Abstract
Obesity-induced inflammation is associated with numerous pathologies and is an independent risk factor of Chronic Kidney Disease (CKD). The prevalence of CKD is escalating and current therapeutic strategies are seriously lacking in efficacy, and immunomodulation has been suggested as a potential new therapeutic approach. Indeed, specialized pro-resolving mediators (SPMs), such as lipoxins (LXs), resolvins and protectins, have demonstrated protection in adipose inflammation, restoring insulin sensitivity and adiponectin production, while modulating leukocyte infiltration and promoting resolution in visceral adipose tissue. Furthermore, SPMs display direct renoprotective effect. Thus we review current evidence of immunomodulation as a potential strategy to subvert obesity-related CKD.
1 Obesity-related pathologies and inflammation
Prolonged obesity is associated with systemic low-grade inflammation, which is related to insulin resistance and increased risk of developing obesity-related pathologies, e.g. Type 2 Diabetes Mellitus (T2DM), atherosclerosis, non-alcoholic fatty liver disease and cancer [1]. Obesity is also an independent risk factor for chronic kidney disease (CKD), even when excluding variables such as diabetes and hypertension [2]. Interestingly, adipose distribution rather than adiposity per se determines the risk of developing obesity-related pathologies, and in this paradigm central obesity and visceral adipose tissue appears to be the major mediator of disease [3]. The prevalence of obesity is increasing rapidly, particularly among children and lower socioeconomic groups [2,4], and understanding how obesity is interlinked with inflammation and CKD is a major priority.
1.2 Adipose tissue inflammation
Adipose tissue is not merely an insulating energy store but rather an endocrine organ regulating appetite, glucose and lipid metabolism, blood pressure and immune function [5]. Prolonged obesity causes adipose hypoxia, hepatic stress responses and systemic hyperglycemia, and the combination of these factors result in adipose tissue inflammation where infiltration of inflammatory macrophages (Mϕ) is a key event [6]. Other leukocytes, including neutrophils, T-cells, B-cells, NK-cells and NK T-cells, also play important regulatory roles in adipose inflammation, as recently reviewed [7,8]. In obesity, the majority of adipose Mϕs are derived from blood monocytes, and recruitment is regulated though chemoattractants such as MCP-1/CCL2 [7,9]. Interestingly FFA derived from adipose lipolysis may also act as a recruitment molecule [10]. The current consensus holds that lean subjects have a basal state of anti-inflammatory M2 Mϕs, whereas obesity causes a recruitment of pro-inflammatory M1 Mϕs [9]. These M1 Mϕs accumulate around dying adipocytes in so called crown like structures (CLS) and produce pro-inflammatory mediators (e.g. TNF-α, IL-1β, IL-6) which are associated with the development of insulin resistance, and subsequent release of free-fatty-acids results in systemic lipotoxicity with detrimental effects [5,6]. In support of this, blocking Mϕ recruitment rescues obesity-induced insulin resistance [11] and PPAR-γ deficient mice displaying impaired M2 phenotype are more susceptible to diet-induced inflammation and insulin resistance [12]. Furthermore, IFN-γ KO mice display improved insulin sensitivity, reduced adipocyte hypertrophy, and a reduced number of adipose M1 Mϕ [13]. However, it should be mentioned that there is ongoing debate in the field. One theory holds that obesity-induced recruitment of Mϕs reflects an adaptive response attempting to retain adipose functionality [7]. Interestingly, Mϕs may help restrict adipocyte hypertrophy as obese CCL2 KO mice lacking Mϕ infiltration display increased adipocyte diameter [7]. It has also been suggested that M1 Mϕs play a beneficial role whereby they phagocytose lipids excreted by adipocytes, importantly without producing pro-inflammatory cytokines, which may suggest that the M1 phenotype is more complex than previously assumed [10]. Similarly the M2 phenotypes are also multifaceted, as M2c Mϕs have been suggested to induce adipose fibrosis through their TGF-β secretion [14]. Thus Mϕs phenotyping is a intricate process that requires careful attention and further characterization, since Mϕs undoubtedly play an important role in adipose inflammation and the onset of obesity-induced pathology, such as CKD.
