Abstract
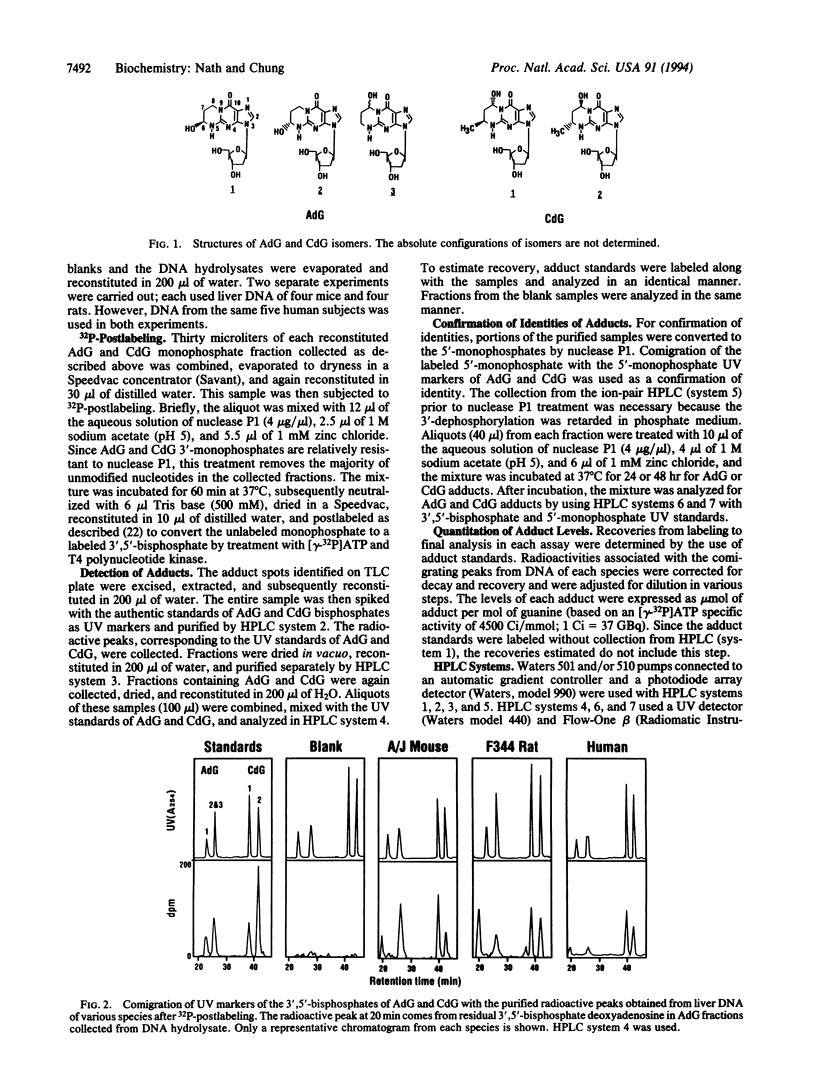
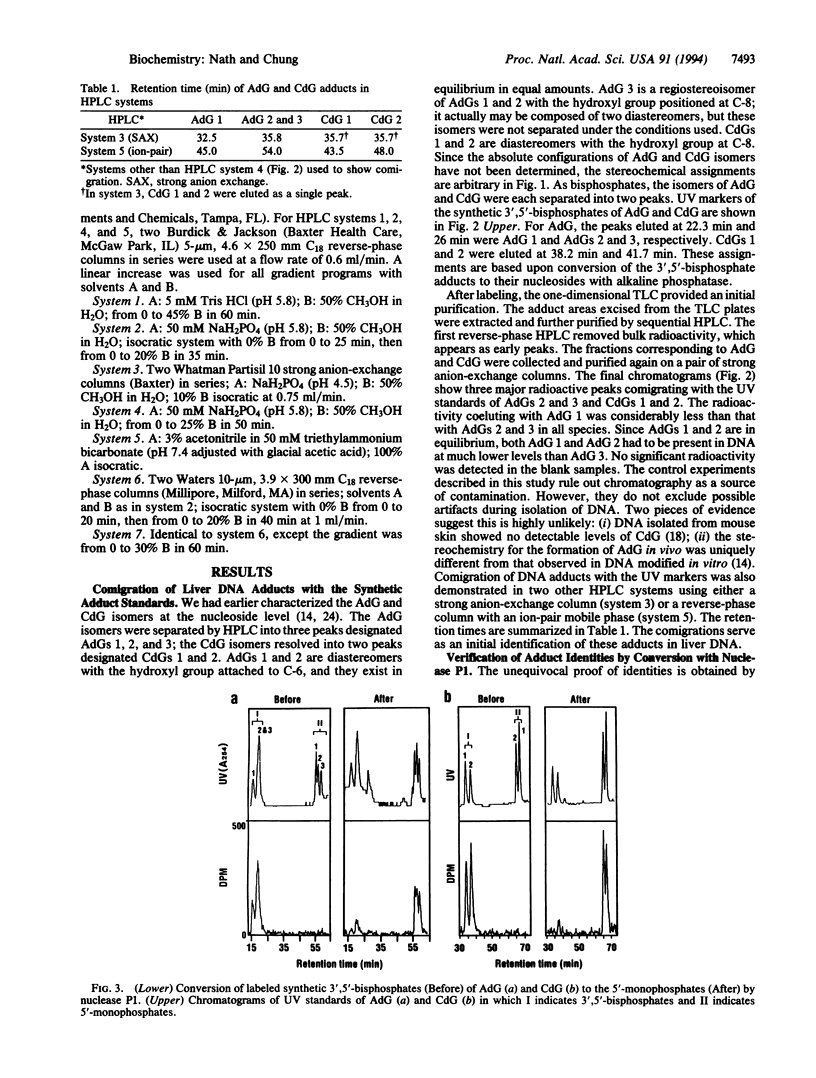
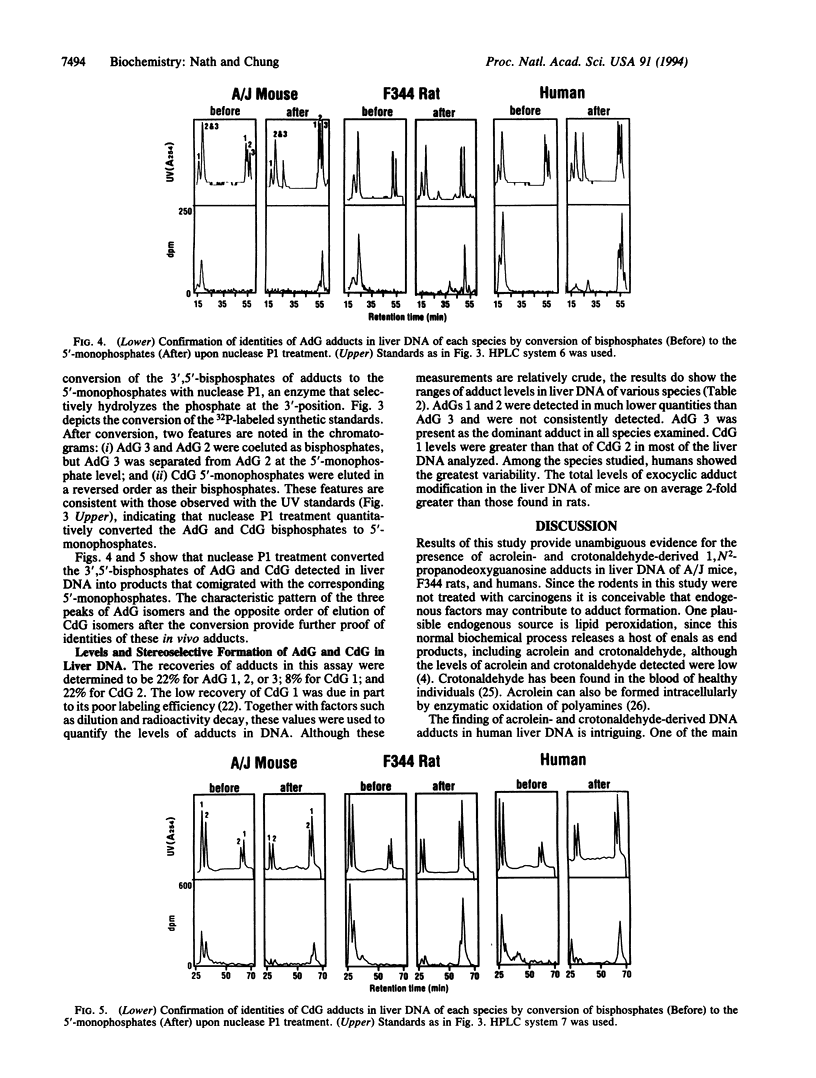
Exocyclic adducts are unique DNA modifications resulting from binding at two sites of bases that normally are involved in hydrogen-bonding for maintaining the double-helical structure of DNA. These adducts have been shown to be formed in rodents upon exposure to carcinogens. Using a sensitive 32P-postlabeling method combined with high performance liquid chromatography, we obtained evidence that 1,N2-propanodeoxyguanosine adducts of acrolein (AdG) and crotonaldehyde (CdG) are present in the liver DNA of humans and rodents without carcinogen treatment. The identities of these adducts were verified by cochromatography with the synthetic adduct standards. Further proof of identities was obtained by conversion mediated by nuclease P1 of the labeled AdG and CdG 3',5'-bisphosphates to their corresponding 5'-monophosphates. This treatment converted the in vivo adducts into products that again cochromatographed in a characteristic pattern with the synthetic 5'-monophosphates of AdG and CdG. Using this assay, we also demonstrated the in vivo stereoselective formation of one of the AdG isomers. The estimated total levels of modification were 1.0-1.7, 0.2-1.0, and 0.3-2.0 adducts in 10(6) guanine bases in the liver DNA of mice, rats, and humans, respectively. The detection of these adducts in relatively high levels without carcinogen treatment suggests that the endogenous factors such as lipid peroxidation may be important for their formation. This study provides evidence for the presence of acrolein- and crotonaldehyde-derived exocyclic adducts as common lesions in the liver DNA of rodents and humans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Hietanen E., Malaveille C. Carcinogenic nitrosamines: free radical aspects of their action. Free Radic Biol Med. 1989;7(6):637–644. doi: 10.1016/0891-5849(89)90144-5. [DOI] [PubMed] [Google Scholar]
