W., Hood L. E., Kent S. B. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986 Jan 10;231(4734):134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- Duclos B., Marcandier S., Cozzone A. J. Chemical properties and separation of phosphoamino acids by thin-layer chromatography and/or electrophoresis. Methods Enzymol. 1991;201:10–21. doi: 10.1016/0076-6879(91)01004-l. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987 Nov 15;262(32):15538–15544. [PubMed] [Google Scholar]
- Hovanessian A. G. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989 Dec;9(6):641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Icely P. L., Gros P., Bergeron J. J., Devault A., Afar D. E., Bell J. C. TIK, a novel serine/threonine kinase, is recognized by antibodies directed against phosphotyrosine. J Biol Chem. 1991 Aug 25;266(24):16073–16077. [PubMed] [Google Scholar]
- Kennedy M. J., Prestigiacomo L. J., Tyler G., May W. S., Davidson N. E. Differential effects of bryostatin 1 and phorbol ester on human breast cancer cell lines. Cancer Res. 1992 Mar 1;52(5):1278–1283. [PubMed] [Google Scholar]
- Koromilas A. E., Roy S., Barber G. N., Katze M. G., Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992 Sep 18;257(5077):1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- Krust B., Galabru J., Hovanessian A. G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984 Jul 10;259(13):8494–8498. [PubMed] [Google Scholar]
- Langland J. O., Jacobs B. L. Cytosolic double-stranded RNA-dependent protein kinase is likely a dimer of partially phosphorylated Mr = 66,000 subunits. J Biol Chem. 1992 May 25;267(15):10729–10736. [PubMed] [Google Scholar]
- Lengyel P. Tumor-suppressor genes: news about the interferon connection. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5893–5895. doi: 10.1073/pnas.90.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Petryshyn R., London I. M. Characterization of double-stranded-RNA-activated kinase that phosphorylates alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha) in reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Feb;77(2):832–836. doi: 10.1073/pnas.77.2.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurides P. A., Akkaraju G. R., Jagus R. Evaluation of protein phosphorylation state by a combination of vertical slab gel isoelectric focusing and immunoblotting. Anal Biochem. 1989 Nov 15;183(1):144–151. doi: 10.1016/0003-2697(89)90182-6. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E. F., Galabru J., Barber G. N., Katze M. G., Hovanessian A. G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine K. S., Henshaw E. C. Serum growth factors cause rapid stimulation of protein synthesis and dephosphorylation of eIF-2 in serum deprived Ehrlich cells. Biochim Biophys Acta. 1989 Dec 14;1014(3):282–288. doi: 10.1016/0167-4889(89)90224-3. [DOI] [PubMed] [Google Scholar]
- Mundschau L. J., Faller D. V. Oncogenic ras induces an inhibitor of double-stranded RNA-dependent eukaryotic initiation factor 2 alpha-kinase activation. J Biol Chem. 1992 Nov 15;267(32):23092–23098. [PubMed] [Google Scholar]
- Rhoads R. E. Regulation of eukaryotic protein synthesis by initiation factors. J Biol Chem. 1993 Feb 15;268(5):3017–3020. [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Safer B., Adams S. L., Anderson W. F., Merrick W. C. Binding of MET-TRNAf and GTP to homogeneous initiation factor MP. J Biol Chem. 1975 Dec 10;250(23):9076–9082. [PubMed] [Google Scholar]
- Samuel C. E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993 Apr 15;268(11):7603–7606. [PubMed] [Google Scholar]
- Sen G. C., Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992 Mar 15;267(8):5017–5020. [PubMed] [Google Scholar]
- SenGupta D. N., Silverman R. H. Activation of interferon-regulated, dsRNA-dependent enzymes by human immunodeficiency virus-1 leader RNA. Nucleic Acids Res. 1989 Feb 11;17(3):969–978. doi: 10.1093/nar/17.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Szyszka R., Kudlicki W., Kramer G., Hardesty B., Galabru J., Hovanessian A. A type 1 phosphoprotein phosphatase active with phosphorylated Mr = 68,000 initiation factor 2 kinase. J Biol Chem. 1989 Mar 5;264(7):3827–3831. [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992 Dec;6(15):3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- Thomis D. C., Samuel C. E. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J Virol. 1993 Dec;67(12):7695–7700. doi: 10.1128/jvi.67.12.7695-7700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]