Multiple Wnt ligands produced by cap cells regulate the expression of Tkv, which acts as a receptor sink to remove excess cap cell–expressed Dpp and to restrict niche-associated Dpp activity, in escort cells.
Abstract
Stem cell self-renewal versus differentiation is regulated by the niche, which provides localized molecules that favor self-renewal. In the Drosophila melanogaster female germline stem cell (GSC) niche, Decapentaplegic (Dpp), a fly transforming growth factor β molecule and well-established long-range morphogen, acts over one cell diameter to maintain the GSCs. Here, we show that Thickveins (Tkv; a type I receptor of Dpp) is highly expressed in stromal cells next to Dpp-producing cells and functions to remove excess Dpp outside the niche, thereby spatially restricting its activity. Interestingly, Tkv expression in these stromal cells is regulated by multiple Wnt ligands that are produced by the niche. Our data demonstrate a self-restraining mechanism by which the Drosophila ovarian GSC niche acts to define its own boundary.
Introduction
Stem cells reside in a tissue-specific microenvironment, termed the niche, which provides localized signaling factors that influence their cell fate decision (Schofield, 1978; Xie and Spradling, 2000). The niche promotes stem cell identity and safeguards against excessive proliferation (Weissman et al., 2001; Fuchs et al., 2004; Li and Xie, 2005; Moore and Lemischka, 2006; Scadden, 2006; Morrison and Spradling, 2008). The loss of niche activity leads to premature stem cell differentiation, whereas ectopic niche activity results in the formation of excess stem cells outside their normal position, possibly leading to tumor formation. Thus, niche activity must be precisely controlled to balance self-renewal versus differentiation of the residing stem cells.
The ovary of Drosophila melanogaster is a well-established system for studying the temporal and spatial regulation of niche activities (Fuller and Spradling, 2007; Chen et al., 2011; Harris and Ashe, 2011; Losick et al., 2011; Xie, 2013). Located at the anterior tip of the germarium, the ovarian niche comprises several types of somatic cells that include terminal filament cells, cap cells, and escort cells (ECs; Fig. 1 A). Each niche supports two to three germline stem cells (GSCs). GSCs undergo asymmetric divisions to generate a GSC daughter within the niche and a cystoblast (CB) daughter that is displaced outside the niche to initiate differentiation. During differentiation, the CB undergoes four synchronized divisions with incomplete cytokinesis and proceeds through 2-, 4-, 8-, and 16-cell cyst stages before giving rise to a mature egg. Both GSCs and CBs possess a spherical intracellular organelle (called spectrosome), whereas the differentiating cyst contains a branched organelle (referred to as fusome) that interconnects individual cystocyte (Lin et al., 1994; de Cuevas et al., 1997). Both spectrosome and fusome are enriched in cytoskeletal proteins such as α-Spectrin.
