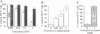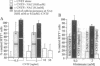Abstract
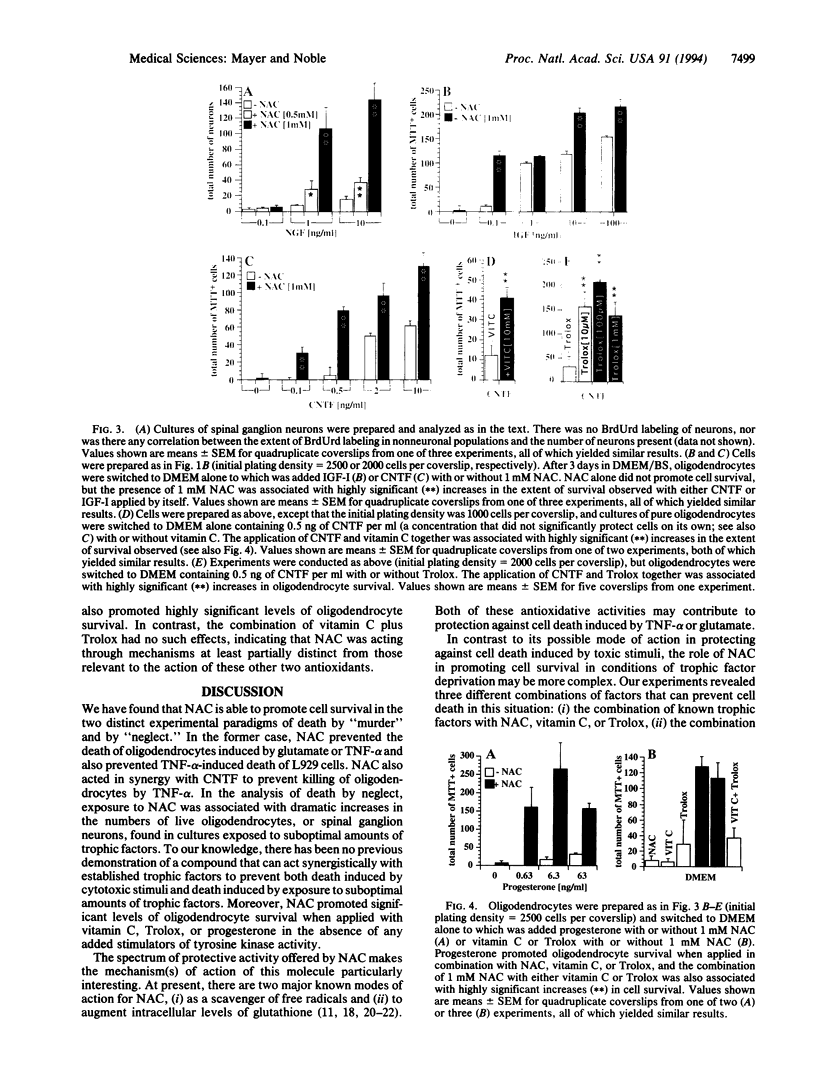
We have discovered that N-acetyl-L-cysteine (NAC) protects cells against death induced by exposure to noxious stimuli and against programmed cell death (apoptosis) associated with exposure to inadequate amounts of trophic factors. NAC prevented glutamate-induced death of oligodendrocytes and tumor necrosis factor alpha (TNF-alpha)-induced death of oligodendrocytes and L929 fibroblasts. Moreover, suboptimal doses of NAC plus ciliary neurotrophic factor (which also protects oligodendrocytes against TNF-alpha-mediated killing) acted synergistically to protect oligodendrocytes against TNF-alpha-induced death. Protection against death by growth factor deprivation was provided by the combination of (i) NAC, vitamin C, or Trolox (a water-soluble analogue of vitamin E) with suboptimal concentrations of protein trophic factors, (ii) NAC, vitamin C, or Trolox with progesterone, and (iii) NAC with either vitamin C or Trolox; these latter experiments suggest that the addition of tyrosine kinase stimulators is not required to promote cell survival. In all paradigms, NAC was either equally or more effective than the other compounds examined. In light of the long history of therapeutic application of NAC, we suggest that use of this compound may be of interest in conditions where certain toxin-mediated forms of cell death and/or apoptosis contribute significantly to disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Schmid R., Sendnter M., Raff M. C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993 May;118(1):283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Bartlett P. F., Noble M. D., Pruss R. M., Raff M. C., Rattray S., Williams C. A. Rat neural antigen-2 (RAN-2): a cell surface antigen on astrocytes, ependymal cells, Müller cells and lepto-meninges defined by a monoclonal antibody. Brain Res. 1981 Jan 12;204(2):339–351. doi: 10.1016/0006-8993(81)90593-x. [DOI] [PubMed] [Google Scholar]
- Boscoboinik D., Szewczyk A., Hensey C., Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991 Apr 5;266(10):6188–6194. [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgunder J. M., Varriale A., Lauterburg B. H. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Carroll S. L., Silos-Santiago I., Frese S. E., Ruit K. G., Milbrandt J., Snider W. D. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992 Oct;9(4):779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992 Oct;9(4):583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Coles H. S., Burne J. F., Raff M. C. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993 Jul;118(3):777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D., Rothman T. P. Enteric glia. Glia. 1991;4(2):195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Groves A. K., Entwistle A., Jat P. S., Noble M. The characterization of astrocyte cell lines that display properties of glial scar tissue. Dev Biol. 1993 Sep;159(1):87–104. doi: 10.1006/dbio.1993.1223. [DOI] [PubMed] [Google Scholar]
