Abstract
Objective:
Although microvascular complications are common in type 1 diabetes mellitus (T1DM), few studies have quantified the severity, risk factors, and implications of cerebral microvascular damage in these patients. As life expectancy in patients with T1DM increases, patients are exposed to age- and disease-related factors that may contribute to cerebral microvascular disease.
Methods:
Severity and volume of white matter hyperintensities (WMH) and infarcts were quantified in 97 middle-aged patients with childhood-onset T1DM (mean age and duration: 50 and 41 years, respectively) and 81 non-T1DM adults (mean age: 48 years), concurrent with cognitive and health-related measures.
Results:
Compared with non-T1DM participants, patients had more severe WMH (Fazekas scores 2 and 3 compared with Fazekas score 1, p < 0.0001) and slower information processing (digit symbol substitution, number correct: 65.7 ± 10.9 and 54.9 ± 13.6; pegboard, seconds: 66.0 ± 9.9 and 88.5 ± 34.2; both p < 0.0001) independent of age, education, or other factors. WMH were associated with slower information processing; adjusting for WMH attenuated the group differences in processing speed (13% for digit symbol, 11% for pegboard, both p ≤ 0.05). Among patients, prevalent neuropathies and smoking tripled the odds of high WMH burden, independent of age or disease duration. Associations between measures of blood pressure or hyperglycemia and WMH were not significant.
Conclusions:
Clinically relevant WMH are evident earlier among middle-aged patients with childhood-onset T1DM and are related to the slower information processing frequently observed in T1DM. Brain imaging in patients with T1DM who have cognitive difficulties, especially those with neuropathies, may help uncover cerebral microvascular damage. Longitudinal studies are warranted to fully characterize WMH development, risk factors, and long-term effects on cognition.
Patients with type 1 diabetes mellitus (T1DM) develop slower information processing speed earlier than adults without diabetes.1 In nondiabetic populations, slower information processing speed is strongly associated with white matter hyperintensities (WMH).2,3 Few studies, however, have characterized WMH in patients with T1DM.4 Most of the knowledge about WMH comes from older populations without diabetes: WMH5 are common in individuals older than 65 years,6 with a prevalence ranging from 60% to 100%,7 and are less frequent in middle-aged adults, with prevalence ranging from 0% to 50%.8 Although they can remain silent, WMH predict greater incidence of stroke, dementia, disability, and death.2,9–11 In addition to older age, chronic exposure to hypertension and hyperglycemia are known risk factors for WMH in adults without diabetes, conditions that may predispose patients with T1DM to develop WMH. Considering the increasing life expectancy in T1DM12 and the 1.5% to 3.0% annual increase in T1DM incidence,13 determining the severity of, and risk factors for, WMH in patients with T1DM who are now aging deserves prompt investigation.
Among the studies investigating WMH in adults with T1DM,14–22 few quantified the relationship between WMH, advancing age, and cardiovascular risk factors or with processing speed; only one study16 examined patients older than 50 years.
This study applied neuroimaging at 3 tesla in middle-aged adults with T1DM to characterize the severity of, and risk factors for, WMH and associations with information processing speed, leveraging an extensive characterization of health-related factors over the prior 20 years.
METHODS
Standard protocol approvals, registrations, and patient consents.
All procedures received local institutional review board approval, and participants provided informed written consent before undergoing research procedures.
Participants.
Patients with T1DM were recruited from the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, an ongoing, prospective study of individuals diagnosed with childhood-onset T1DM between January 1, 1950, and May 31, 1980, and seen within 1 year of diagnosis at the Children's Hospital of Pittsburgh. The baseline assessment in 1986–1988 included 658 patients (mean age and duration: 28 and 19 years, respectively) who underwent biennial examinations through 1996–1998, with an additional examination in 2004–2006 and biennial questionnaires from 1998 to present. In 2010, the 263 EDC study participants living in the Pittsburgh area were invited to participate in a neuroimaging study: 106 (mean age 48 years, 50% female, 98% Caucasian) received brain imaging from 2010 to 2013, 81 were not interested, 37 were ineligible for MRI (e.g., claustrophobic, metal implants), and 39 did not reply or failed to show for their scheduled MRI (figure e-1 on the Neurology® Web site at Neurology.org). Of the 106 participants undergoing MRI, 6 did not complete the fluid-attenuated inversion recovery (FLAIR) sequence and 3 were excluded from analyses because of poor coverage or head movement in the scanner, resulting in an analytic sample of n = 97.
Adults without T1DM, participating in a study of the effects of prehypertension on cerebral structure and function, served as a comparison group. Inclusion criteria were age 35 to 60 years, living in the Pittsburgh area, and blood pressure inside 120–139/80–89 mm Hg (appendix e-1). Of the 414 who responded to mailing/advertisement and were screened to participate, 230 were enrolled (mean age 46 years), 110 were MRI ineligible, 60 changed their mind, and 14 withdrew. To mirror the EDC study racial distribution, all Caucasian participants with complete WMH data as of June 2013 (n = 81) were included in these analyses.