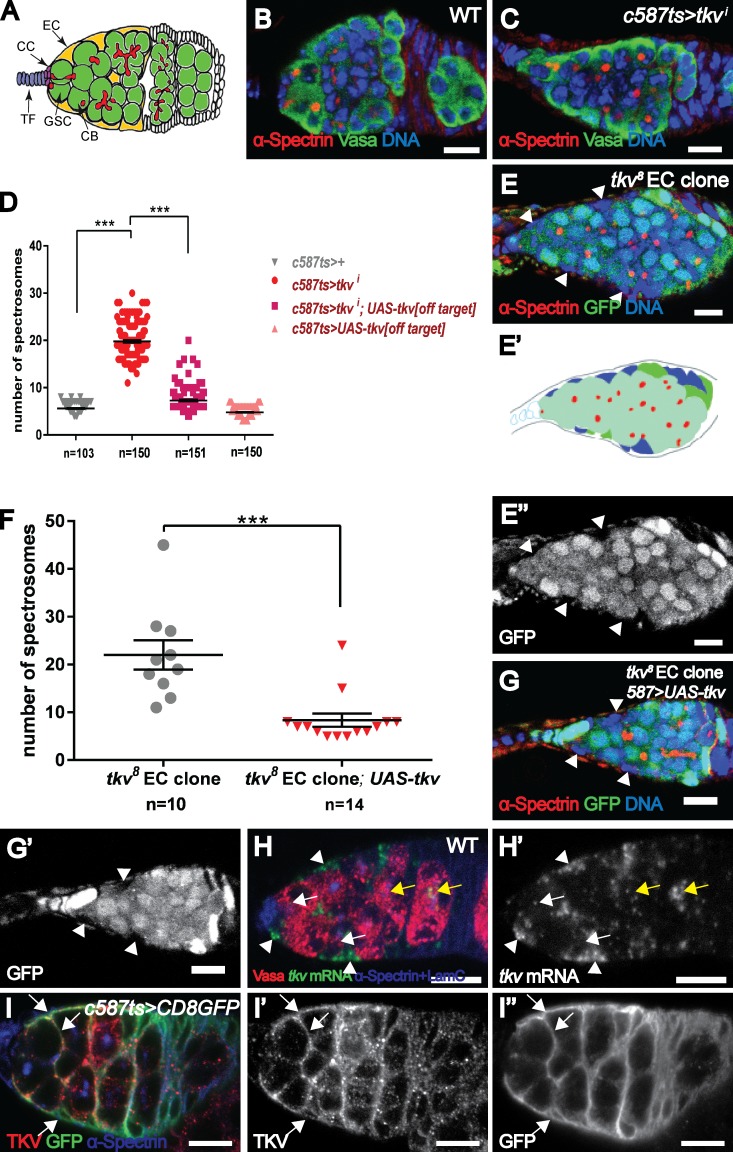
Figure 1.
Tkv acts in ECs to restrict germline proliferation. (A) Schematic of a Drosophila germarium. (B and C) Compared with a control (c587ts/+) germarium (B), a tkvi (C; BL40937) germarium contains ectopic spectrosome-containing cells. Vasa (green) is a germ cell marker. (D) Statistical data showing the number of spectrosome-containing cells in control (c587ts/+), tkvi (c587ts;tkvi[BL-40937]), off-target construct rescue (c587ts;UAS-tkv[off-target]), or c587ts;tkvi/UAS-tkv[off-target] germaria. (E–G) A germarium carrying tkv8 mutant ECs (lack of GFP signal marked by arrowheads) exhibits more spectrosome-containing cells (E and F), which is rescued by restoring tkv expression (G). (E′) Cartoon model to illustrate the position of mutant EC clones (blue). The genotype of E is c587.UAS-flp; FRT40A.ubiGFP/FRT40A.tkv8. The genotype of G is c587.UAS-flp; FRT40A.ubiGFP/FRT40A.tkv8;UAS-tkv. (H) In addition to the germline expression (white arrows indicate GSCs and CBs and yellow arrows indicate developing oocytes), tkv mRNA is strongly detected in the ECs (arrowheads). (I) A c587ts;UAS-CD8GFP germarium exhibits strongly colocalized Tkv and GFP staining in ECs (arrows). Bars, 10 µm. ***, P < 0.001.
Decapentaplegic (Dpp) is the primary niche-derived signal that maintains GSCs (Xie and Spradling, 1998; Xie and Spradling, 2000). As a morphogen, Dpp can act over a long distance (many cell diameters) to influence cell fate specification, whereas in the germarium it functions as a short-range signal (one-cell-diameter range) to regulate GSC self-renewal (Tabata and Takei, 2004; Losick et al., 2011). Several mechanisms involving both somatic and germline cells act in concert to spatially restrict Dpp activity within the niche (Harris and Ashe, 2011; Losick et al., 2011; Xie, 2013). The primary regulatory mechanism derived from the somatic cells involves division abnormally delayed (dally). Dally, a glypican specifically expressed in cap cells, binds and stabilizes Dpp on the extracellular matrix. Ectopic Dally expression in ECs caused Dpp signal activation outside the niche (Guo and Wang, 2009; Hayashi et al., 2009; Liu et al., 2010).