- Holdiness M. R. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991 Feb;20(2):123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Nye S. H., Boulton T. G., Davis S., Taga T., Li Y., Birren S. J., Yasukawa K., Kishimoto T., Anderson D. J. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992 Jun 26;69(7):1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Gorin P. D., Brandeis L. D., Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980 Nov 21;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Leviton A., Paneth N. White matter damage in preterm newborns--an epidemiologic perspective. Early Hum Dev. 1990 Oct;24(1):1–22. doi: 10.1016/0378-3782(90)90002-z. [DOI] [PubMed] [Google Scholar]
- Louis J. C., Magal E., Takayama S., Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993 Jan 29;259(5095):689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Mayer M., Bhakoo K., Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994 Jan;120(1):143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- Mayer M., Bögler O., Noble M. The inhibition of oligodendrocytic differentiation of O-2A progenitors caused by basic fibroblast growth factor is overridden by astrocytes. Glia. 1993 May;8(1):12–19. doi: 10.1002/glia.440080103. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E., Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr. 1986;5(2):137–151. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- Merrill J. E. Effects of interleukin-1 and tumor necrosis factor-alpha on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13(3):130–137. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- Mihm S., Ennen J., Pessara U., Kurth R., Dröge W. Inhibition of HIV-1 replication and NF-kappa B activity by cysteine and cysteine derivatives. AIDS. 1991 May;5(5):497–503. doi: 10.1097/00002030-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Cotgreave I. A., Berggren M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration. 1986;50 (Suppl 1):31–42. doi: 10.1159/000195086. [DOI] [PubMed] [Google Scholar]
- Moody S. A., Quigg M. S., Frankfurter A. Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J Comp Neurol. 1989 Jan 22;279(4):567–580. doi: 10.1002/cne.902790406. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oka A., Belliveau M. J., Rosenberg P. A., Volpe J. J. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993 Apr;13(4):1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M., Staal F. J., Raju P. A., Ela S. W., Herzenberg L. A., Herzenberg L. A. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein M. J., Knapp G. L., Kulig K. W., Rumack B. H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988 Dec 15;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Spofford B. T., Daynes R. A., Granger G. A. Cell-mediated immunity in vitro: a highly sensitive assay for human lymphotoxin. J Immunol. 1974 Jun;112(6):2111–2116. [PubMed] [Google Scholar]
- Staal F. J., Ela S. W., Roederer M., Anderson M. T., Herzenberg L. A., Herzenberg L. A. Glutathione deficiency and human immunodeficiency virus infection. Lancet. 1992 Apr 11;339(8798):909–912. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
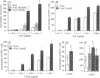
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., Marafino B. J., Jr, Chan A., Landre P., Winkelhake J. L. The role of oxidant injury in tumor cell sensitivity to recombinant human tumor necrosis factor in vivo. Implications for mechanisms of action. J Immunol. 1989 Feb 15;142(4):1405–1409. [PubMed] [Google Scholar]
- van de Bor M., Guit G. L., Schreuder A. M., Wondergem J., Vielvoye G. J. Early detection of delayed myelination in preterm infants. Pediatrics. 1989 Sep;84(3):407–411. [PubMed] [Google Scholar]