Measures of interest.
Comparable data collection methods were used for both cohorts for brain MRI and concurrent assessment of cognition, blood pressure, lipids, and glucose, except when noted (see reference 23 and table e-1). For participants with T1DM, prevalence of diabetes-related complications and other health and lifestyle factors were assessed periodically from 1986–1988 to 2004–2006 via questionnaire, physical examination, and medical records23; skin intrinsic fluorescence (SIF) was measured in 2004–2006 via skin fluorescence spectrometer to the left volar forearm.24 The time interval from the most recent EDC study assessments of T1DM-related complications occurred, on average, 5 years before MRI. Blood pressure, weight, height, serum glucose, and glycated hemoglobin (HbA1C) were obtained periodically for patients with T1DM from 1986–1988 to time of MRI, and study 20-year averages were computed. Three seated blood pressure readings were taken with a random-zero sphygmomanometer on day of MRI, with an average of the second and third readings used per the Hypertension Detection and Follow-up Program protocol. Smoking status was self-reported as current, past, or never smoking 100+ cigarettes, then was dichotomized as ever vs never smoking. Estimated physical activity for the past week (kcal) was determined via Paffenbarger questionnaire.
Brain MRI.
Both cohorts underwent imaging protocols on the same 3T Siemens Trio TIM scanners (Siemens AG, Erlangen, Germany) at the Magnetic Research Center, University of Pittsburgh. Details on MRI acquisition and WMH segmentation are provided in appendix e-2.
A certified neuroradiologist (J.M.) differentiated WMH from chronic lacunar-type infarcts, cerebral strokes, and perivascular spaces by careful review. WMH demonstrated no parenchymal loss and did not suppress centrally on the T2/FLAIR. Infarcts were defined as T2 hyperintense/T1 hypointense lesions with