Abstract
Obesity has emerged as one of the most critical health care problems globally that is associated with the development of insulin resistance, type 2 diabetes mellitus, metabolic dysfunction and cardiovascular disease. Central adiposity with intra-abdominal deposition of visceral fat, in particular, has been closely linked to cardiometabolic consequences of obesity. Increasing epidemiological, clinical and experimental data suggest that both adipose tissue quantity and perturbations in its quality termed “adiposopathy” contribute to mechanisms of cardiometabolic disease. The current review discusses regional differences in adipose tissue characteristics and highlights profound abnormalities in vascular endothelial function and angiogenesis that are manifest within the visceral adipose tissue milieu of obese individuals. Clinical data demonstrate up-regulation of pro-inflammatory and pro-atherosclerotic mediators in dysfunctional adipose tissue that may support pathological vascular changes not only locally in fat but also in multiple organ systems, including coronary and peripheral circulations, potentially contributing to mechanisms of obesity-related cardiovascular disease.
Keywords: adiposopathy, endothelium, obesity, vascular disease, visceral fat
Introduction
Obesity has emerged as one of the most critical health care problems worldwide as nearly 1.5 billion of the world’s population is either overweight or obese [1]. The cost of battling obesity is estimated to be nearly $2 trillion, ranking third after smoking and military conflicts among the social burdens that impact global gross domestic product. Alarmingly, adult and childhood obesity rates, particularly in categories of severe obesity, are continuing to rise globally with significant short- and long-term health, social and economic consequences [2, 3]. Obesity represents a disease state characterized by chronic systemic inflammation that appears to be derived largely from adipose tissue inflammation and overproduction of pro-inflammatory cytokines such as TNF-α, MCP-1, and IL-6 and activation of NFκB-dependent pathways that are strongly implicated in mechanisms of systemic insulin resistance [4]. Obesity is a strong predictor of all-cause mortality and is closely associated with a number of chronic diseases such as insulin resistance, type 2 diabetes mellitus, cancer and ischemic heart disease. Cardiovascular disease is currently the major cause of mortality in this population [5–8]. While cardiometabolic risk increases with rising body mass index (BMI), intra-abdominal deposition of ectopic visceral fat has been more closely associated with cardiovascular risk, metabolic syndrome and type 2 diabetes [9, 10]. In this brief review we will discuss qualitative and quantitative differences between fat depots focusing on ectopic visceral fat as a potential negative regulator of vascular function and whole-body cardio-metabolic disease.
Visceral adiposopathy
Adipose tissue is a complex highly secretory endocrine organ capable of physiological modulation of signals that regulate appetite, energy expenditure, insulin sensitivity, endocrine and reproductive functions, bone metabolism, inflammation and immunity [11–13]. Consisting mostly of adipocytes, fat contains several other cell types including pre-adipocytes, endothelial cells, fibroblasts, mesenchymal cells, macrophages and other leukocytes that reside in the stromal vascular fraction. Although there are complex genetic and environmental components to the development of obesity, the resulting expansion in fat mass appears to occur largely due to an imbalance of food intake and energy expenditure [14]. Clinical studies suggest that subcutaneous adipose tissue accumulation may in part represent a physiological buffer for nutrient surplus, acting as a potential metabolic sink for excess free fatty acids (FFA) and triglycerides. However, in the face of persistent obesogenic stress and limited capacity for regional adipocyte hypertrophy or hyperplasia, adipose tissue storage is forced into ectopic regions in and around specific organs or compartments of the body [15]. Ectopic fat is defined by excess adipose tissue accumulation in locations not classically associated with adipose storage. As such, subcutaneous fat is categorized as a non-ectopic depot and visceral fat as the classic ectopic depot [16]. Certainly, omentum and mesenteric visceral fat is present in normal weight individuals and play an important physiological role. However, the expansion of these depots which are not teleologically designed to accommodate significant adipose storage is associated with functional abnormalities referred to as adiposopathy, or “sick fat” [17]. While we have chosen to focus on ectopic visceral fat specifically for the purposes of this review, accumulation of fat in other ectopic regions such as muscle, kidney, heart and liver have also been linked to adverse cardiometabolic risk and reviewed thoroughly elsewhere [9, 15, 16, 18, 19].
