Supplemental Digital Content is available in the text.
Keywords: CADASIL; cerebral small vessel diseases; genetic association studies; stroke, lacunar
Abstract
Background and Purpose—
The most common monogenic cause of cerebral small-vessel disease is cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, caused by NOTCH3 gene mutations. It has been hypothesized that more common variants in NOTCH3 may also contribute to the risk of sporadic small-vessel disease. Previously, 4 common variants (rs10404382, rs1043994, rs10423702, and rs1043997) were found to be associated with the presence of white matter hyperintensity in hypertensive community-dwelling elderly.
Methods—
We investigated the association of common single nucleotide polymorphisms (SNPs) in NOTCH3 in 1350 patients with MRI-confirmed lacunar stroke and 7397 controls, by meta-analysis of genome-wide association study data sets. In addition, we investigated the association of common SNPs in NOTCH3 with MRI white matter hyperintensity volumes in 3670 white patients with ischemic stroke. In each analysis, we considered all SNPs within the NOTCH3 gene, and within 50-kb upstream and downstream of the coding region. A total of 381 SNPs from the 1000 genome population with a mean allele frequency >0.01 were included in the analysis. A significance level of P<0.0015 was used, adjusted for the effective number of independent SNPs in the region using the Galwey method.
Results—
We found no association of any common variants in NOTCH3 (including rs10404382, rs1043994, rs10423702, and rs1043997) with lacunar stroke or white matter hyperintensity volume. We repeated our analysis stratified for hypertension but again found no association.
Conclusions—
Our study does not support a role for common NOTCH3 variation in the risk of sporadic small-vessel disease.
Cerebral small-vessel disease (SVD) accounts for nearly one quarter of all ischemic strokes and is an important cause of dementia. Lacunar infarction and white matter hyperintensities (WMH) on MRI are lesions commonly seen in SVD. Genetic factors have been suggested to play an important role in SVD.1–3 Several monogenic causes of SVD have been described, the most common of which is cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). CADASIL is caused by mutations in the NOTCH3 gene, and among the main features are recurrent ischemic strokes and white matter lesions on MRI.4 Besides CADASIL causing mutations, it has been suggested that more common variants in NOTCH3 may also contribute to the risk of sporadic SVD.5 This study in a community-dwelling elderly cohort, the Austrian Stroke Prevention Study, found 4 common single nucleotide polymorphism (SNP) polymorphisms at the NOTCH3 gene (rs10404382, rs1043994, rs10423702, and rs1043997) to be associated with the presence of WMH. However, these associations seemed to be restricted to hypertensive subjects. In contrast, another study in 120 patients with lacunar stroke found no association between 2 common NOTCH3 SNPs (rs3815188 and rs1043994) and the presence of WMH.6 One other study investigated the association between common NOTCH3 variation and ischemic stroke in white patients.7 This study identified the SNP rs78501403 to be associated with ischemic stroke, but power was lacking to investigate this association in the SVD subtype. Lacunar infarcts are small and frequently not seen on computed tomography; therefore, MRI is important for accurate diagnosis.
To test the hypothesis that common NOTCH3 variation is associated with SVD, we investigated the association of common variants in NOTCH3 with both clinical and MRI-confirmed lacunar stroke and with WMH lesion volume quantified on MRI.
Methods
Lacunar Stroke Population
Lacunar stroke cases were obtained from cohorts from the United Kingdom, Germany, and Belgium (n=1350; aged, 60 years [SD, 11]; 68% men; Table I in the online-only Data Supplement). Lacunar stroke was defined as a clinical lacunar syndrome8 with a compatible lesion on MRI (subcortical infarct ≤15 mm in diameter). Exclusion criteria were as follows: stenosis >50% in the extra- or intracranial cerebral vessels; cardioembolic source of stroke, defined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria9 as high or moderate probability; subcortical infarct >15 mm in diameter, as these can be caused by embolic mechanisms (striatocapsular infarcts); any other specific cause of stroke (eg, lupus anticoagulant, cerebral vasculitis, and dissection). A description of all cohorts is given in the online-only Data Supplement. Controls (n=7397) for the United Kingdom and German analyses were derived from population cohorts and were therefore not confirmed to be stroke free. Belgian controls were ascertained from the local population.