irregular borders and central parenchymal loss that suppressed on T2/FLAIR. Perivascular spaces, which can demonstrate central T1 hypointensity and T2/FLAIR suppression, were distinguished from infarcts by smooth borders, a linear orientation, and absence of adjacent T2 hyperintensity. WMH volume was normalized to total brain volume (sum of gray and white matter volumes, via the automated labeling pathway).25 All images were rated using the Fazekas scale26 blinded to case status (weighted κ: 0.825, p = 0.00001 and 0.782, p = 0.0000114 for intrarater and interrater reliability, respectively).
Cognitive tests.
General intelligence was estimated using the North American Adult Reading Test (NAART). Information processing speed was assessed using the Digit Symbol Substitution Test (DSST, number correct in 90 seconds) and the Grooved Pegboard task (dominant hand, time to insert pegs, in seconds).
Statistical analysis.
Group differences were assessed using t test for normally distributed continuous variables, Fisher exact test for categorical variables, and Wilcoxon rank sum test for nonnormal data. Demographic and other factors were compared between patients by MRI status (table e-1), between participants with and without T1DM (table 1), and between patients by WMH volume (table 2). Fisher exact test assessed between-group differences in WMH severity (dichotomized Fazekas score 1 compared with Fazekas scores 2 and 3) (table 1, figure 1).
Table 1.
Characteristics of participants with and without T1DM
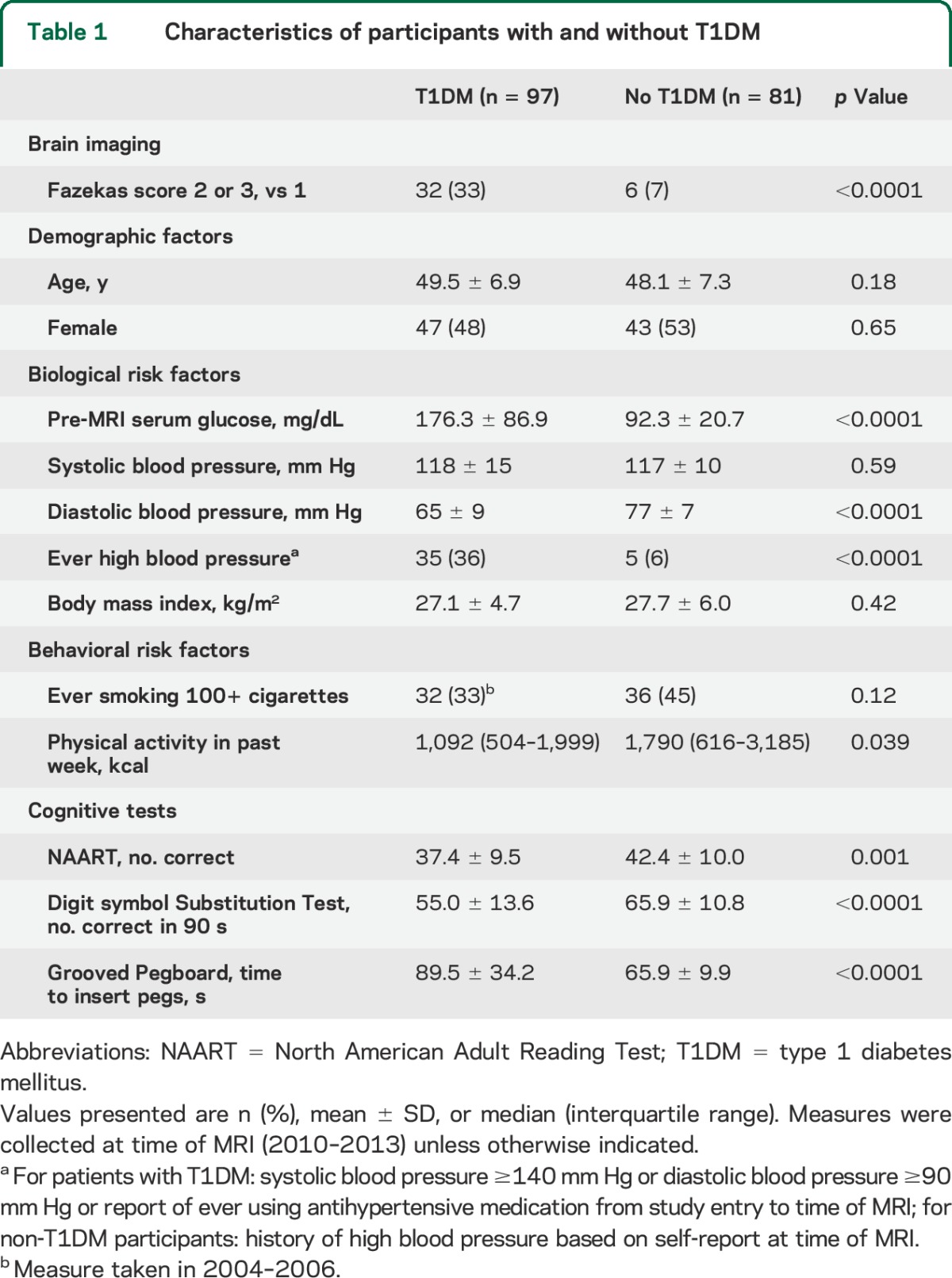
Table 2.
Characteristics of patients with T1DM
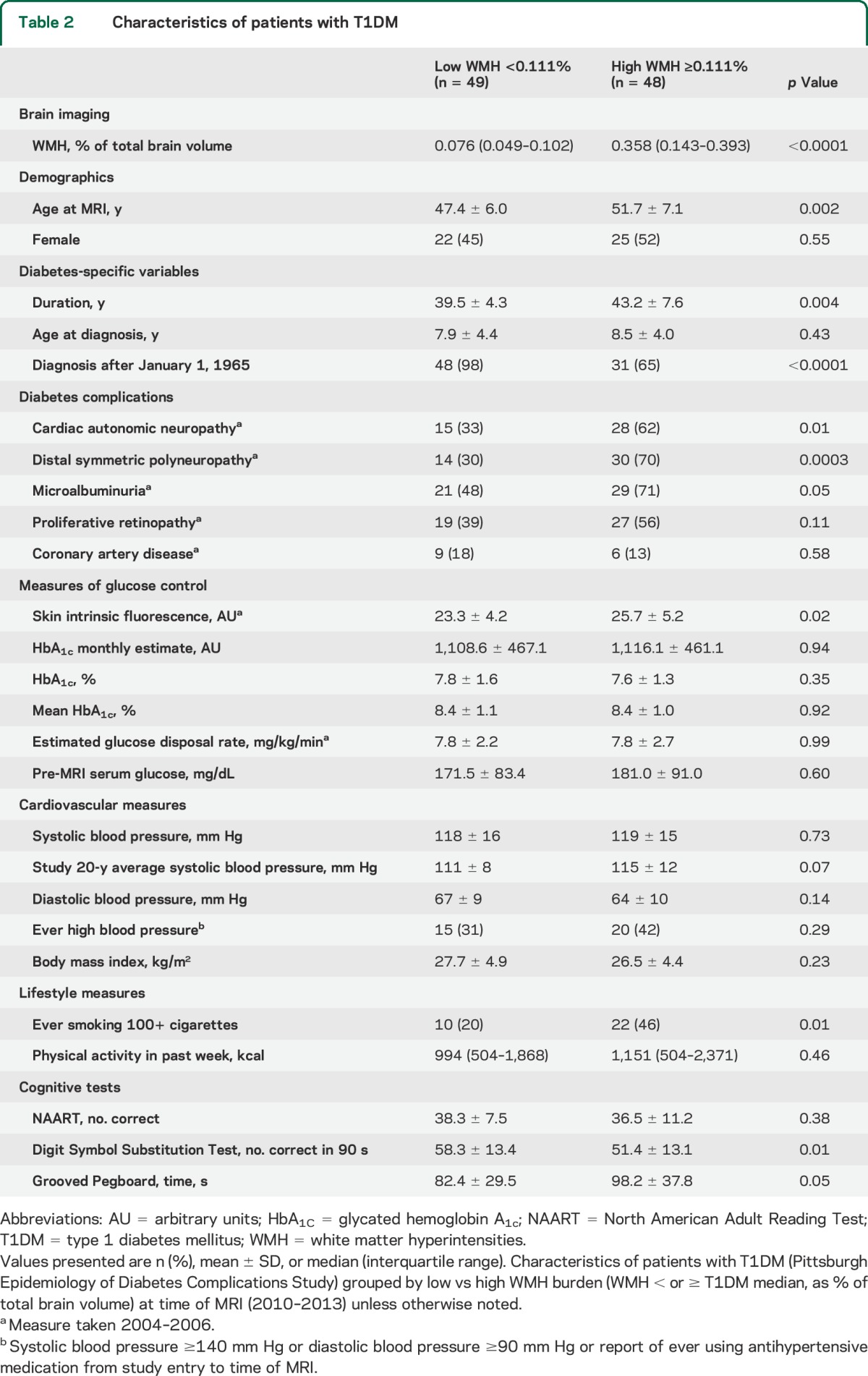
Figure 1. Between-group differences in severity of white matter hyperintensities.
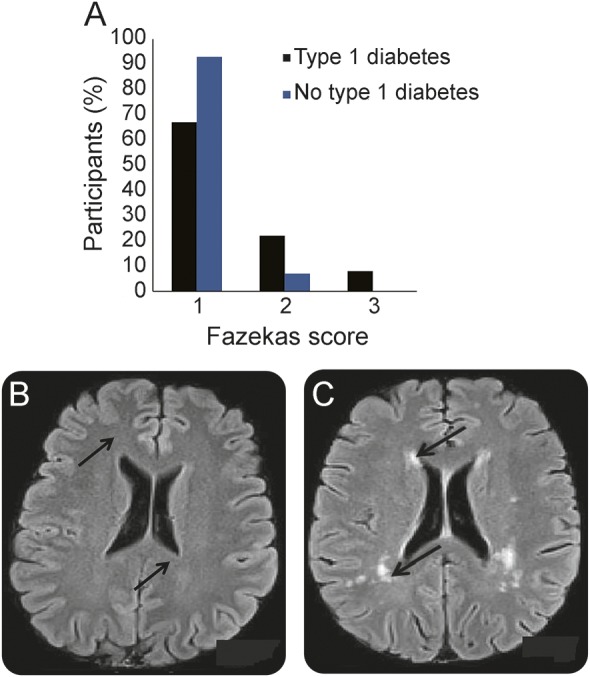