Adiposopathy is classically described as pathogenic adipose tissue changes that occur due to the toxic combination of positive caloric energy balance, sedentary lifestyle and genetic predisposition that results in dysfunctional endocrine, metabolic and immune adaptations [20]. While this occurs in all fat depots to some extent, abnormalities tend to be more prominent in visceral fat. Visceral and subcutaneous adipose depots arise from different origins during development, which may in part explain the propensity for visceral fat to develop differing metabolic, inflammatory, angiogenic and lypolytic properties compared to subcutaneous fat in obesity [21–25]. Subcutaneous fat comprises approximately 80% of total body fat mass, with abdominal visceral adipose tissue accounting for 5%–20% [26]. Despite not being the dominant adipose depot, clinical studies have demonstrated that FFA, interleukin (IL)-6, C-reactive protein (CRP) and tumor necrosis factor (TNF)-α circulate at higher concentrations in patients with greater deposition of visceral fat. These cytokines and mediators likely exert direct pro-inflammatory effects on target organs and play a role in hepatic insulin resistance [27–34]. Elevated circulating levels of CRP and IL-6 are also independent predictors for the development of type 2 diabetes and risk of myocardial infarction [35, 36]. Alarmingly, systemic inflammation is already evident in obese children [37–40] and associated with endothelial dysfunction and cardiovascular risk factors at early ages [38] recognized in toddlers as young as 3 years old. This is particularly concerning given the long-term disease exposure and potential impact on future metabolic and vascular health [41].
It has been well described in animal models and in some clinical studies that the source of pro-inflammatory cytokines originates largely from non-adipose cells that reside and/or infiltrate the stromal-vascular fraction of fat compartments in obesity [13, 27, 42–45]. The bulk of the immune response appears to be largely macrophage driven, primarily by pro-inflammatory M1 phenotype cells in animal models, although M2 macrophages have also been shown to be increased in clinical studies [46, 47]. The degree of adipose inflammation, however, tends to exhibit greater heterogeneity in clinical studies with lower degrees of adiposopathy being associated with healthier systemic cardiometabolic parameters in obese subjects [48–52]. Data from the Framingham Heart Study show that inflammatory markers correlate significantly with degree of fat burden in both subcutaneous and visceral compartments, but visceral reserves appear to have a stronger relation [53]. Transcriptomic studies of human tissue specimens from our group and others suggest a more atherogenic gene expression profile in visceral compared to subcutaneous fat, characterized by greater expression of pro-inflammatory, oxidative stress-related and anti-angiogenic genes [23, 46, 47, 54–62]. We have recently shown the pivotal role of non-canonical Wnt signaling in obesity-induced adipose inflammation and metabolic dysfunction [56]. Additionally, studies demonstrate that visceral fat releases increased amounts of IL-6, IL-8, vascular endothelial growth factor (VEGF), plasminogen activator inhibitor (PAI)-1, TNF-α and vasoconstrictor prostaglandins compared with subcutaneous adipose tissue, while anti-atherogenic factors such as adiponectin are reduced in obesity [27, 47, 55, 63]. A summary of mediators elaborated by adipose tissue that have been implicated in cardiovascular disease mechanisms is listed in Table 1.
Table 1.
Adipose-derived mediators implicated in cardiovascular disease mechanisms.
| Adiponectin |
| Angiopoietin-like 2 (ANGPTL-2) |
| Angiopoietin-like 4 (ANGPTL-4) |
| Angiotensinogen |
| Apelin |
| C-reactive protein (CRP) |
| Chemokine (C-C motif) ligand-5 (CCL-5) |
| Free fatty acids (FFA) |
| Intercellular adhesion molecule-1 (ICAM-1) |
| Interleukin-18 (IL-18) |
| Interleukin-6 (IL-6) |
| Leptin |
| Matrix metalloproteinase |
| Monocyte chemotactic protein-1 (MCP-1) |
| Nuclear factor kappa B (NFκB) |
| Omentin |
| Plasminogen activator inhibitor-1 (PAI-1) |
| Prostaglandins |
| P-selectin |
| Rentionol binding protein 4 (RBP-4) |
| Resistin |
| Serum amyloid A (SAA) |
| Toll-like receptor-4 (TLR-4) |
| Tumor necrosis factor-alpha (TNF-α) |
| Vascular cell adhesion molecule-1 (VCAM-1) |
| Vascular endothelial growth factor-A (VEGF-A) |
| Vascular endothelial growth factor- A165b (VEGF-A165b) |
| Visfatin |
| Wnt5a |
Adiposopathy and vascular dysfunction
Pro-inflammatory mechanisms represent the key mechanistic underpinnings of cardiovascular disease progression from the early stages of endothelial dysfunction to atherothrombosis leading to adverse clinical events [64]. It is tempting to hypothesize that as a consequence of adiposopathy and altered biology of adipose tissue, increased synthesis and release of fat-derived pro-atherogenic mediators might promote the development of atherosclerosis in obesity. Although direct causal links have not yet been definitively established that would allow for therapeutic targets, clinical studies are presently investigating pathogenic adipose-vascular connections. The vascular endothelium plays a critical role in the regulation of arterial tone, blood flow, inflammation and thrombosis. Endothelial phenotype serves as a barometer of overall vascular health and displays impairment in insulin-resistant states as an early sign of atherosclerosis [65–67]. Furthermore, the severity of dysfunction in coronary and peripheral vessels independently predicts future cardiovascular events [68–73]. Recent work from our laboratory demonstrated a significant association between histological adipose tissue inflammation and systemic vascular endothelial function assessed by brachial artery flow-mediated dilation (FMD) [48]. The findings built on prior data demonstrating a relation between adipose quantity and risk of arterial disease, as macrovascular function is significantly impaired with increasing weight burden in adults and children [74, 75]. Microvascular vasodilation to intra-arterial infusion of endothelium-dependent agonist acetylcholine is also blunted in obese subjects and tracks measures of insulin sensitivity and central adiposity independently of other cardiovascular risk factors [76]. Imaging computed tomography (CT) or magnetic resonance imaging studies of fat compartments identify visceral fat volume to be more highly associated with impaired flow-mediated vasodilation compared to subcutaneous [77, 78].