1.3 Adipose inflammation and CKD
Importantly, obesity-induced adipose inflammation alters the adipokine profile, where leptin and fetuin-A play important roles and correlate with pathologies such as CKD [2,6]. Additionally, adipose inflammation attenuates production of the protective hormone adiponectin, contributing to insulin resistance, inflammation and oxidative stress [15]. Indeed T2DM patients with hypoadiponectinemia display more severe renal damage compared to controls [16]. In mice adiponectin regulates vasodilation via induction of eNOS and NO and displays renoprotective properties by reversing loss of podocyte foot processes, through induction of AMPK activation and attenuation of Nox4 and ROS production [2]. In addition to the adipokines, adipose tissue also expresses the components of the renin-angiotensin (RAAS) system, locally affecting adipose glucose homeostasis, lipid metabolism and inflammation [17]. Obesity-induced upregulation of adipose RAAS may contribute to as much as 30 % of circulating angiotensinogen, causing a paracrine effect linked to kidney disease and inflammation [18]. Interestingly activation of Antigotensin-receptor-1a appears to be an important mediator of inflammation and renal injury in obesity-induced CKD [19].
CKD is characterized by progressive loss of renal function with an accumulation of pro-fibrotic extracellular matrix (ECM) leading to glomerulosclerosis and tubulointerstitial fibrosis (TIF) [2]. Consequent loss of parenchyma and disease progression is further propagated by inflammation, insulin resistance and oxidative stress [2,20]. Although CKD is typically diagnosed well before it reaches end-stage kidney disease, there is as of yet no treatment that halts or reverses the decline in renal function and current therapeutics merely focus on slowing disease progression through blood pressure and glycaemic control. As such there is an acute need for novel anti-fibrotic and pro-resolving therapeutics.
Immunomodulation has been suggested as an alternatively therapeutic path, as the obesity-induced adipose and systemic inflammation are central to CKD development [6,21]. However, in order to successfully use immunomodulation as a therapeutic tool, we must first appreciate how the intricate inflammatory process is regulated. Thus the resolution of inflammation is described below, followed by current evidence that promoting the resolution of inflammation may be beneficial in obesity and CKD, respectively.
2 Resolution of inflammation
Inflammation is a fundamental part of normal physiology, shielding the host from pathogens and tissue injury. However, this dynamic process must be tightly regulated to avoid chronic inflammation and pathology. Indeed, failure of inflammatory resolution may result in severe conditions, including abscess formation and fibrosis as evident in arthritis, diabetes and atherosclerosis [21]. It is likely that the resolution of inflammation is tightly regulated by specialized pro-resolving mediators (SPMs). These include lipids mediators e.g. lipoxins (LXs), resolvins and protectins, but also peptides such as Annexin-1 [22].
Inflammation is initiated by pro-inflammatory leukotrienes (LTs) and prostaglandins (PGs), causing vasodilation and recruitment of inflammatory cells that battle the inflammatory insult (Figure 1). Interestingly, in physiological acute inflammation, e.g. normal wound healing, the same mediators initiating the process also program its resolution, as PGE2 enhance the production of SPMs via the induction of 15-Lipoxygenase (LO) [23]. In an intricate network, SPMs enhance resolution by reducing vascular permeability and attenuating production of inflammatory cytokines and chemokines, while stimulating pro-resolving mediators such as IL-10 [21,22]. Interestingly, SPMs have profoundly different roles on leukocytes of varying origin and while they reduce vascular permeability and inhibit PMN recruitment, they promote infiltration of monocytes and furthermore shift their phenotype from inflammatory (M1) to resolving (M2). An important SPM characteristic is that they promote efferocytosis, i.e. the non-phlogistic phagocytosis of apoptotic PMN by Mϕs, which is a crucial process in resolution [6,22,24]. As such, SPMs exhibit protection from numerous disease processes though manipulation of leukocytes, as reviewed [20,25]. Importantly, SPMs also affect cells of non-myeloid origin, such as endothelial and mesangial cells [20]. In addition, recent studies demonstrate that SPMs may exert protective properties via microRNAs. Indeed, RvD1 has been shown to promote resolution of acute inflammation via activation of microRNA [26] and similarly LipoxinA4 (LXA4) attenuates chronic renal inflammation by activation of let7 [27].
Figure 1. Lipid mediators regulating the onset and resolution of inflammation.