- Bartsch H. The role of cyclic nucleic acid base adducts in carcinogenesis and mutagenesis. IARC Sci Publ. 1986;(70):3–14. [PubMed] [Google Scholar]
- Benamira M., Singh U., Marnett L. J. Site-specific frameshift mutagenesis by a propanodeoxyguanosine adduct positioned in the (CpG)4 hot-spot of Salmonella typhimurium hisD3052 carried on an M13 vector. J Biol Chem. 1992 Nov 5;267(31):22392–22400. [PubMed] [Google Scholar]
- Cheng K. C., Preston B. D., Cahill D. S., Dosanjh M. K., Singer B., Loeb L. A. The vinyl chloride DNA derivative N2,3-ethenoguanine produces G----A transitions in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9974–9978. doi: 10.1073/pnas.88.22.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F. L., Hecht S. S. Formation of cyclic 1,N2--adducts by reaction of deoxyguanosine with alpha-acetoxy-N-nitrosopyrrolidine, 4-(carbethoxynitrosamino)butanal, or crotonaldehyde. Cancer Res. 1983 Mar;43(3):1230–1235. [PubMed] [Google Scholar]
- Chung F. L., Tanaka T., Hecht S. S. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986 Mar;46(3):1285–1289. [PubMed] [Google Scholar]
- Chung F. L., Young R., Hecht S. S. Detection of cyclic 1,N2-propanodeoxyguanosine adducts in DNA of rats treated with N-nitrosopyrrolidine and mice treated with crotonaldehyde. Carcinogenesis. 1989 Jul;10(7):1291–1297. doi: 10.1093/carcin/10.7.1291. [DOI] [PubMed] [Google Scholar]
- Chung F. L., Young R., Hecht S. S. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984 Mar;44(3):990–995. [PubMed] [Google Scholar]
- Cohen S. M., Garland E. M., St John M., Okamura T., Smith R. A. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992 Jul 1;52(13):3577–3581. [PubMed] [Google Scholar]
- Comporti M. Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest. 1985 Dec;53(6):599–623. [PubMed] [Google Scholar]