We have recently demonstrated that in BMI-matched obese individuals, reduced adipose tissue inflammation was associated with improved insulin sensitivity, decreased pro-atherogenic gene expression and preserved vascular function similar to lean subjects [50]. In multivariate analyses, both waist circumference and adipose inflammation were independent predictors of FMD, suggesting that in addition to obesity burden qualitative features of adipose tissue may be an important determinant of cardiovascular disease risk. This notion is supported by other clinical data demonstrating extensive adipose tissue inflammatory changes in insulin-resistant but not BMI-matched insulin-sensitive subjects [79]. Additionally, recent data from the Framingham Heart Study showed that lower CT radiodensity attenuation of adipose tissue, as measured by Hounsfield units (HU), was closely linked to adverse metabolic parameters such as insulin resistance beyond quantification of total fat volume [80]. Thus, CT imaging differentiation of tissue HU may provide a non-invasive and indirect measure of adipose tissue composition and quality. As such, adipose tissue with lower lipid content, smaller adipocytes, altered fibrosis and higher vascularity may exhibit less negative HU [80, 81]. Clinical studies continue to emerge supporting a relationship between adiposopathy and systemic disease, and it appears likely that collectively quality, quantity and location of adipose accumulation all relate to whole-body disease processes, but pathogenic mechanisms and their relative contributions remain poorly understood.
Vasomotor dysfunction in visceral fat
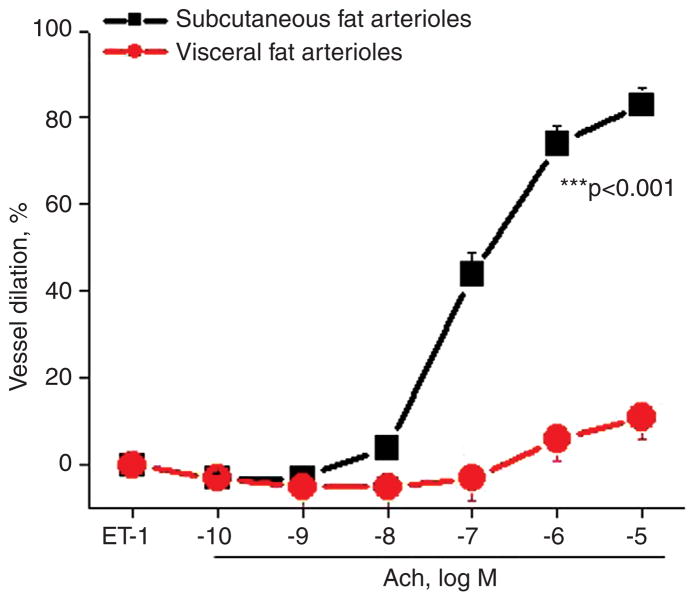
We recently considered that if adipose tissue is a regulator of vascular function with visceral milieu seemingly more pro-atherogenic, then differences in vasomotor function should be manifest in arterioles examined from different fat compartments within the body. Inflammatory cytokines over-expressed in visceral fat may impair vasoregulatory and anti-atherogenic properties owing in part to reduced endothelial nitric oxide synthase (eNOS) and loss of nitric oxide (NO) bioactivity, leading to vasomotor dysfunction. In this regard, our group [47, 55] and others [82–89] have started to examine these mechanistic interactions by directly studying physiological properties of microvessels within human fat by utilizing videomicroscopy and culture myograph techniques. In recent experiments, we collected paired subcutaneous and visceral adipose tissue biopsy samples from obese subjects during planned bariatric surgery, isolated tiny microvessels (75–250 μm in diameter) from different fat compartments and assessed vasodilator function using videomicroscopy. Endothelium-dependent, acetylcholine-mediated vasodilation was severely impaired in visceral arterioles compared to the subcutaneous depot [47]. The degree of vasomotor impairment is profound and consistent across varying systemic phenotypes. Our most recent cumulative data from a cohort of 104 obese subjects are displayed in Figure 1. Treatment with Nω-nitro-L-arginine methyl ester significantly reduced acetylcholine-mediated vasodilation by 40% in subcutaneous arterioles, whereas no significant effect was observed in visceral microvessels that already exhibited severe dysfunction, suggesting impairment in vascular NO bioavailability. Complementary to physiological studies, we observed significant impairment in acetylcholine-mediated activation of eNOS at the phosphorylation site serine 1177 in vascular endothelial cells isolated from visceral fat [55]. Responses to non-endothelium-dependent agonists papaverine and sodium nitroprusside were preserved in both depots, indicating intact smooth muscle responses and thus selective impairment in endothelial function. Similar findings have been confirmed by others who reported arteriolar dysfunction in visceral fat [82] and demonstrated that the impairment is specific to the state of obesity as arterioles isolated from visceral tissue of lean subjects exhibit preserved vasomotor function [83, 84].