The Wnt pathway participates in diverse processes and plays an essential role in regulating stem cell activity during development. Deregulation of this signaling pathway is associated with a wide range of human diseases (Clevers and Nusse, 2012). In the absence of Wnt ligands, a cytoplasmic destruction complex composed of glycogen synthase kinase 3, casein kinase 1α, Axin, and adenomatous polyposis coli targets β-catenin (or Armadillo [Arm] in fly) for 26S proteasome-mediated degradation. The binding of Wnt to its cognate receptors activates Dishevelled (Dsh), which in turn represses the destruction complex, thereby stabilizing β-catenin and promoting its nuclear entry. In the nucleus, β-catenin forms a transcription complex with T cell factor (TCF)/lymphoid enhancer factors Pygopus (Pygo) and Legless (Lgs) to regulate target gene expression (Logan and Nusse, 2004; Angers and Moon, 2009; Mosimann et al., 2009). In the Drosophila germarium, Wingless (Wg; the fly Wnt homologue) was initially reported to be produced in the cap cells and to regulate the activity of follicle stem cells located at the germarial 2a/2b boundary (Forbes et al., 1996; Song and Xie, 2003). Recent data suggest that Wg is also expressed in ECs and that this expression may also be important for follicle stem cell maintenance (Sahai-Hernandez and Nystul, 2013).
Recently, several lines of evidence revealed that ECs participate in restricting germline proliferation in a non cell-autonomous manner (Liu et al., 2010; Eliazer et al., 2011; Kirilly et al., 2011; Wang et al., 2011). To understand the underlying mechanisms, we conducted a genetic screen by knocking down the functions of genes specifically in ECs. Using this approach, we reveal that EC-expressed Thickveins (Tkv) acts as a receptor sink to remove excess cap cell–expressed Dpp, thereby restricting niche-associated Dpp activity and promoting germ cell differentiation independently of the canonical Dpp signaling transduction pathway. We further demonstrate that the expression level of Tkv is transcriptionally regulated by multiple Wnt ligands produced by cap cells. Therefore, the Drosophila ovarian stem cell niche uses a self-restraining mechanism to maintain germline homeostasis.
Results
Tkv functions in ECs to promote germline homeostasis
In a small-scale RNAi screen, we found that germaria with compromised Tkv function in ECs by a shRNA construct (termed tkvi germaria and thereafter i superscript is referred to as knocking down gene of interest in the ECs) contained an excess of spectrosome-containing cells. In controls, each germarium exhibited 5.6 ± 0.1 spectrosome-containing cells; however, each tkvi germarium contained 19.8 ± 0.3 spectrosomes (Fig. 1, B–D). To verify this result, we generated a transgene carrying a tkv variant, which cannot be targeted by this shRNA construct (referred to as off-target variant; see Materials and methods) and found that it could rescue tkvi phenotype (Fig. 1 D and Fig. S1 A). Similar phenotypes were observed in germaria with compromised Tkv function in ECs using several RNAi constructs targeting different regions (Fig. S1 B and not depicted). To confirm this result, we removed Tkv function from the ECs using two null mutants (tkv4 and tkv8 [Nellen et al., 1994; Penton et al., 1994]). As expected, these germaria contained more spectrosome-containing cells (Fig. 1, E and F, 22.0 ± 3.1; and Fig. S1 C). Importantly, the formation of ectopic spectrosome-containing cells in these germaria was suppressed by restoring Tkv expression in the ECs (Fig. 1, F and G, 8.4 ± 1.4), indicating that Tkv functions in the ECs to promote germline differentiation.