(A) Percentage of participants with type 1 diabetes (black bars) and without type 1 diabetes (blue bars) for each level of WMH severity per Fazekas score; 1 = mild; 2 = moderate; 3 = severe. All differences are statistically significant (p < 0.01). (B) FLAIR image from a 49-year-old woman without type 1 diabetes with a notable absence of WMH in periventricular (indicated by arrows) or subcortical areas or other areas. (C) FLAIR image from a 49-year-old woman with type 1 diabetes since age 10 (Epidemiology of Diabetes Complications Study), with arrows indicating substantial WMH localized in anterior and posterior periventricular areas (arrows) as well as in subcortical areas. FLAIR = fluid-attenuated inversion recovery; WMH = white matter hyperintensity.
Logistic regression models with WMH severity as outcome and T1DM status as main covariate were adjusted for factors with significant between-group differences (table 1, p < 0.05). Multivariable linear regression models, controlling for age, NAART, and factors with significant between-group differences (table 1, p < 0.05) tested the hypothesis that T1DM was related to cognitive test scores and whether WMH modified the associations between T1DM and information processing speed. Analyses were repeated excluding participants with a history of high blood pressure to ensure this WMH risk factor was not the source of between-group differences.
In analyses restricted to patients, WMH volume was dichotomized as “low” or “high” (i.e., < or ≥ theT1DM median of 0.111% ± 0.31%). Additional analyses were repeated using log-transformed WMH and using WMH severity (table e-2). Associations between factors that differed by WMH volume (table 2, p < 0.05) were included in logistic regression models controlling for diabetes duration at MRI (table 3) or age at MRI (table e-3) to minimize collinearity between these 2 variables. The time interval from the 2004–2006 EDC study examination to time of MRI was added to these models to control for the time gap between the last assessment of diabetes-related factors and MRI acquisition.