Figure 1.
Acetylcholine-mediated, endothelium-dependent vasodilation in blood vessels isolated from visceral fat is severely impaired compared to arterioles isolated from subcutaneous adipose tissue (p < 0.001, n = 104 obese subjects).
There are likely multiple mechanisms that negatively regulate vascular responses in visceral obesity. Cytokinedriven inflammation likely plays a key role as we and others have demonstrated the adipose secretome and transcriptome to be markedly pro-inflammatory in visceral depots. Experimental studies in mice demonstrate that transplantation of inflamed visceral fat accelerates atherosclerosis in Apo-E knockout mice [90]. Adipose gene expression of inflammatory mediators correlate inversely with acetylcholine- mediated vasodilation of human microvessels [47, 55]. Endothelial cells isolated from visceral fat exhibit upregulated expression of pro-inflammatory mediators such as CCL-5, IL-6, TNF-α and toll-like receptor-4 [47]. More direct evidence that inflammatory mechanisms are involved is provided by experimental studies demonstrating histological vascular inflammation and reversal of vasomotor dysfunction following treatment with IL-6 and TNF-α antagonists [83, 88]. However, other pathogenic processes that involve oxidative stress, mitochondrial dysfunction and endoplasmic-reticulum stress are likely intertwined and may contribute to obesity-related vascular disease. For example, recent data demonstrated evidence of impaired NO-dependent vasodilation, mitochondrial hyperpolarization, reduced mitochondrial mass and increased mitochondrial superoxide production in the adipose tissue of type-2 diabetic subjects [87]. We recently identified increased expression of cyclooxygenase (COX)-mediated vasoconstrictor prostanoids in visceral fat that contribute to endothelial dysfunction. Treatment with indomethacin, a COX-specific inhibitor, significantly improved endothelium-dependent vasodilation by twofold. This improvement was associated with phosphorylation and stimulation of eNOS at serine 1177 in visceral endothelial cells, supporting a contribution of the eicosanoid/cyclooxgenase pathway to adipose microvascular dysfunction in obesity [55]. Vasodilator responses in the adipose microvasculature have been shown to correlate with cardiovascular risk factors and brachial arterial responses; thus, investigation of the adipose microenvironment may provide novel translational information relevant to systemic vascular disease mechanisms [85, 89].
Vascular insulin resistance
Insulin resistance represents a highly prevalent metabolic disturbance in obesity. In particular, regional adiposity with central accumulation of visceral fat has been closely associated with insulin resistance, endothelial dysfunction and cardiovascular disease [12, 16]. Although insulin resistance generally implies diminished actions of insulin in mediating glucose uptake in target organs such as fat, liver and muscle, insulin also exerts important physiological actions upon the vasculature that regulate metabolism and blood flow via eNOS activation and endothelial NO production [91, 92]. In animals, endothelium-specific deletion of the insulin receptor impairs eNOS bioavailability, promotes atherogenesis and is associated with whole-body insulin resistance, hypertension and ischemia [93]. Recent work from our group also demonstrated impaired insulin-stimulated eNOS phosphorylation, inflammation and vasodilator dysfunction of endothelial cells isolated from the vascular wall of obese diabetics [94]. Under conditions of obesogenic stress, insulin signaling in the vasculature becomes impaired promoting vascular inflammation, vasoconstriction and progression of atherosclerotic plaques [91, 93]. Compelling evidence from animal and clinical studies support a close link between insulin resistance and development of vascular disease, as preservation of insulin signaling represents a fundamental homeostatic mechanism of blood vessels [95–98]. Currently, however, regulatory mechanisms that govern these pathogenic processes are incompletely understood.