Tkv is known to play a role in mediating Dpp signaling in GSCs for their maintenance (Xie and Spradling, 1998). Consistent with this, tkv transcripts examined by RNA in situ hybridization were detected in the germline cells including GSCs (Fig. 1 H, white arrows). In addition, we also detected tkv mRNA in ECs (Fig. 1 H, arrowheads). tkv-lacZ (tkvk16713), a tkv transcription reporter that recapitulates its expression in wing imaginal discs (del Álamo Rodríguez et al., 2004), was similarly detected in ECs (Fig. S1 D). In agreement with the in situ data, immunostaining with anti-Tkv antibodies also detected strong expression in the germarial region (Fig. S1 E). To address whether the high levels of Tkv in the germarium reflected its expression in ECs, we expressed the membrane marker CD8. GFP specifically in the ECs. Indeed, Tkv extensively colocalized with the CD8. GFP marker and also decorated the cellular extensions that wrap GSCs and germline cysts (Fig. 1 I). These EC-associated signals were not detected in tkvi germarium or tkv8 mutant ECs, whereas germline signals were still present (Fig. S1, F–H), confirming the specificity of these signals. Collectively, these data show that Tkv acts in ECs to non cell-autonomously restrict germline proliferation.
Tkv functions independently of the canonical Dpp signaling pathway
Tkv acts as a type I receptor of the Dpp pathway to mediate downstream signaling. We next investigated whether Tkv in the ECs reflects the function of Dpp signaling. To address this possibility, we used RNAi constructs to knock down various Dpp pathway components in the ECs. We did not observe more spectrosome-containing cells in germaria with compromised function of Put (Punt, the Type II receptor; Fig. S2, A and E, 5.9 ± 0.1), Sax (Saxophone, another type I receptor; Fig. S2, B and E, 5.5 ± 0.1), Mad (Mothers against dpp; Fig. S2, C and E, 5.5 ± 0.1 for BL31315 and 5.2 ± 0.1 for BL35648), and Med (Medea, the coSmad; Fig. S2, D and E, 5.6 ± 0.1) in ECs. Consistent with these results, germaria devoid of mad function from the ECs using a null mutant (mad12) did not contain more