SVD stroke subtype, classified using the TOAST criteria,9 and leukoaraiosis grading using the semiquantitative Fazekas scale was performed with central review of all MRI scans by 1 physician (H.S.M.). The Fazekas scale has been shown to reflect pathological severity of SVD in a postmortem validation study.10 In addition, lacunar infarcts were determined as high signal lesions <1.5 cm diameter on acute diffusion-weighted imaging sequences or fluid attenuated inversion recovery or low signal lesions on T1 sequences.
A preplanned secondary analysis was performed in those SVD cases with confluent leukoaraiosis (Fazekas grade >2 or multiple lacunar infarcts (n=717; 53%), as cases of CADASIL present with this phenotype, and compared with the controls (n=7397).
WMH Volumes Population
The WMH volumes population (n=3670) was derived from ischemic stroke cohorts from United Kingdom, Italy, Belgium, Germany, Australia, and United Stated (Table II in the online-only Data Supplement). Inclusion criteria were as follows: aged >18 years, self-reported European ancestry, and a diagnosis of ischemic stroke. Exclusion criteria were CADASIL, vasculitis, and demyelinating and mitochondrial disorders. For the present study, we included all available patients with ischemic stroke from each cohort who met the inclusion and exclusion criteria and had MRI and genome-wide association study data available. MRI scans were acquired as a part of routine clinical practice for evaluation of ischemic stroke. Fluid attenuated inversion recovery sequences were primarily used for leukoaraiosis analysis; however, in their absence, T2 sequences were used. All scans were quantitatively graded to obtain a WMH volume, which was normalized for intracranial volume. WMH volume was measured in the hemisphere contralateral to the infarcts and doubled to obtain whole brain volumes. All neuroimaging analyses have been previously described.11
Genotyping
Genotyping of all cohorts was performed on commercially available arrays from Affymetrix or Illumina. All cohorts performed extensive quality control steps before imputation, removing SNPs showing significant departure from Hardy-Weinberg equilibrium, high levels of missingness or low minor allele frequency. Individuals were removed that did not segregate with Hapmap II European populations based on ancestry informative principal component (PC) analysis using EIGENSTRAT software package or multidimensional scaling in PLINK software package.12,13 In addition, individuals with high levels of missingness or heterozygosity were excluded. All data sets were imputed to 1000 genomes integrated variant set (March 2012) using IMPUTE version 2.14
Lacunar Stroke Analyses
We analyzed binary case/control status for each lacunar stroke population using a score test, as implemented in SNPTEST version 2.5. Imputed genotype probabilities were taken into account using a missing data likelihood score test or an expectation-maximization method for SNPs with low mean allele frequency or high uncertainty. The first 2 ancestry informative PCs, age and sex were included as covariates in the model where possible (sex and PC1, PC2 only in the UK-Wellcome Trust Case-Control Consortium-2 and Germany-Wellcome Trust Case-Control Consortium-2 studies; Table I in the online-only Data Supplement). We meta-analyzed the 4 cohorts using a fixed-effects inverse variance-weighted method, as implemented in METAL.15 We first performed analyses using an additive model and then under dominant and recessive models.
WMH Volumes Analysis
The association between WMH volume and each autosomal SNP was determined by performing linear regression of WMH volume on genotype dosages using PLINK version 1.07.13 SNPs with PLINK INFO (information content metric) score >0.7 or mean allele frequency <0.01 were removed from further analyses. We used genomic inflation to evaluate inflation of test statistics in each center.16 Results across all centers were combined using a fixed-effects inverse variance-weighted method using METAL.15 Heterogeneity was assessed using Cochran q statistic. After the meta-analysis, we considered only SNPs present in >12 centers, and with heterogeneity P>0.001, for analysis.