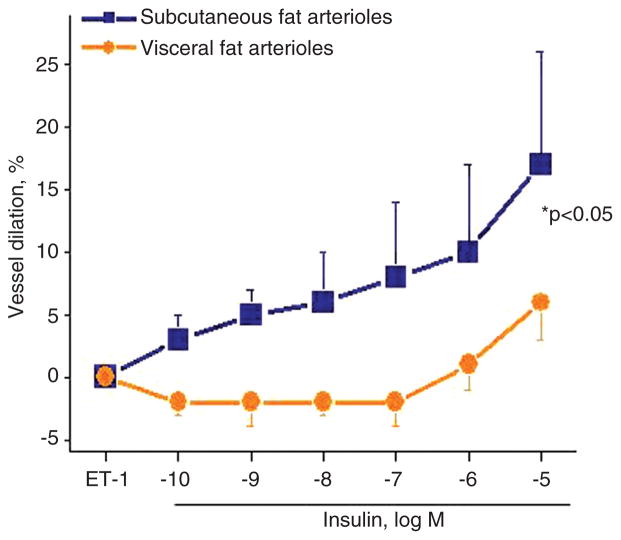
In our videomicroscopy experiments, we observed significant impairment in insulin-mediated vasodilation of visceral compared to subcutaneous adipose arterioles harvested from obese subjects as shown in Figure 2. The response to insulin was severely blunted in visceral compared to subcutaneous microvessels indicating a profound collapse of vascular homeostasis. This is consistent with responses to other agonists discussed above, demonstrating impairment of visceral arterioles to a broad range of physiological and pharmacological stimuli that modulate normal vascular function. We found evidence of down-regulated components of the insulin signaling cascade and reduced insulin-mediated activation and phosporylation of Akt in visceral fat. Disruption of this pathway polarizes insulin’s actions toward mitogen-activated protein kinase and proliferative pathways that support atherogenesis [99]. Mediators involved in promoting vascular dysfunction in adipose tissue may also have systemic pathophysiological actions that contribute to cardiometabolic disease and atherosclerosis, although specific therapeutic targets remain unclear. There is growing interest in targeting insulin sensitivity to combat obesity-related cardiovascular disease, especially in light of recent clinical data linking improved long-term cardiovascular survival following bariatric weight loss primarily to parameters of insulin resistance [100]. In line with these findings, we have recently shown that improved endothelial function with weight loss was directly tied to recovery of insulin sensitivity [101].
Figure 2.
Insulin-mediated, endothelium-dependent vasodilation is significantly impaired in arterioles from visceral fat compared to blood vessels isolated from subcutaneous adipose tissue (p < 0.05, n = 23 obese subjects).
Angiogenesis and visceral adiposity
Angiogenesis, the generation of new blood vessels, is critical for adequate fat expansion and adipose tissue remodeling. As adipose tissue expands and regresses with weight change, tightly controlled regulation of angiogenesis within adipose tissue is required to maintain metabolic and oxygen exchange that is critical for maintaining whole body homeostasis [102]. Experimental studies suggest that expanding adipose tissue may “outgrow” its blood supply in obesity possibly owing to deficient angiogenesis that triggers a vicious cycle of ischemia, hypoxia, necrosis and inflammation within the adipose milieu that promotes whole body metabolic dysfunction [103–106]. Capillary dropout and deficient vascularization occur in the adipose depots of animals and humans, particularly in visceral fat, and is associated with inflammation and metabolic dysfunction [57, 104–108]. Experimental studies demonstrate that adipose-specific deletion of VEGF-A induces adipose hypoxia, apoptosis, inflammation and metabolic abnormalities including insulin resistance and hyperlipidemia [109], while its over-expression promotes neovascularization and improves glucose metabolism [110]. These data prompt speculation that qualitative features of fat and altered tissue homeostasis as a function of impaired vascular support may play a role in shaping metabolic health.
We and others have recently shown that subcutaneous adipose tissue exhibits higher capillary density and angiogenic capacity compared to the visceral depot despite paradoxically higher expression of several proangiogenic factors including VEGF-A [47, 57, 111–113]. Affymetrix microarray analysis reported significant differences in gene transcripts associated with angiogenesis between visceral and subcutaneous fat in obese humans [57]. Among several mediators, pro-angiogenic ANGPTL-4 is down-regulated in visceral fat and may play an important role [108]. We recently described a splice variant isoform of VEGF-A, anti-angiogenic VEGF-A165b, that is over-expressed in human visceral fat and associated with impaired adipose tissue angiogenesis [111]. Targeted VEGF-A165b inhibition restored pro-angiogenic VEGF receptor activation and vascularization. Circulating VEGF-A165b blood levels were elevated in obese compared to lean subjects and decreased significantly following bariatric weight loss. This latter finding has potential clinical implications as up-regulation of systemic VEGF-A165b in the state of obesity raises the possibility that this anti-angiogenic isoform could contribute to vascular disease and ischemia beyond the adipose environment. In this regard, our group recently described the key role of anti-angiogenic VEGF-A165b in mechanisms of peripheral arterial disease in animal models and humans [114]. It is thus becoming increasingly clear that qualitative features of adipose tissue, including its vascularity, could play an important role in the pathogenesis of obesity-induced cardio-metabolic complications. However, whether modulation of adipose tissue angiogenesis may alter clinical consequences of human obesity remains an open question.