- Farmer P. B., Bailey E., Naylor S., Anderson D., Brooks A., Cushnir J., Lamb J. H., Sepai O., Tang Y. S. Identification of endogenous electrophiles by means of mass spectrometric determination of protein and DNA adducts. Environ Health Perspect. 1993 Mar;99:19–24. doi: 10.1289/ehp.939919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtke N., Boucheron J. A., Walker V. E., Swenberg J. A. Vinyl chloride-induced DNA adducts. II: Formation and persistence of 7-(2'-oxoethyl)guanine and N2,3-ethenoguanine in rat tissue DNA. Carcinogenesis. 1990 Aug;11(8):1287–1292. doi: 10.1093/carcin/11.8.1287. [DOI] [PubMed] [Google Scholar]
- Foiles P. G., Akerkar S. A., Chung F. L. Application of an immunoassay for cyclic acrolein deoxyguanosine adducts to assess their formation in DNA of Salmonella typhimurium under conditions of mutation induction by acrolein. Carcinogenesis. 1989 Jan;10(1):87–90. doi: 10.1093/carcin/10.1.87. [DOI] [PubMed] [Google Scholar]
- Foiles P. G., Akerkar S. A., Miglietta L. M., Chung F. L. Formation of cyclic deoxyguanosine adducts in Chinese hamster ovary cells by acrolein and crotonaldehyde. Carcinogenesis. 1990 Nov;11(11):2059–2061. doi: 10.1093/carcin/11.11.2059. [DOI] [PubMed] [Google Scholar]
- Goel S. K., Lalwani N. D., Reddy J. K. Peroxisome proliferation and lipid peroxidation in rat liver. Cancer Res. 1986 Mar;46(3):1324–1330. [PubMed] [Google Scholar]
- Marnett L. J., Hurd H. K., Hollstein M. C., Levin D. E., Esterbauer H., Ames B. N. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res. 1985 Jan-Feb;148(1-2):25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- Matijasevic Z., Sekiguchi M., Ludlum D. B. Release of N2,3-ethenoguanine from chloroacetaldehyde-treated DNA by Escherichia coli 3-methyladenine DNA glycosylase II. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9331–9334. doi: 10.1073/pnas.89.19.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R. G., Chen H. J., Nishikawa A., Young-Sciame R., Chung F. L. A 32P-postlabeling method for simultaneous detection and quantification of exocyclic etheno and propano adducts in DNA. Carcinogenesis. 1994 May;15(5):979–984. doi: 10.1093/carcin/15.5.979. [DOI] [PubMed] [Google Scholar]