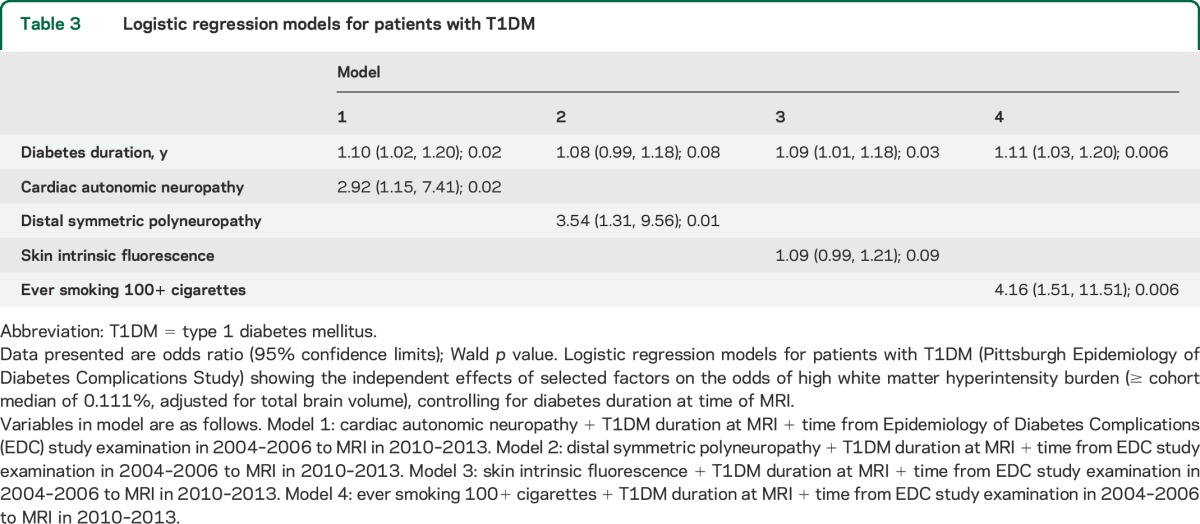
Table 3.
Logistic regression models for patients with T1DM
Analyses were repeated excluding the 18 patients diagnosed before 1965. This sensitivity analysis assessed survival bias, as mortality in the EDC study cohort was relatively negligible for those diagnosed post-1965 (15%) compared with those diagnosed pre-1965 (44%). SAS 9.3 (SAS Institute, Cary, NC) and SPSS 21.0 (IBM Corp., Armonk, NY) were used for analyses.
RESULTS
Compared with the 157 patients without MRI (table e-1), the 106 patients with MRI were significantly younger, more likely to be male, with a lower prevalence of complications and lower body mass index.
Compared with non-T1DM participants (figure 1, table 1), patients were significantly more likely to have Fazekas scores of 2 or 3, indicating more severe WMH (p < 0.0001). Five patients had at least one lacunar infarct and 4 had a history of clinical stroke, whereas none of the non-T1DM participants had either. Between-group differences in glucose, physical activity levels, and diastolic blood pressure were significant, but controlling for these variables did not modify the difference in WMH severity; regardless of which variables were added to the model, T1DM remained significantly associated with more severe WMH.
Compared with non-T1DM participants, patients had lower DSST score and longer pegboard times (table 1). In multivariable models, having T1DM was associated with worse performance on DSST and pegboard independently of NAART and age (standardized β: −0.31, p < 0.0001 and 0.40, p < 0.0001, respectively). Controlling for factors with significant between-group differences (table 1) did not modify these associations. However, adjustment for WMH severity attenuated the association of T1DM with DSST by 13% and with pegboard by 11% (standardized β: −0.27, p = 0.0001 and 0.36, p < 0.0001, respectively). In these models with T1DM status, age, and NAART, severe WMH were associated with worse performance on DSST and pegboard (standardized β: −0.14, p = 0.04 and 0.15, p = 0.05, respectively). Results were similar after excluding patients with a history of high blood pressure, lacunar infarcts, stroke, or T1DM diagnosis before 1965 and one patient with extremely high WMH volume.
Among patients, high WMH volume was significantly and positively associated with age, disease duration, prevalent cardiac autonomic and peripheral neuropathies, SIF, and smoking (table 3). Inverse associations between high WMH volume and cognitive tests were also significant. By contrast, high WMH volume was not significantly associated with markers of glucose control, hypertension, or other factors. A borderline association was found with higher 20-year average systolic blood pressure (p = 0.06). Distributions of factors were overall similar when comparing patients with T1DM by WMH severity (table e-2).
Associations between high WMH volume and prevalent cardiac autonomic and peripheral neuropathies and smoking were similar after adjustment for duration (table 3) or age (table e-3). The association of high WMH volume with SIF was borderline significant after adjustment for duration (table 3) and became nonsignificant after adjustment for age (table e-3). Results were similar when using log-transformed WMH or adjusting for the interval between assessment of complications and MRI. Results were similar when excluding those with lacunar infarcts, stroke, or T1DM diagnosis pre-1965.