NOTCH3 SNPs Analyzed and Assessment of Statistical Significance
In each analysis, we considered all SNPs within the NOTCH3 gene, and within 50-kb upstream and downstream of the coding region. A total of 381 SNPs from the 1000 genomes population with mean allele frequency >0.01 were included in the analysis. We used the Galwey method to estimate the effective number of independent SNPs in the region,17 based on the linkage disequilibrium patterns from European individuals in the 1000 genomes population.14 This method has been shown to give the best agreement with random permutations. Using the method, we estimated there to be 34 effective independent SNPs in the region. Therefore, we set our P value threshold for each analysis to P<0.0015. Power calculations were conducted using the Genetic Power Calculator for a case–control study of discrete traits under an additive disease risk model and a disease prevalence of 0.2% for lacunar stroke.18
Results
Lacunar Stroke Analyses
We first tested for an association of any NOTCH3 SNP with lacunar stroke under an additive model. No SNP met our criteria for statistical significance for association with lacunar stroke. Results for all SNPs in the region by chromosomal position are given in Figure 1A.
Figure 1.
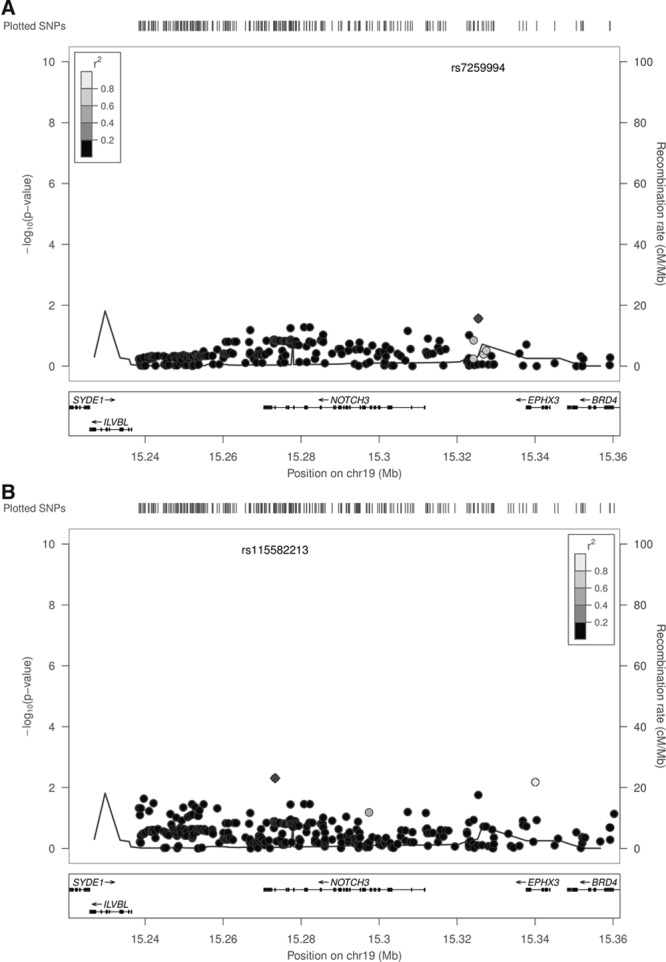
Association of common NOTCH3 variants with lacunar stroke (A) and lacunar stroke with leukoaraiosis or multiple lacunar infarcts (B). SNP indicates single nucleotide polymorphism.
We then performed secondary analyses under recessive and dominant models. Again, none of these SNPs met our criteria for statistical significance (Figure IA and IB in the online-only Data Supplement). All SNPs had P>0.005 in all analyses.