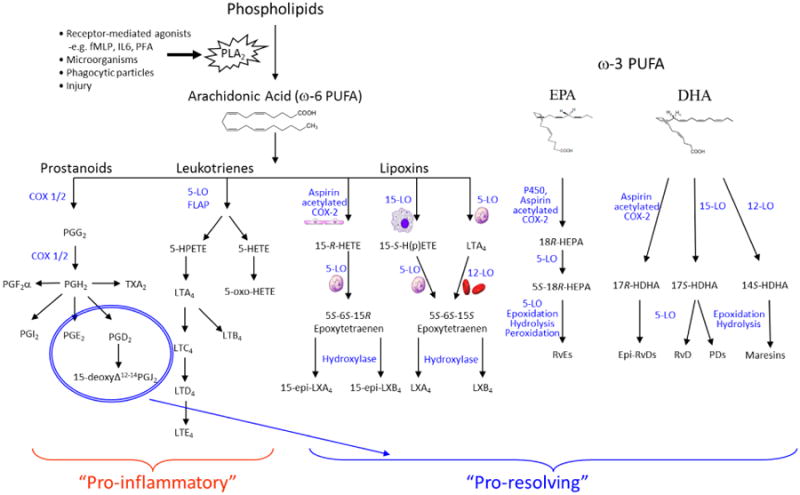
The initiation and resolution of inflammation is regulated by numerous lipid mediators. Upon injury or insult, PLA2 cleaves membrane phospholipids to yield arachidonic acid derived prostaniods and leukotriens, which induce vasodilation and act as recruiting chemokines for infiltrating leukocyte. Prostaglandin (PG)E2 and 15Δ-PGJ2 may also act as pro-resolving mediators, initiating the production of specialized pro-resolving mediators (SPMs) though induction of 15-lipoxygenase (LO) expression. Lipoxins (LXs) are also generated in a trans-cellular manner involving neutrophils, plateles and resident tissue cells, such as epithelial cells. LO thus transforms AA into 15-Hydroxyeicosatetraenoic acid (HETE) and subsequently LXA4 or LXB4. Aspirin may also induce production of subsequently epi-LXs by acetylates cyclooxygenase (COX)-2 and shifting its activity from that of an endoperoxidase to a lipoxygenase, yielding 15-HETE and 15-epi-LXA4 or 15-epi-LXB4 though the action of 5-LO. ω-3 PUFA may also give rise to pro-resolving lipid mediators. Eicosapentaenoic acid (EPA) is converted by cytochrome P450 or acetylated COX-2 into 18R-HEPA, which can be further transformed by enzymatic epoxidation and 5-LO in leukocytes to form E series resolvins (RvE). Docosahexaenoic acid (DHA) may be converted into D series resolvins (RvD) by the sequential activation of 15-LO or acetylated COX-2 into 17R-HDHA, which is then transformed by enzymatic epoxidation and 5-LO to form D series resolvins (RvD). Protectins are similarly to resolvins generated from DHA, but via a separate pathway involving 15-LO and enzymatic epoxydation and hydrolysis, where 17S-H(p)-DHA serves as the intermediate product. MΦ mediator in resolving inflammation (maresin) are in human cells generated from DHA by a 12-LO, which forms 14S-HDHA. This product is then further modified into maresins by epoxidation and hydrolysis.
Lipoxins are arachidonic acid (AA) derived eicosanoids, produced at local sites of inflammation in a transcellular manner by the sequential action of 5-LO and either 12-LO or 15-LO, between neutrophils, platelets and resident tissue cells, e.g. epithelial cells (Figure 1). Formation of epi-LXs may also be induced though aspirin-mediated acetylation of cyclooxygenase (COX)-2 [22]. LipoxinA4 (LXA4) and its positional isomer lipoxinB4 (LXB4) are the principal mammalian LX species. LXA4 binds the G-protein coupled receptor (GPCR) receptor FPR2/ALX, identified in numerous cell types, including monocytes and Mϕs, T-cells, fibroblasts, renal mesangial cells and murine adipocytes [20,28]. LXA4 also interacts with GPR32 [29], whereas the LXB4 receptor remains to be identified. In addition to LXs, several other SPMs have been identified, including the ω-3 derived resolvins, protectins and marseins [6,22]. Resolvins may be synthesized from either eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA), and are thus divided into ‘E series’ and ‘D series’ [6,22]. Resolvins are generated in a transcellular manner by the sequential action of LO, whereas protectins and maresins are generated by single cells (Figure 1). In neutrophils RvE1 has been shown to bind BLT1, whereas in Mϕ and dendritic cells RvE1 bind ChemR23 [22]. RvD1 has also been reported to interact both with FPR2/ALX and GPR32 in phagocytes, but it is currently unknown which receptor the protectins and maresins act through [22]. SPMs display their anti-inflammatory effect in numerous inflammatory disorders, including kidney disease, peritonitis, asthma and atherosclerosis [6,21,22].