Weight loss and visceral adiposity
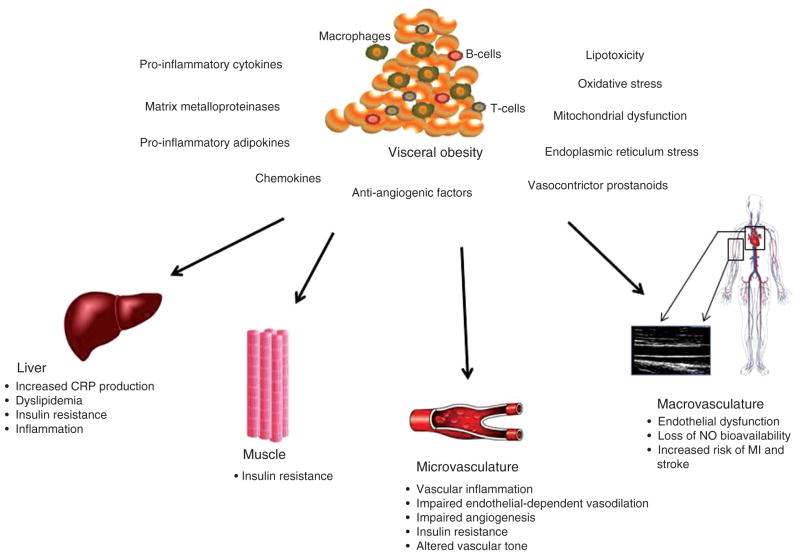
There is great interest in promoting weight loss for the reversal of many obesity-related complications. Several short-term studies have shown that weight reduction improves cardiovascular function [115–120]. Bariatric surgery currently represents the most effective and durable weight loss intervention. It is also the sole weight reduction method shown to reduce long-term (> 10 year) cardiovascular mortality, largely owning to decreased myocardial infarction risk [100, 121, 122]. Specific mechanisms for this improvement in cardiovascular health remains largely unclear, though recent data from the Swedish Obesity Study identified plasma insulin levels as the primary predictor of risk reduction [100]. We have similarly shown that improvement in systemic vascular function following significant weight loss from bariatric surgery is specifically tied to insulin sensitivity [101]. Few studies have examined the effect of bariatric weight loss on ectopic fat and relation to overall cardiometabolic risk and reported greater reduction in visceral compared to other ectopic regions and subcutaneous fat depots [123, 124]. Weight loss in insulin-dependent, type-2 diabetic subjects incurred by calorie reduction also showed preferential loss in visceral compared to subcutaneous adiposity in parallel with improved cardiovascular risk factors [125]. Serial imaging studies by CT in the multi-ethnic study of atherosclerosis study show that only visceral fat volume and its longitudinal changes independent of BMI were strongly associated with metabolic phenotypes [126]. While the concept that visceral fat “quantity” links to cardiometabolic risk is well accepted, essentially nothing is known about weight loss-induced “qualitative” alterations in visceral fat in relation to systemic disease. The literature suggests that bariatric surgery favorably remodels adipose tissue by attenuating macrophage-mediated inflammation and cytokine production [127, 128], and improved microvascular function has also been reported in subcutaneous fat [129]. However, additional studies are needed to examine the relative contributions of visceral adiposity and adiposopathy to human disease. A summary concept schematic illustrating the role of obesity in cardiometabolic disease is provided in Figure 3.
Figure 3.
Role of adiposopathy in cardiometabolic disease.
Conclusions
Obesity will remain one of the most important heath care challenges worldwide, and improving our understanding of mechanisms of obesity-related vascular disease is critical. Clinical, epidemiological and experimental data suggest that visceral adiposity is more closely linked to obesity-related cardiovascular disease. We have provided evidence that the visceral adipose tissue microenvironment is associated with profound abnormalities in vascular homeostasis. With clinical data consistently linking visceral adiposity burden to cardiovascular risk, characterization of pathophysiological mechanisms learned from the adipose microenvironment may provide valuable translational clues to mechanisms of systemic disease in human obesity.
Acknowledgments
Dr. Gokce is supported by National Institutes of Health (NIH) grants HL081587, HL1145675 and HL126141.