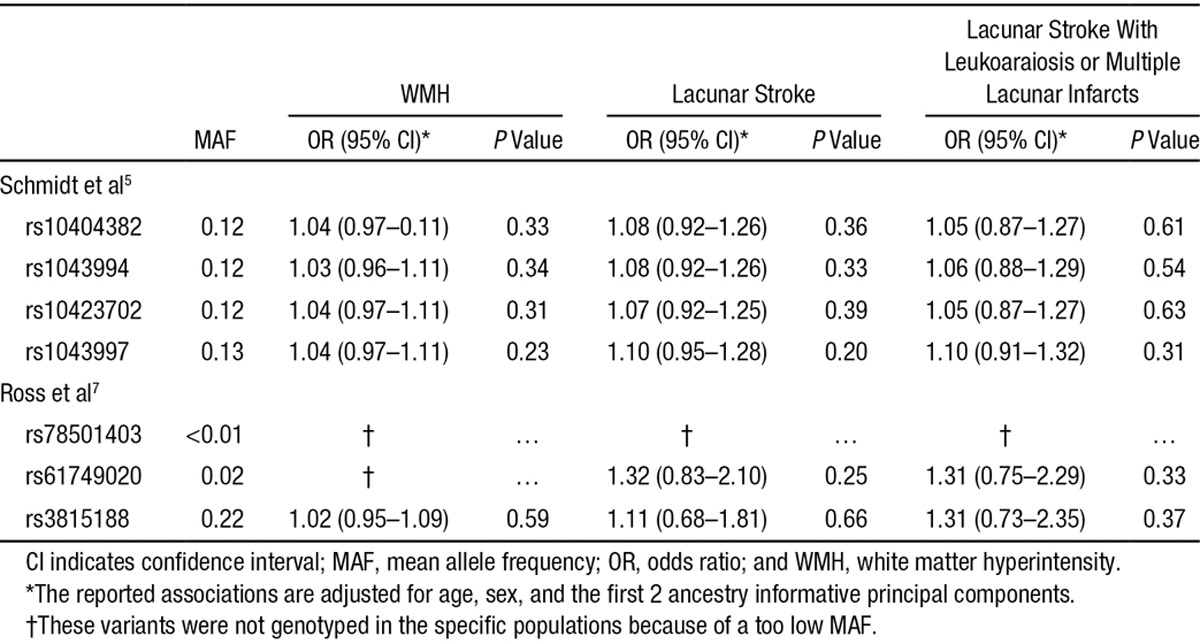
We next tested for association of any NOTCH3 SNP in those patients with lacunar stroke who also had confluent leukoaraiosis or multiple lacunar infarcts (n=717) under an additive model. No SNP met our criteria for statistical significance for association with lacunar stroke with confluent leukoaraiosis or multiple lacunar infarcts (Figure 1B). The associations for the SNPs that were identified in previous studies are given in the Table. Power calculations showed that we had >95% power to replicate these findings (Table III in the online-only Data Supplement).
Table.
Association of Single Nucleotide Polymorphisms Reported in Previous Publications With WMH and Lacunar Stroke
Secondary analyses under recessive and dominant models also revealed no significant associations (Figure IIA and IIB in the online-only Data Supplement). All SNPs had P>0.005 in all analyses.
WMH Volumes Analysis
Similarly, no SNP met our criteria for statistical significance for association with WMH volumes. Results for all SNPs in the region by genomic position are given in Figure 2. Forest plots showing the associations of rs10404382, rs1043994, rs10423702, and rs1043997 with WMH per cohort are shown in the Figure IIIA–IIID in the online-only Data Supplement.
Figure 2.
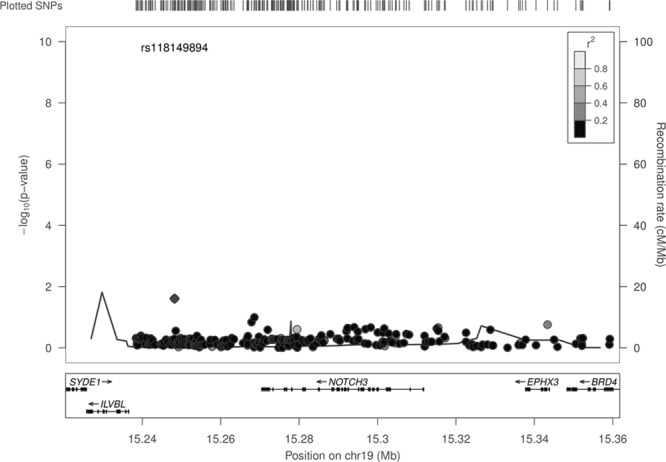
Association of common NOTCH3 variants with white matter hyperintensity volumes. SNP indicates single nucleotide polymorphism.
We repeated the latter analysis in only hypertensive subjects (n=2466), but again none of the SNPs met our criteria for statistical significance (Figure IV in the online-only Data Supplement).
Discussion
Mutations in the NOTCH3 gene cause CADASIL, a hereditary form of SVD. Common variants in the NOTCH3 gene have been suggested to also confer risk of sporadic SVD. To test this hypothesis, we analyzed NOTCH3 in an imputed genome-wide association study data set of 1350 cases and 7397 controls. We found no evidence that common variants in notch 3 associated with risk of lacunar stroke or WMH.