In the context of inflammatory resolution it is also noteworthy to mention PMN-derived micro-particles (MPs), which enhance resolution by attenuating PMN recruitment while enhancing efferocytosis [30-32]. Human MPs activate the ALX/FPR2 receptor and have been shown to contain SPM precursors and Annexin-1 [30,31]. Interestingly, MPs have been manipulated into so called Humanized nano-proresolving medicines (NPRMs), where additional SPMs are incorporated into MPs to augment their pro-resolving effect [30]. Indeed, NPRMs have proven to attenuate leukocyte trafficking in vitro [33], as well as zymoza-induced peritonitis, wound healing and inflammatory joint disorders in mice [30]. NPRMs thus display an interesting therapeutic potential in relation to inflammatory resolution and delivery of SPMs.
3 Proresolving lipid mediators in adipose inflammation
Promoting resolution of adipose inflammation would likely to be a beneficial therapeutic approach, reducing the risk of developing obesity-associated complications [6,21,34]. Research is currently attempting to address this hypothesis, primarily using genetic or high-fat-diet models of obesity in rodents. However, it is noteworthy that genetic obesity does not impact adipose 12/15-LO expression, whereas diet-induced obesity causes a significant decrease [35]. Interestingly, 5-LOX expression remained unaltered by obesity in both models [35]. As the LO enzymes are essential for SPM synthesis this may account for some difference between experimental models of obesity. As reported by Neuhofer et al, the SPMs are differently expressed in genetic vs. diet-induced obesity, although they often appear to follow similar trends.
Production of specialized pro-resolving mediators (SPMs) appears deficient in obese visceral adipose tissue, and genetic models of obesity attenuate endogenous production of SPMs [35]. To our knowledge it has not yet been demonstrated whether weight loss restores SPM production, which would be an important contribution to the field. In this context it would also be important to differentiate between short-term and long-term weight loss. Although caloric restriction quickly restores insulin sensitivity, it somewhat surprisingly appears that short-term (3-7 days) weight loss increases lipolysis, thus accelerating Mϕ recruitment, although these Mϕs appear non-phlogistic based on the fact that they phagocytose lipids without promoting inflammation [10]. Similarly 3 wk caloric restriction restores insulin sensitivity and although the number of CD11c+ Mϕ remains unaltered, they display decreased expression of TNF-α and IL-1β, suggesting phenotype placidity [36]. Longer duration of caloric restriction (6wk) in mice attenuate Mϕ infiltration [10]. In human studies 12 wk caloric restriction attenuates TNF-α while increasing adiponectin [37] and 3 months after surgically-induced weight-loss there is reduced adipose tissue M1/M2 ratio [38]. Another interesting aspect would be to investigate whether glucagon-like-peptide-1 (GLP-1) affects SPMs production, as GLP-1 inhibits adipose tissue Mϕ infiltration and inflammation in ob/ob mice [39], and the GLP-1R agonist Exenatide has been shown to increase myocardial LX [40].
3.1 Omega-3 derived SPMs in adipose tissue inflammation
Obesity is associated with attenuation of resolvins and dietary EPA and DHA supplementation increase insulin sensitivity and adiponectin levels, while attenuating adipose inflammation and adipose CLSs [35]. The beneficial effect of ω-3 PUFA in obesity is well established [6] and ω-3 PUFA increases SPM levels in high-fat-diet induced obesity [41]. Transgenic restoration of long-chain ω-3 PUFA also alleviates obesity-linked inflammation and insulin resistance [42]. In ob/ob mice both ω-3 PUFA and RvE1 increased expression of genes involved in glucose transport (GLUT-4), insulin signaling (IRS-1) and insulin sensitivity (PPAR-γ). Furthermore, they increased adiponectin levels, as did PD1 when incubated with adipose explants from ob/ob mice [43]. Interesting, the resolution of acute inflammation has been described as impaired in type 2 diabetes, as db/db mice with peritonitis present with impaired wound closure and increased leukocyte infiltration and impaired efferocytosis [44]. Furthermore, endogenous production of resolvins appeared impaired, and RvD1 increased wound closure and enhanced the peritonitis-related resolution[44]. Similarly the pro-resolving lipid mediator 14S,21R-diHDHA has also been shown to restore Mϕ-mediated wound healing in diabetic mice [45].