Footnotes
Conflict of interest statement
Disclosures: There are no conflicts of interest to disclose.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, Huang T, Marsh T, Moodie ML. Changing the future of obesity: science, policy, and action. Lancet. 2011;378:838–47. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. J Am Med Assoc. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitahara CM, Flint AJ, Berrington de GA, Bernstein L, Brotzman M, MacInnis RJ, Moore SC, Robien K, Rosenberg PS, Singh PN, Weiderpass E, Adami HO, Anton-Culver H, Ballard-Barbash R, Buring JE, Freedman DM, Fraser GE, Beane Freeman LE, Gapstur SM, Gaziano JM, Giles GG, Hakansson N, Hoppin JA, Hu FB, Koenig K, Linet MS, Park Y, Patel AV, Purdue MP, Schairer C, Sesso HD, Visvanathan K, White E, Wolk A, Zeleniuch-Jacquotte A, Hartge P. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11:e1001673. doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrington de GA, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the obesity society. Circulation. 2013;129:S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 10.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–9. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol. 2014;34:1820–6. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–41. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 17.Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating “sick fat” through improving fat function with antidiabetes therapies. Am J Cardiol. 2012;110(9 Suppl):4B–12B. doi: 10.1016/j.amjcard.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–51. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathieu P, Boulanger MC, Despres JP. Ectopic visceral fat: a clinical and molecular perspective on the cardiometabolic risk. Rev Endocr Metab Disord. 2014;15:289–98. doi: 10.1007/s11154-014-9299-3. [DOI] [PubMed] [Google Scholar]
- 20.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–75. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karastergiou K, Fried SK. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atheroscler Rep. 2013;15:361. doi: 10.1007/s11883-013-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von ZT, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–8. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 25.Virtanen KA, Lonnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Ronnemaa T, Huupponen R, Nuutila P. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87:3902–10. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 27.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 28.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13:674–82. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 32.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 33.Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA, Katsilambros N. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism. 1999;48:1332–5. doi: 10.1016/s0026-0495(99)90277-9. [DOI] [PubMed] [Google Scholar]
- 34.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. J Am Med Assoc. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 35.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Am Med Assoc. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 37.Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2003;108:1053–8. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- 38.Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, Jilma B, Roggla G, Wolzt M, Widhalm K, Wagner OF. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26:2541–6. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 39.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–9. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 41.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 42.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–30. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 43.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–23. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 47.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol. 2012;32:467–73. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–9. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A, Joseph L, Apovian CM, Gokce N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes. 2012;2:e30. doi: 10.1038/nutd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, Gokce N. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, Beale E, Xie C, Greenberg AS, Allayee H, Goran MI. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes. 2011;60:2802–9. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–60. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 54.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010;18:879–83. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 55.Farb MG, Tiwari S, Karki S, Ngo DT, Carmine B, Hess DT, Zuriaga MA, Walsh K, Fetterman JL, Hamburg NM, Vita JA, Apovian CM, Gokce N. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity (Silver Spring) 2014;22:349–55. doi: 10.1002/oby.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuster JJ, Zuriaga MA, Thi-Minh ND, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Non-canonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2014 doi: 10.2337/db14-1164. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–94. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, Bluher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–83. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 60.Ledoux S, Queguiner I, Msika S, Calderari S, Rufat P, Gasc JM, Corvol P, Larger E. Angiogenesis associated with visceral and subcutaneous adipose tissue in severe human obesity. Diabetes. 2008;57:3247–57. doi: 10.2337/db07-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–9. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 62.Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 64.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 66.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–30. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 67.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 68.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–9. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 69.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 70.Gokce N. Clinical assessment of endothelial function: ready for prime time? Circ Cardiovasc Imaging. 2011;4:348–50. doi: 10.1161/CIRCIMAGING.111.966218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamburg NM, Larson MG, Vita JA, Vasan RS, Keyes MJ, Widlansky ME, Fox CS, Mitchell GF, Levy D, Meigs JB, Benjamin EJ. Metabolic syndrome, insulin resistance, and brachial artery vasodilator function in Framingham Offspring participants without clinical evidence of cardiovascular disease. Am J Cardiol. 2008;101:82–8. doi: 10.1016/j.amjcard.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–9. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arkin JM, Alsdorf R, Bigornia S, Palmisano J, Beal R, Istfan N, Hess D, Apovian CM, Gokce N. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. 2008;101:98–101. doi: 10.1016/j.amjcard.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–7. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 76.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–65. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 77.Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, O’Donnell CJ, Vasan RS, Mitchell GF, Hoffmann U, Fox CS, Benjamin EJ. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 2009;17:2054–9. doi: 10.1038/oby.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, Yoshizumi M, Toba K, Ouchi Y. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disord. 1998;22:477–84. doi: 10.1038/sj.ijo.0800620. [DOI] [PubMed] [Google Scholar]