Our observation is in contrast to a recent study in a community-dwelling elderly cohort, the Austrian Stroke Prevention study, which found 4 common variants at the NOTCH3 gene to be associated with the presence of WMH although only in hypertensive subjects.5 The SNP that showed the strongest association, rs10404382, was replicated within the Cohorts for Heart and Ageing Research in Genomic Epidemiology (CHARGE) Consortium. The associations found in the Austrian Stroke Prevention study were all confined to hypertensive subjects. In the present study, we failed to replicate any of these findings in the present study, even when only hypertensive patients were studied. There might be several explanations for the discrepancy between the results of the Austrian Stroke Prevention study and the present study. First, our negative finding might be because of a type II error. The 4 SNPs associated with the presence of WMH in hypertensive patients had an odds ratio between 2.1 and 3.2. We performed power calculations, and we had an estimated 100% power to detect these associations in our study (Table III in the online-only Data Supplement). This makes type II error unlikely.
Second, there are differences in the populations studied. WMH lesion volume in our study was measured in clinical ischemic stroke populations, whereas the populations in the Austrian Stroke Prevention study and the CHARGE consortium were community-dwelling elderly free from stroke. WMH are more frequent in patients with a history of stroke than in healthy age-matched individuals.19 It might be that the underlying pathology of WMH differs between patients with a history of stroke and community-dwelling elderly free from stroke. However, it is likely that, at least to some extent, there is an overlap in pathology because the WMH-associated locus 17q25, which was previously identified in the CHARGE consortium, was successfully replicated in the WMH populations used in the present study.11
Third, methods to assess WMH volume differed between studies. Grading of WMH was done in a similar manner in the present study and Austrian Stroke Prevention study because both used the Fazekas scale and the same cutoff for the presence of WMH.
WMH volume measurements in the present study were done using a similar semiautomatic method in all cohorts, with a good agreement between the 2 main reading centers (intraclass correlation coefficient, 0.95; 95% confidence interval, 0.91–0.97; n=50). Also in the Austrian Stroke Prevention study, a semiautomatic method was used to measure the WMH volume. In the CHARGE consortium, WMH volume measurements were done using 2 different approaches; in most cohorts, either an automatic or a semiautomatic method is used, but in some cohorts, a semiquantitative rating scale was used.
Fourth, statistical analysis differed between the studies. In the Austrian Stroke Prevention, analyses were adjusted for several potential confounding risk factors: age, sex, diabetes mellitus, and cardiac disease. We repeated the analysis for association with the 4 SNPs detected in the previous study, adjusted for age, sex, hypertension, and diabetes mellitus in a subset of the patients with lacunar stroke and available data on hypertension and diabetes mellitus status (Table IV in the online-only Data Supplement). The additional adjustment for hypertension and diabetes mellitus did not significantly change the estimates. In addition, population structure is an important source of confounding to account for in genetic association studies.12 In contrast to the analysis reported to be done in Austrian Stroke Prevention study and its replication in CHARGE, we accounted for population structure in our analysis by including 2 ancestry informative PCs in every analysis.
Furthermore, we applied a correction for multiple testing in the analysis of the present study, based on the effective number of independent SNPs in the studied region (Galwey method).17 In the Austrian Stroke Prevention study, no correction for multiple testing was used in their analyses, which enhances the possibility of false-positive findings.
One other study investigated the association between common NOTCH3 variants and ischemic stroke and revealed an association for 1 SNP, rs78501403.7 Unfortunately, we could not investigate this SNP in our study because the minor allele frequency of this SNP is generally <0.01% in white populations and therefore the SNP was not present in the 1000 genomes population to which we imputed our data to.14 Surprisingly, this SNP had a minor allele frequency of 3.3% in the white population in this previous study. Consistent with our finding, 1 previous study in lacunar stroke found no association between 2 common NOTCH3 SNPs and the presence of WMH.6
There are several limitations in this study. We used the approach of genotyping and then imputing all cohorts to the 1000 genomes population. Although this method provides good performance for identifying common variants, the quality of imputation can drop at mean allele frequency <5%, meaning we cannot rule out associations at these frequencies. Similarly, the size of some of the WMH cohorts was small, meaning low-frequency variants could not be assessed in this analysis.
In summary, our results do not support a role for common NOTCH3 variation in the risk of sporadic SVD.