RvD1 also improves insulin sensitivity in the db/db model, correlating with restored levels of pAktSer473 in adipose tissue and aorta, although skeletal muscle, liver and heart tissue remained unaffected [46]. Furthermore, RvD1 reduce adipose CLS while increasing adipose M2 (MGL-1+) to M1 (CD11c+) ratio [46], although it should be mentioned that the role of MGL-1+ Mϕs in obesity has been debated [47]. Other studies confirm that RvD1 shift the phenotype of peritoneal Mϕs from M1 to M2 and stimulate efferocytosis [48]. In db/db mice RvD1 increase plasma adiponectin and adipose pAMPK, while circulating resistin and adipose PPAR-γ expression remained unaltered [46]. Interestingly, RvD1 inhibited ATM IL-6 [46], previously shown to attenuate adiponectin expression in 3T3-L1 adipocytes [49], which may provide mechanistic insights [46]. RvD1 and RvD2 also restore high-fat-diet induced attenuation of adiponectin while inhibiting leptin, TNF-α, IL-6 and IL-1β secretion, as well as monocyte adherence to adipocytes and trans-adipose migration [28].
3.2 Omega-6 derived SPMs in adipose tissue inflammation
The direct effect of LXA4 on obesity-induced adipose inflammation remains to be investigated. However, in a model of age-associated inflammation, LXA4 attenuates adipose IL-6 while increasing IL-10, which correlated with restoration of adipose GLUT-4 and IRS-1 expression [50]. Furthermore, LXA4 in vitro rescue Mϕ induced attenuation of adipose glucose uptake in response to insulin. In this system LXA4 attenuated Mϕ production of inflammatory cytokines (TNF-α and MCP-1), while restoring insulin-induced pAkt and GLUT-4 upregulation to the plasma membrane in 3T3-L1 adipocytes [50]. LXA4 has also been shown to restore diet-induced attenuation of adiponectin [28].
4 Proresolving lipid mediators in kidney disease
The role of SPMs in obesity-induced CKD remains to be evaluated. It is however clearly established that inflammation plays a crucial role in kidney disease and immunomodulation and may provide a beneficial tool in subverting CKD [21]. Interestingly, attenuation of the pro-resolving cytokine IL-10 from the spleen has been implicated as an initiator in obesity-induced CKD [51]. SPMs have been shown to directly attenuate renal injury, as reviewed [4,21]. The effect of SPMs was first displayed in models of acute renal injury, e.g. ischemia-reperfusion where LXs, protectins and resolvins attenuate neutrophil influx and Mϕ activation to the effect of attenuated kidney injury [20]. In vitro SPMs have also displayed potential to modulate inflammatory and fibrotic responses in podocytes, mesangial and epithelial cells [21]. More recently, SPMs have also been evaluated in experimental CKD, utilizing the unilateral ureteric obstruction (UUO) model. Indeed, LXA4 and its synthetic analog benzo-LXA4 attenuate UUO-induced renal fibrosis, as displayed by reduced renal collagen deposition and attenuated activation of MAP kinases, Akt and Smads [52]. Importantly, the LXs shifted the inflammatory milieu toward resolution, inhibiting TNF-α and IFN-γ expression, while stimulating pro-resolving IL-10. In vitro it was specifically demonstrated that LXs modulate fibroblast activation, inhibiting TGF-β1-induced activation of Smad2 and MAP-kinases [52]. RvE1 and RvD1 have also demonstrated protection in rodent UUO models, attenuating collagen deposition and PDGF-BB expression, as well as Mϕ infiltration [53]. The resolvins diminished myofibroblasts accumulation and fibroblast proliferation (Ki67+/α-SMA+) both in vivo and in vitro, via activation of ChemR23. Similarly to LXs [52], resolvins attenuated UUO-induced activation ERK and AKT signaling pathways [53]. As illustrated in Figure 1, 15Δ-PGJ2 may similarly to the SPMs contribute to the resolution of inflammation [6,54]. It is thus noteworthy that 15Δ-PGJ2 was recently shown to induce HO-1 expression and increase antioxidant response though Nrf2 in mesangial cells [55]. Annexin-1 has also been shown to be protective in ischemia-reperfusion injury in the rat [56], although to our knowledge its effect as of yet has not been determined in CKD. Interestingly, LXs and RvE1 enhance survival following kidney transplantation in mice [57].