- 79.Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–15. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 80.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762–71. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, Murabito JM, Hoffmann U, Fox CS. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100:227–34. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grizelj I, Cavka A, Bian JT, Szczurek M, Robinson A, Shinde S, Nguyen V, Braunschweig C, Wang E, Drenjancevic I, Phillips SA. Reduced flow-and acetylcholine-induced dilations in visceral compared to subcutaneous adipose arterioles in human morbid obesity. Microcirculation. 2015;22:44–53. doi: 10.1111/micc.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, Salvetti A, Pinchera A, Taddei S. Vascular generation of tumor necrosis factor- alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–47. doi: 10.1016/j.jacc.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 84.Virdis A, Duranti E, Rossi C, Dell’agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu072. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 85.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens. 2012;25:528–34. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115:525–32. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2012;32:2531–9. doi: 10.1161/ATVBAHA.112.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 89.Grassi G, Seravalle G, Scopelliti F, Dell’Oro R, Fattori L, Quarti-Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 2010;18:92–8. doi: 10.1038/oby.2009.195. [DOI] [PubMed] [Google Scholar]
- 90.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol. 2008;93:158–63. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 92.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–91. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 93.Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052–9. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–82. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 96.Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, Kearney MT. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–14. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–81. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 98.Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, Luo H, van BC. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–23. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 99.Fulton DJ. Mechanisms of vascular insulin resistance: a substitute Akt? Circ Res. 2009;104:1035–7. doi: 10.1161/CIRCRESAHA.109.198028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 101.Bigornia SJ, Farb MG, Tiwari S, Karki S, Hamburg NM, Vita JA, Hess DT, Lavalley MP, Apovian CM, Gokce N. Insulin status and vascular responses to weight loss in obesity. J Am Coll Cardiol. 2013;62:2297–305. doi: 10.1016/j.jacc.2013.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rojas-Rodriguez R, Gealekman O, Kruse ME, Rosenthal B, Rao K, Min S, Bellve KD, Lifshitz LM, Corvera S. Adipose tissue angiogenesis assay. Methods Enzymol. 2014;537:75–91. doi: 10.1016/B978-0-12-411619-1.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 104.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 105.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–5. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227–35. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 108.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–64. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 110.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, Lavalley MP, Hamburg NM, Vita JA, Hess DT, Walsh K, Gokce N. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation. 2014;130:1072–80. doi: 10.1161/CIRCULATIONAHA.113.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villaret A, Galitzky J, Decaunes P, Esteve D, Marques MA, Sengenes C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumie A. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–63. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–54. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 114.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–71. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ashrafian H, le Roux CW, Darzi A, Athanasiou T. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- 116.Bigornia SJ, Mott MM, Hess DT, Apovian CM, McDonnell ME, Duess MA, Kluge MA, Fiscale AJ, Vita JA, Gokce N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring) 2010;18:754–9. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M, Lipinska I, Keaney JF, Jr, Apovian CM. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–8. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 118.Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, Gallagher J, Williams Z, Preece K, Gundersen N, Strong MB, Pendleton RC, Segerson N, Cloward TV, Walker JM, Farney RJ, Gress RE, Adams TD, Hunt SC, Litwin SE. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol. 2011;57:732–9. doi: 10.1016/j.jacc.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–8. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 120.Sciacqua A, Candigliota M, Ceravolo R, Scozzafava A, Sinopoli F, Corsonello A, Sesti G, Perticone F. Weight loss in combination with physical activity improves endothelial dysfunction in human obesity. Diabetes Care. 2003;26:1673–8. doi: 10.2337/diacare.26.6.1673. [DOI] [PubMed] [Google Scholar]
- 121.Romeo S, Maglio C, Burza MA, Pirazzi C, Sjoholm K, Jacobson P, Svensson PA, Peltonen M, Sjostrom L, Carlsson LM. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care. 2012;35:2613–7. doi: 10.2337/dc12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 123.Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clement K, Bernard M, Dutour A. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60:1381–9. doi: 10.1016/j.jacc.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 124.van Schinkel LD, Sleddering MA, Lips MA, Jonker JT, de RA, Lamb HJ, Jazet IM, Pijl H, Smit JW. Effects of bariatric surgery on pericardial ectopic fat depositions and cardiovascular function. Clin Endocrinol (Oxf) 2014;81:689–95. doi: 10.1111/cen.12402. [DOI] [PubMed] [Google Scholar]
- 125.Snel M, Jonker JT, Hammer S, Kerpershoek G, Lamb HJ, Meinders AE, Pijl H, de RA, Romijn JA, Smit JW, Jazet IM. Long-term beneficial effect of a 16-week very low calorie diet on pericardial fat in obese type 2 diabetes mellitus patients. Obesity (Silver Spring) 2012;20:1572–6. doi: 10.1038/oby.2011.390. [DOI] [PubMed] [Google Scholar]
- 126.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 128.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–69. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 129.De CC, Porteri E, Rizzoni D, Corbellini C, La BE, Boari GE, Pilu A, Mittempergher F, Di BE, Casella C, Nascimbeni R, Rosei CA, Ruggeri G, Caimi L, Rosei EA. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension. 2011;58:29–36. doi: 10.1161/HYPERTENSIONAHA.111.171082. [DOI] [PubMed] [Google Scholar]