Acknowledgments
We thank all study staff and participants for their important contributions. Study-specific acknowledgments are reported in the online-only Data Supplement.
Sources of Funding
Collection of the UK Young Lacunar Stroke DNA Study (DNA lacunar) was primarily supported by the Wellcome Trust (WT072952) with additional support from the Stroke Association (TSA 2010/01). Genotyping of the DNA lacunar samples, and Dr Traylor, was supported by a Stroke Association Grant (TSA 2013/01). Funding for the genotyping at Massachusetts General Hospital was provided by the Massachusetts General Hospital-Deane Institute for the Integrative Study of Atrial Fibrillation and Stroke and the National Institute of Neurological Disorders and Stroke (U01 NS069208). Dr Rutten-Jacobs was supported by a project grant from the Stroke Association/British Heart Foundation grant (TSA BHF 2010/01). Dr Adib-Samii was supported by a Medical Research Council (United Kingdom) training fellowship. Drs Markus and Bevan were supported by the National Institute for Health Research Cambridge University Hospitals Comprehensive Biomedical Research Centre. Dr Markus was supported by a National Institute for Health Research Senior Investigator award. Dr Thijs was supported by a Clinical Investigator Grant from the scientific research fund, Fonds Wetenschappelijk Onderzoek Flanders. Dr Rost was supported by a National Institute of Neurological Disorders and Stroke grant (R01 NS082285-01). The sponsors of the study had no role in the study design, data collection, data analysis, interpretation, writing of the article, or the decision to submit the article for publication.
Disclosures
None.
Supplementary Material
Footnotes
Guest Editor for this article was Miguel A. Perez-Pinzon, PhD.
Drs Rutten-Jacobs and Traylor contributed equally.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.114.008540/-/DC1.
References
- 1.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, et al. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35:1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- 2.Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, et al. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29:1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- 3.Turner ST, Jack CR, Fornage M, Mosley TH, Boerwinkle E, de Andrade M. Heritability of leukoaraiosis in hypertensive sibships. Hypertension. 2004;43:483–487. doi: 10.1161/01.HYP.0000112303.26158.92. doi: 10.1161/01.HYP.0000112303.26158.92. [DOI] [PubMed] [Google Scholar]
- 4.Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23:269–276. doi: 10.1177/0891988710383570. doi: 10.1177/0891988710383570. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt H, Zeginigg M, Wiltgen M, Freudenberger P, Petrovic K, Cavalieri M, et al. CHARGE consortium Neurology working group. Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease. Brain. 2011;134(Pt 11):3384–3397. doi: 10.1093/brain/awr252. doi: 10.1093/brain/awr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Hassan A, Zhang Z, Huber D, Dalageorgou C, Markus HS. Yield of screening for CADASIL mutations in lacunar stroke and leukoaraiosis. Stroke. 2003;34:203–205. doi: 10.1161/01.str.0000048162.16852.88. [DOI] [PubMed] [Google Scholar]
- 7.Ross OA, Soto-Ortolaza AI, Heckman MG, Verbeeck C, Serie DJ, Rayaprolu S, et al. NOTCH3 variants and risk of ischemic stroke. PLoS One. 2013;8:e75035. doi: 10.1371/journal.pone.0075035. doi: 10.1371/journal.pone.0075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 11.Adib-Samii P, Rost N, Traylor M, Devan W, Biffi A, Lanfranconi S, et al. Australian Stroke Genetics Collaborative; Wellcome Trust Case-Control Consortium-2 (WTCCC2); METASTROKE; International Stroke Genetics Consortium. 17q25 Locus is associated with white matter hyperintensity volume in ischemic stroke, but not with lacunar stroke status. Stroke. 2013;44:1609–1615. doi: 10.1161/STROKEAHA.113.679936. doi: 10.1161/STROKEAHA.113.679936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 13.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 17.Galwey NW. A new measure of the effective number of tests, a practical tool for comparing families of non-independent significance tests. Genet Epidemiol. 2009;33:559–568. doi: 10.1002/gepi.20408. doi: 10.1002/gepi.20408. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122:171–185. doi: 10.1007/s00401-011-0851-x. doi: 10.1007/s00401-011-0851-x. [DOI] [PubMed] [Google Scholar]