Supplementation of anti-inflammatory ω-3 PUFA has been suggested as a beneficial strategy in advanced kidney disease [58]. In CKD patients, higher doses of ω-3 PUFA increase subcutaneous adiponectin and leptin production, while attenuating MMP9 and CD68 levels, indicating some protection against inflammation although eGFR remained unaltered [59]. Similarly in an 8 week study with CKD stage 2-5 patients, ω-3 PUFA supplementation decreased levels of pro-inflammatory LTB4 and 5-HETE, although renal creatinine clearance and proteinuria did not improve [60]. Renoprotective effects of ω-3 PUFA, DHA and EPA have however been demonstrated in experimental models of kidney disease, reducing upregulation of pro-inflammatory and pro-fibrotic pathways and attenuating TIF [20,21]. As Diabetic Nephropathy is a prevalent form of CKD, it is noteworthy that the TZD drug Pioglitazone increase formation of 15-epi-LXA4 in diabetic patients, which was associated with decreasing fasting glucose and increased adiponectin levels [61]. Furthermore, LXA4 appears to be an important mediator of resolution in spontaneously resolving poststreptococcal glomerulonephritis [62].
5 Conclusion
Obesity is associated with chronic inflammation and is a potent contributor to CKD. The resolution of inflammation is tightly regulated by SPMs, which display protective effects in obesity-induced adipose inflammation and several models or kidney disease. The direct impact of SPMs on obesity-induced CKD remains to be determined. However, immunomodulation through the use of SPMs may be an important tool in developing novel therapeutic pathways to battle obesity-induced pathologies such as CKD.
Highlights.
Adipose and renal inflammation play a central role in obesity-induced CKD
Inflammatory resolution is regulated by SPMs (e.g. lipoxins, resolvins, protectins)
SPMs attenuate obesity-induced adipose inflammation and restore insulin sensitivity
SPMs reduce renal fibrosis and inflammation in experimental CKD
Immunomodulation may be a therapeutic strategy to subvert obesity-induced CKD
Acknowledgments
Work in Prof Sharma's laboratory is supported by VA MERIT Award and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Awards, specifically U01 DK060995, DP3 DK094352-01 and DK083142. Dr Börgeson is a recipient of Marie Curie international outgoing fellowship.
Abbreviations
- AA
Arachidonic acid
- ATLs
Aspirin triggered lipoxins
- BAT
Brown adipose tissue
- CVD
Cardiovascular disease
- CKD
Chronic kidney disease
- COX-2
Cyclooxygenase-2
- DHA
Docosahexaenoic acid
- GFR
Glomerular filtration rate
- GPCR
G-protein coupled receptor
- EPA
Eicosapentaenoic acid
- ECM
Extracellular matrix
- LTs
Leukotriene
- LXs
Lipoxins
- LO
Lipoxygenase
- PGs
Prostaglandins
- RAAS
Renin-angiotensin
- SPMs
Specialized pro-resolving mediators
- TIF
Tubulointerstitial fibrosis
- T2DM
Type 2 Diabetes Mellitus
- WAT
White adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol. 2010;108:735–743. doi: 10.1152/japplphysiol.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew AV, Okada S, Sharma K. Obesity related kidney disease. Curr Diabetes Rev. 2011;7:41–49. doi: 10.2174/157339911794273928. [DOI] [PubMed] [Google Scholar]
- 3.Noori N, Hosseinpanah F, Nasiri AA, Azizi F. Comparison of overall obesity and abdominal adiposity in predicting chronic kidney disease incidence among adults. J Ren Nutr. 2009;19:228–237. doi: 10.1053/j.jrn.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10:306–315. doi: 10.1007/s11892-010-0122-6. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Periz A, Claria J. Resolution of adipose tissue inflammation. ScientificWorldJournal. 2010;10:832–856. doi: 10.1100/tsw.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–314. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583–2592. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 9.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14:341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism. 2012;61:1152–1161. doi: 10.1016/j.metabol.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kacso I, Lenghel A, Bondor CI, Moldovan D, Rusu C, Nita C, Hancu N, Gherman Caprioara M, Kacso G. Low plasma adiponectin levels predict increased urinary albumin/creatinine ratio in type 2 diabetes patients. Int Urol Nephrol. 2012;44:1151–1157. doi: 10.1007/s11255-011-0064-1. [DOI] [PubMed] [Google Scholar]