- Palejwala V. A., Simha D., Humayun M. Z. Mechanisms of mutagenesis by exocyclic DNA adducts. Transfection of M13 viral DNA bearing a site-specific adduct shows that ethenocytosine is a highly efficient RecA-independent mutagenic noninstructional lesion. Biochemistry. 1991 Sep 10;30(36):8736–8743. doi: 10.1021/bi00100a004. [DOI] [PubMed] [Google Scholar]
- Randerath K., Li D., Nath R., Randerath E. Exogenous and endogenous DNA modifications as monitored by 32P-postlabeling: relationships to cancer and aging. Exp Gerontol. 1992 Sep-Dec;27(5-6):533–549. doi: 10.1016/0531-5565(92)90008-n. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Lim Y. P., Farber E., Ghoshal A. K. Rapid lipid peroxidation in the nuclear fraction of rat liver induced by a diet deficient in choline and methionine. Cancer Lett. 1984 Oct;24(3):251–255. doi: 10.1016/0304-3835(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Russo A., Mitchell J. B., DeGraff W., Friedman N., Gamson J. Depletion of cellular glutathione by exogenous spermine in V79 cells: implications for spermine-induced hyperthermic sensitization. Cancer Res. 1985 Oct;45(10):4910–4914. [PubMed] [Google Scholar]
- Shamberger R. J. Increase of peroxidation in carcinogenesis. J Natl Cancer Inst. 1972 May;48(5):1491–1497. [PubMed] [Google Scholar]
- Shuker D. E., Prevost V., Friesen M. D., Lin D., Ohshima H., Bartsch H. Urinary markers for measuring exposure to endogenous and exogenous alkylating agents and precursors. Environ Health Perspect. 1993 Mar;99:33–37. doi: 10.1289/ehp.939933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Antoccia A., Basu A. K., Dosanjh M. K., Fraenkel-Conrat H., Gallagher P. E., Kuśmierek J. T., Qiu Z. H., Rydberg B. Both purified human 1,N6-ethenoadenine-binding protein and purified human 3-methyladenine-DNA glycosylase act on 1,N6-ethenoadenine and 3-methyladenine. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9386–9390. doi: 10.1073/pnas.89.20.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Williamson D. S., Cerny R. L., Cohen S. M. Detection of 1,N6-propanodeoxyadenosine in acrolein-modified polydeoxyadenylic acid and DNA by 32P postlabeling. Cancer Res. 1990 May 15;50(10):3005–3012. [PubMed] [Google Scholar]
- Sodum R. S., Chung F. L. 1,N2-ethenodeoxyguanosine as a potential marker for DNA adduct formation by trans-4-hydroxy-2-nonenal. Cancer Res. 1988 Jan 15;48(2):320–323. [PubMed] [Google Scholar]
- Sodum R. S., Chung F. L. Stereoselective formation of in vitro nucleic acid adducts by 2,3-epoxy-4-hydroxynonanal. Cancer Res. 1991 Jan 1;51(1):137–143. [PubMed] [Google Scholar]
- Swenberg J. A., Fedtke N., Ciroussel F., Barbin A., Bartsch H. Etheno adducts formed in DNA of vinyl chloride-exposed rats are highly persistent in liver. Carcinogenesis. 1992 Apr;13(4):727–729. doi: 10.1093/carcin/13.4.727. [DOI] [PubMed] [Google Scholar]
- Wang M. Y., Chung F. L., Hecht S. S. Identification of crotonaldehyde as a hepatic microsomal metabolite formed by alpha-hydroxylation of the carcinogen N-nitrosopyrrolidine. Chem Res Toxicol. 1988 Jan-Feb;1(1):28–31. doi: 10.1021/tx00001a005. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Foiles P. G., Chung F. L., Povey A. C., Frank A. A., Harris C. C. Detection of acrolein and crotonaldehyde DNA adducts in cultured human cells and canine peripheral blood lymphocytes by 32P-postlabeling and nucleotide chromatography. Carcinogenesis. 1991 Aug;12(8):1483–1490. doi: 10.1093/carcin/12.8.1483. [DOI] [PubMed] [Google Scholar]
- Zlatkis A., Poole C. F., Brazeli R., Bafus D. A., Spencer P. S. Volatile metabolites in sera of normal and diabetic patients. J Chromatogr. 1980 May 9;182(2):137–145. doi: 10.1016/s0378-4347(00)81617-5. [DOI] [PubMed] [Google Scholar]