DISCUSSION
This study revealed significantly greater severity of WMH and slower information processing in middle-aged patients with T1DM compared with similarly aged non-T1DM adults, independent of hyperglycemia, hypertension, and other risk factors. While the design of this study does not allow determination of whether these traditional risk factors contributed to the development of WMH, it indicates that these factors were not related to the degree of WMH severity already present in these participants at time of MRI. This study also identified diabetes-related neuropathies and ever smoking as 5-year predictors of high WMH volume among patients. These findings suggest that WMH are an emerging complication of patients with T1DM who are now living longer, advancing our understanding of T1DM-related WMH in several ways.
First, our findings indicate that severe WMH can be visualized on MRI at an earlier age than is typically seen in middle-aged non-T1DM adults. These results are consistent with one small case-control study of WMH volumes14; however, other case-control studies reported nonsignificant between-group differences16,18,22 or an absence of detectable WMH in both T1DM and non-T1DM participants.15,17 Population characteristics varied across these studies, possibly contributing to the contradictory findings. First, patients in previous studies had a shorter T1DM duration than our patients; second, participants in all but one previous study16 were younger than our patients. Furthermore, studies17,18,20,21 excluded patients with microvascular complications, thereby potentially lowering WMH prevalence. Individuals without microvascular complications are unlikely to represent the typical adult patient with long-standing T1DM. Our results indicate that age and duration are both strong correlates of WMH, hence studies of younger patients, with shorter disease duration and lower prevalence of microvascular complications, are less likely to detect between-group differences in WMH, especially at lower resolution.27
Second, we found that WMH severity correlated with the slower information processing speed observed in T1DM compared with non-T1DM participants, supporting a mechanistic role of WMH on slower information processing. The relationship of WMH with information processing speed is consistent with previous findings in elderly participants without diabetes28 but has not been reported for patients with T1DM. Additional studies are needed to identify strategies that prevent or slow the progression of WMH, possibly reducing the negative impact of WMH accrual on health outcomes including cognition. This is especially important for patients entering their fifth or sixth decade of life, in whom T1DM-related brain changes overlap with age-related brain changes. Of note, the association between T1DM and slower information processing speed remained independent of WMH, indicating the need to include other neuroimaging markers of brain integrity to fully understand the pathogenesis of slower information processing in these patients.
Among the risk factors for WMH in patients with T1DM, neuropathies had a predominant role. This is a novel finding, as most prior studies of WMH in T1DM excluded patients with neuropathy16–18 or had very low rates of neuropathy.19 It is possible that these neuropathies are epiphenomena of WMH and these conditions all reflect underlying microvascular disease burden. Although there is a biological plausibility for associations between neuropathies in the central, peripheral, or autonomic nervous systems, mechanisms underlying these associations need further investigation. If confirmed, clinicians should consider neuroimaging in patients with T1DM presenting with complaints of cognitive difficulties, particularly if accompanied with neuropathies or other microvascular complications.
Smoking is another risk factor for WMH that deserves further study. We found an association of smoking with WMH, robust to adjustment for other complications, that attenuated the associations between neuropathies and WMH. While the relationship between smoking and WMH has been previously shown in older adults without diabetes,29,30 only one prior study examined the association in T1DM,18 finding no significant relationship. However, the patients of this prior work were younger (average age 32 years), with disease duration almost half that of our patients.
This study's negative findings deserve attention. Differences in WMH severity between patients and non-T1DM participants were not accounted for by factors known to be associated with WMH in older populations, including hypertension and hyperglycemia.7,11,29,31–34 Among patients with T1DM, WMH associations with glucose or blood pressure were not significant and the association between SIF and WMH volume was weaker than those observed with other factors. Effect sizes of these relationships tend to be small in nondiabetic adults,7 hence the lack of statistical associations could be attributable to the relatively small sample size of the 2 cohorts. Of note, patients did display a difference in hypertension prevalence by WMH volume (31% and 42%) as well as in 20-year average systolic blood pressure by WMH volume (111 vs 115 mm Hg), although neither reached statistical significance. The well-controlled risk factor profile of patients at the time of MRI could also contribute to these findings.
Previous studies also reported no significant association between severe hypoglycemia and WMH,15–20 although animal studies show that hypoglycemia inhibits oligodendrocyte development, inducing apoptosis of oligodendrocyte precursor cells35; this relationship is further complicated by study results implicating posthypoglycemic reactive hyperglycemia in neuronal death.36 Other diabetes-related factors such as proliferative retinopathy and age at diagnosis were not related to WMH volume, similar to earlier studies.15,16,18,19,21,22 The 5-year interval between SIF measurement and ascertainment of diabetes-related complications and MRI acquisition may have contributed to these null findings because it may have led to an underestimation of these associations. However, glycated hemoglobin measures and blood pressure were taken periodically through time of MRI.