- 17.Feraco A, Armani A, Mammi C, Fabbri A, Rosano GM, Caprio M. Role of mineralocorticoid receptor and renin-angiotensin-aldosterone system in adipocyte dysfunction and obesity. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011;79:162–168. doi: 10.1038/ki.2010.391. [DOI] [PubMed] [Google Scholar]
- 19.Ma LJ, Corsa BA, Zhou J, Yang H, Li H, Tang YW, Babaev VR, Major AS, Linton MF, Fazio S, et al. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am J Physiol Renal Physiol. 2011;300:F1203–1213. doi: 10.1152/ajprenal.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgeson E, Godson C. Molecular circuits of resolution in renal disease. ScientificWorldJournal. 2010;10:1370–1385. doi: 10.1100/tsw.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgeson E, Godson C. Resolution of inflammation: therapeutic potential of pro-resolving lipids in type 2 diabetes mellitus and associated renal complications. Front Immunol. 2012;3:318. doi: 10.3389/fimmu.2012.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 24.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41:366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. First study to demonstrate that resolvins regulated specific miRNAs target genes involved in resolution, establishing a novel resolution circuit involving RvD1 receptor-dependent regulation of specific miRNAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan EP, Nolan KA, Borgeson E, Gough OS, McEvoy CM, Docherty NG, Higgins DF, Murphy M, Sadlier DM, Ali-Shah ST, et al. Lipoxins Attenuate Renal Fibrosis by Inducing let-7c and Suppressing TGFbetaR1. J Am Soc Nephrol. 2013;24:627–637. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. This article demonstrates that resolvins of the D-series counteract obesity-induced adipose inflammation and that adipose tissue expresses the SPM receptors ALX/FPR2, ChemR23, and GPR32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. A study reporting on a new therapeutic approach whereby SPMs are incorporated into micro-particles to augment their pro-resolving effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 32.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, Irimia D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci U S A. 2012;109:20560–20565. doi: 10.1073/pnas.1210269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filep JG. Resolution pathways in inflammation: The devil in the adipose tissues and in the details. Focus on “Diversity of lipid mediators in human adipose tissue depots”. Am J Physiol Cell Physiol. 2013 doi: 10.1152/ajpcell.00063.2013. [DOI] [PubMed] [Google Scholar]
- 35*.Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, Hochbrugger EE, Fraisl P, Cinti S, Serhan CN, et al. Impaired local production of pro-resolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013 doi: 10.2337/db12-0828. A novel report showing that obesity impairs biosynthesis of SPMs. The article also makes important comparisons between genetic and diet-induced obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee IS, Shin G, Choue R. A 12-week regimen of caloric restriction improves levels of adipokines and pro-inflammatory cytokines in Korean women with BMIs greater than 23 kg/m2. Inflamm Res. 2010;59:399–405. doi: 10.1007/s00011-009-0113-8. [DOI] [PubMed] [Google Scholar]
- 38.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 39.Lee YS, Park MS, Choung JS, Kim SS, Oh HH, Choi CS, Ha SY, Kang Y, Kim Y, Jun HS. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55:2456–2468. doi: 10.1007/s00125-012-2592-3. [DOI] [PubMed] [Google Scholar]
- 40.Ye Y, Qian J, Castillo AC, Ling S, Ye H, Perez-Polo JR, Bajaj M, Birnbaum Y. Phosphodiesterase-3 inhibition augments the myocardial infarct size-limiting effects of exenatide in mice with type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304:H131–141. doi: 10.1152/ajpheart.00609.2012. [DOI] [PubMed] [Google Scholar]
- 41.Flachs P, Ruhl R, Hensler M, Janovska P, Zouhar P, Kus V, Macek Jilkova Z, Papp E, Kuda O, Svobodova M, et al. Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia. 2011;54:2626–2638. doi: 10.1007/s00125-011-2233-2. [DOI] [PubMed] [Google Scholar]
- 42.White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59:3066–3073. doi: 10.2337/db10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. Faseb J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian H, Lu Y, Shah SP, Hong S. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. Am J Pathol. 2011;179:1780–1791. doi: 10.1016/j.ajpath.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 49.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 50*.Borgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF, Maderna P, Roche HM, Godson C. Lipoxin A4 attenuates adipose inflammation. Faseb J. 2012;26:4287–4294. doi: 10.1096/fj.12-208249. The first report on lipoxins attenuating adipose inflammation and macrophage-induced insulin resistance, in a model of age-associated inflammation. [DOI] [PubMed] [Google Scholar]
- 51.Gotoh K, Inoue M, Masaki T, Chiba S, Shiraishi K, Shimasaki T, Matsuoka K, Ando H, Fujiwara K, Fukunaga N, et al. Obesity-related chronic kidney disease is associated with spleen-derived IL-10. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfs440. [DOI] [PubMed] [Google Scholar]
- 52**.Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O'Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. Faseb J. 2011;25:2967–2979. doi: 10.1096/fj.11-185017. The first report of how lipoxins protecting against experimental chronic kidney disease, where lipoxins attenuated both UUO-induced inflammation and fibrosis. [DOI] [PubMed] [Google Scholar]
- 53**.Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol. 2012 doi: 10.1002/path.4050. An interesting study reporting protective effect of resolvins in chronic kidney disease, where the SPMs attenuated both macrophage infiltration and fibrosis. [DOI] [PubMed] [Google Scholar]
- 54.Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci U S A. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez AE, Sanchez-Gomez FJ, Diez-Dacal B, Oeste CL, Perez-Sala D. 15-Deoxy-Delta(12,14)-prostaglandin J2 exerts pro- and anti-inflammatory effects in mesangial cells in a concentration-dependent manner. Inflamm Allergy Drug Targets. 2012;11:58–65. doi: 10.2174/187152812798889349. [DOI] [PubMed] [Google Scholar]
- 56.Facio FN, Jr, Sena AA, Araujo LP, Mendes GE, Castro I, Luz MA, Yu L, Oliani SM, Burdmann EA. Annexin 1 mimetic peptide protects against renal ischemia/reperfusion injury in rats. J Mol Med (Berl) 2011;89:51–63. doi: 10.1007/s00109-010-0684-4. [DOI] [PubMed] [Google Scholar]
- 57.Levy BD, Zhang QY, Bonnans C, Primo V, Reilly JJ, Perkins DL, Liang Y, Amin Arnaout M, Nikolic B, Serhan CN. The endogenous pro-resolving mediators lipoxin A4 and resolvin E1 preserve organ function in allograft rejection. Prostaglandins Leukot Essent Fatty Acids. 2011;84:43–50. doi: 10.1016/j.plefa.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman AN. Omega-3 fatty acid supplementation in advanced kidney disease. Semin Dial. 2010;23:396–400. doi: 10.1111/j.1525-139X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 59.Guebre-Egziabher F, Debard C, Drai J, Denis L, Pesenti S, Bienvenu J, Vidal H, Laville M, Fouque D. Differential dose effect of fish oil on inflammation and adipose tissue gene expression in chronic kidney disease patients. Nutrition. 2013 doi: 10.1016/j.nut.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Maaloe T, Schmidt EB, Svensson M, Aardestrup IV, Christensen JH. The effect of n-3 polyunsaturated fatty acids on leukotriene B(4) and leukotriene B(5) production from stimulated neutrophil granulocytes in patients with chronic kidney disease. Prostaglandins Leukot Essent Fatty Acids. 2011;85:37–41. doi: 10.1016/j.plefa.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez AD, Sathyanarayana P, Konduru S, Ye Y, Birnbaum Y, Bajaj M. The effect of pioglitazone treatment on 15-epi-lipoxin A4 levels in patients with type 2 diabetes. Atherosclerosis. 2012;223:204–208. doi: 10.1016/j.atherosclerosis.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Wu SH, Liao PY, Yin PL, Zhang YM, Dong L. Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. Am J Pathol. 2009;174:115–122. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]