It is possible that long-term exposure to these factors may have a greater effect on WMH earlier, during the development of WMH, rather than later in life. Cerebral WMH develop over years of “incubation”; once cerebral WMH become manifest, these factors may no longer have a substantial role. Longitudinal neuroimaging studies of younger patients, ideally beginning near time of T1DM diagnosis, with imaging methodologies capturing early white matter abnormalities, such as diffusion tensor imaging, and with careful characterization of trajectories of risk factors and complications are needed to test this hypothesis.
The results of this study may not be entirely generalizable to other adults with childhood-onset T1DM. For instance, 99% of participants with T1DM were Caucasian while 93% of people diagnosed with T1DM in Allegheny County from 1965 to 1979 were Caucasian.37 In addition, a survivor bias may be present in that these patients have survived with diabetes for a substantial period of time, unlike patients who died before the MRI study. A selection bias is also noted in that the patients participating in this MRI study are, in general, much healthier than the parent EDC study cohort. The major causes of mortality and of morbidity preventing participation in this MRI study share a similar pathogenesis with that of WMH, potentially underestimating the true prevalence of WMH in middle-aged patients with T1DM.
Strengths of this study include using a well-defined cohort with more than 20 years of data, thus allowing computation of long-term trajectories of risk factor profiles, and using a higher-resolution neuroimaging protocol than most previous studies. By incorporating the use of a WMH visual rating scale along with a volumetric assessment of WMH, the importance of this study's findings should be easily appreciated by clinicians who would not have access to WMH volumes.
Our results underscore the relevance of studying early detection, risk factors, and long-term consequences of WMH in middle-aged patients with childhood-onset T1DM. Longitudinal neuroimaging studies, with first MRI shortly after diagnosis, are needed to understand the natural history of WMH in T1DM. Studies with larger sample sizes and wider age ranges are also needed to fully examine the contribution of chronological age and age of T1DM onset to WMH development. For these patients, preventing microvascular complications may help reduce or delay the progression of WMH. Strategies to improve myelination during the critical years of white matter production, which continue well into the fourth decade of life, are also important, because they could compensate for the detrimental effects of WMH on cognition.38,39
Supplementary Material
ACKNOWLEDGMENT
The authors thank the study participants for their time, Dr. Joseph Mettenburg for reviewing brain images, Anne Ritter for T1DM neuroimaging data management and study coordination, and Dave Fine and Jeff Krystek for processing WMH images and volumes.
GLOSSARY
- DSST
Digit Symbol Substitution Test
- EDC
Epidemiology of Diabetes Complications
- FLAIR
fluid-attenuated inversion recovery
- NAART
North American Adult Reading Test
- SIF
skin intrinsic fluorescence
- T1DM
type 1 diabetes mellitus
- WMH
white matter hyperintensity
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.A. Nunley analyzed the data, rated WMH, interpreted results, and wrote the manuscript. C.M. Ryan provided the neuropsychological test battery, provided guidance to manuscript development, and reviewed and edited the manuscript. T.J. Orchard, the principal investigator of the cohort with type 1 diabetes that provided data on population characteristics, contributed guidance on manuscript development, and edited the manuscript. H.J. Aizenstein processed MRIs, provided interpretation of brain imaging data, rated WMH, and edited the manuscript. J.R. Jennings is the principal investigator of the study that provided neuroimaging and other data on the adults without type 1 diabetes and reviewed the manuscript. J. Ryan reviewed and edited the manuscript. J.C. Zgibor provided guidance to manuscript development and edited the manuscript. R.M. Boudreau provided oversight of statistical analyses and edited the manuscript. T. Costacou provided guidance to manuscript development and statistical approach, and edited the manuscript. J.D. Maynard conducted skin intrinsic fluorescence assessments on participants with type 1 diabetes, interpreted SIF results, and reviewed the manuscript. R.G. Miller provided guidance regarding statistical analyses, managed the type 1 diabetes dataset, and reviewed the manuscript. C. Rosano is the principal investigator of the neuroimaging study of patients with type 1 diabetes that provided neuroimaging data; she also analyzed data, interpreted results, and wrote the manuscript.
STUDY FUNDING
This research was supported in part by individual NIH grants to the following: R01 DK089028, principal investigator (PI): Dr. Rosano; R01 DK034818-25, PI: Dr. Orchard; and R01 HL101959, PI: Dr. Jennings.
DISCLOSURE
K. Nunley, C. Ryan, and T. Orchard report no disclosures relevant to the manuscript. H. Aizenstein's research is funded in part by NIH grants MH076079, AG025516, AG037451, and AG044474. J. Jennings is funded by the following NIH grants: NHLBI 1RO1, NIMH 5RO1 MH084938, NIMH MH085722, NHLBI 5R01 HL105647, NHLBI 5R01 HL111802, NIMH 1R01 MH101088, and NIA R01 AG014116. J. Ryan, J. Zgibor, R. Boudreau, and T. Costacou report no disclosures relevant to the manuscript. J. Maynard is an independent medical device and diagnostics consultant. His consulting clients include Miraculins, Inc., the manufacturer of the SCOUT DS device. R. Miller and C. Rosano report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ryan CM. Diabetes, aging, and cognitive decline. Neurobiol Aging 2005;26(suppl 1):21–25. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs HIL, Leritz EC, Williams VJ, et al. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp 2013;34:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower Digit Symbol Substitution Test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc 2008;56:1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 2014;63:2244–2252. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostrup E, Gouw AA, Vrenken H, et al. The spatial distribution of age-related white matter changes as a function of vascular risk factors: results from the LADIS study. Neuroimage 2012;60:1597–1607. [DOI] [PubMed] [Google Scholar]
- 8.Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp 2009;30:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosano C, Chang YF, Kuller LH, et al. Long-term survival in adults 65 years and older with white matter hyperintensity: association with performance on the Digit Symbol Substitution Test. Psychosom Med 2013;75:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005;53:649–654. [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study cohort. Diabetes 2012;61:2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care 2013;36:1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejgaard A, Gade A, Larsson H, Balle V, Parving A, Parving HH. Evidence for diabetic encephalopathy. Diabetes Med 1991;8:162–167. [DOI] [PubMed] [Google Scholar]
- 15.Yousem D, Tasman W, Grossman RI. Proliferative retinopathy: absence of white matter lesions at MR imaging. Radiology 1991;179:229–230. [DOI] [PubMed] [Google Scholar]
- 16.Brands AM, Kessels RP, Hoogma RP, et al. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 2006;55:1800–1806. [DOI] [PubMed] [Google Scholar]
- 17.Lobnig BM, Kromeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing type 1 diabetic patients without macrovascular complications. Diabetic Med 2006;23:32–39. [DOI] [PubMed] [Google Scholar]
- 18.Weinger K, Jacobson AM, Musen G, et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia 2007;51:417–425. [DOI] [PubMed] [Google Scholar]
- 19.Perros P, Deary I, Sellar R, Best J, Frier BM. Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care 1997;20:1013–1018. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson SC, Blane A, Wardlaw J, et al. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 2005;28:1431–1437. [DOI] [PubMed] [Google Scholar]
- 22.Woerdeman J, van Duinkerken E, Wattjes MP, et al. Proliferative retinopathy in type 1diabetes is associated with cerebral microbleeds, which is part of generalized microangiopathy. Diabetes Care 2014;37:1165–1168. [DOI] [PubMed] [Google Scholar]
- 23.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469. [DOI] [PubMed] [Google Scholar]
- 24.Conway BN, Aroda VR, Maynard JD, et al. Skin intrinsic fluorescence is associated with coronary artery disease in individuals with long duration of type 1 diabetes. Diabetes Care 2012;35:2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp 2006;27:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Neuroradiol 1987;8:421–426. [DOI] [PubMed] [Google Scholar]
- 27.Olsson E, Klasson N, Berge J, et al. White matter lesion assessment in patients with cognitive impairment and healthy controls: reliability comparisons between visual rating, a manual, and an automatic volumetrical MRI method: the Gothenburg MCI Study. J Aging Res 2013;2013:198471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 2000;14:224–232. [DOI] [PubMed] [Google Scholar]
- 29.Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 2005;237:251–257. [DOI] [PubMed] [Google Scholar]
- 30.Jorm A, Anstey K, Christensen H, et al. MRI hyperintensities and depressive symptoms in a community sample of individuals 60–64 years old. Am J Psychiatry 2005;162:699–704. [DOI] [PubMed] [Google Scholar]
- 31.Soderlund H, Nyberg L, Adolfsson R, Nilsson G, Launer LJ. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex 2003;39:1093–1105. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth WT, Arnold AM, Beauchamp NJ, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 33.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure: brain atrophy, WMH change and blood pressure. J Neurol 2007;254:713–721. [DOI] [PubMed] [Google Scholar]
- 34.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging 2007;28:1307–1315. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Rivkees SA. Hypoglycemia influences oligodendrocyte development and myelin formation. Neuroreport 2006;17:55–59. [DOI] [PubMed] [Google Scholar]
- 36.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 2007;117:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ. Mortality trends in type 1 diabetes: the Allegheny County (Pennsylvania) registry 1965–1999. Diabetes Care 2001;24:823–827. [DOI] [PubMed] [Google Scholar]
- 38.Ladouceur CD, Peper JS, Crone EA, Dahl RE. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev Cogn Neurosci 2012;2:36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 2012;60:340–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