spectrosomes (Fig. S2 F). Furthermore, blocking Dpp signal transduction in the ECs using TkvDN, a dominant-negative form of Tkv lacking the GS boxes and the kinase domain (Haerry et al., 1998), did not lead to the formation of ectopic spectrosome-containing cells (Fig. S2, E and G, 4.9 ± 0.1). Altogether, these data indicate that Tkv functions in ECs to restrict germline proliferation independent of the canonical Dpp signaling pathway.
EC-expressed Tkv prevents ectopic Dpp signal activation outside the niche
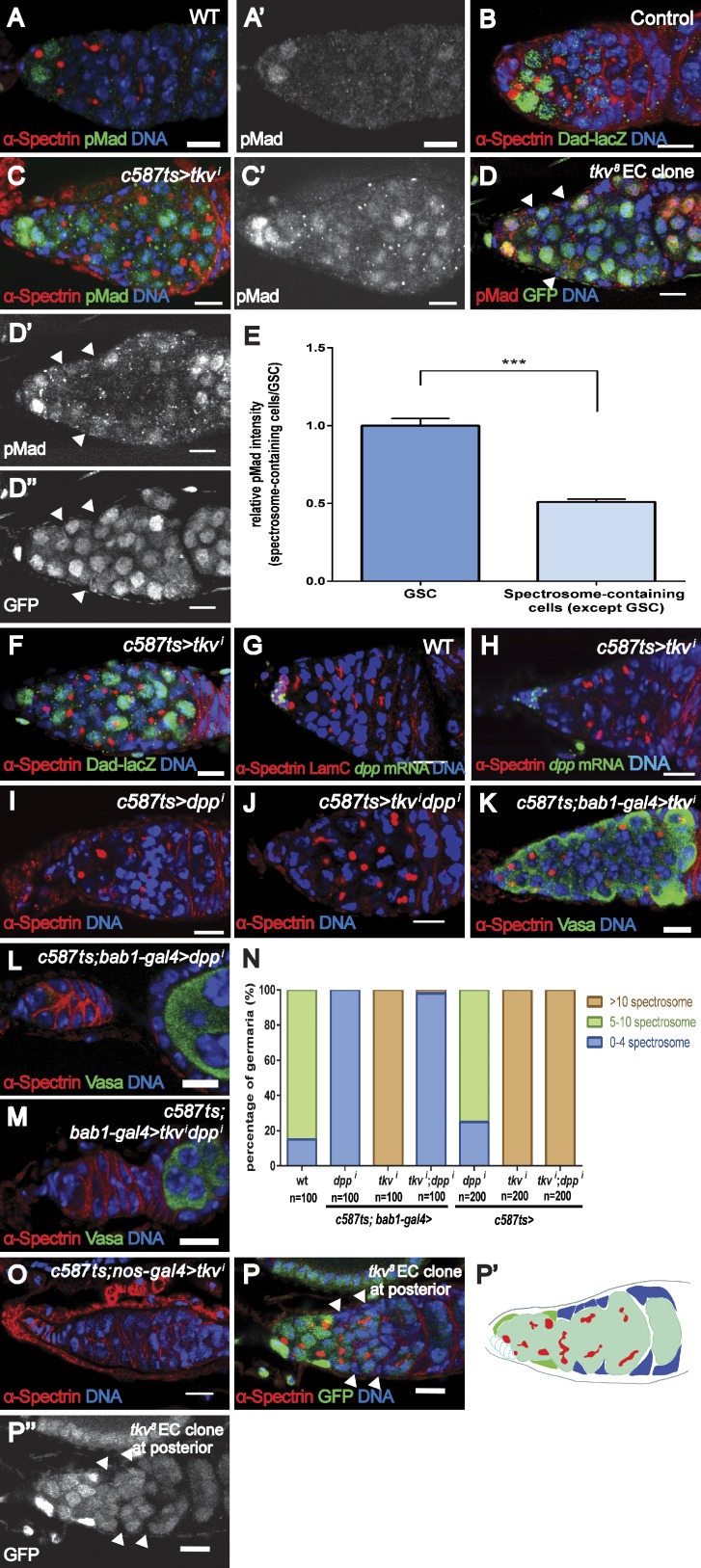
To elucidate the role of Tkv in the ECs, we investigated the cell fate of those ectopic spectrosome-containing cells in the tkvi germarium. In wild-type (WT) germaria, as a result of local Dpp signal activation, phosphorylated Mad (pMad) was detected in the GSCs but not in the CBs (Fig. 2 A), whereas Dad-lacZ (a LacZ reporter for Daughters against dpp, which is a target of Dpp signaling) was expressed at high levels in the GSCs and at lower levels in the CBs (Fig. 2 B). In tkvi germaria, some ectopic spectrosome-containing cells outside the niche (defined by non cap cell contacting) expressed pMad (Fig. 2 C, 18.7 ± 0.7, n = 100, in tkvi germaria compared with 2.2 ± 0.1, n = 100, for controls; P < 0.001). As expected, pMad expression was also detected in some ectopic spectrosome-containing cells in germaria carrying tkv8 mutant EC clones (Fig. 2 D). Consistent with this finding, tkvi germaria contained more Dad-lacZ–positive spectrosome-containing cells (Fig. 2 F, 21.4 ± 0.4, n = 101, for tkvi germaria vs. 6.0 ± 0.1, n = 115, for control germaria; P < 0.001). These results indicate an ectopic Dpp signal activation outside the niche in tkvi germaria.
Figure 2.
Tkv prevents ectopic Dpp signaling. (A) A WT germarium contains two pMad-positive GSCs within the niche. (B) A control (Dad-lacZ/+) germarium contains five Dad-lacZ–positive cells. (C) A tkvi (Bl-40937) germarium exhibits more pMad-positive spectrosome-containing cells. (D) A germarium carrying tkv8 ECs (arrowheads) also contains more pMad-positive cells. Genotype: c587.UAS-flp; FRT40A.ubiGFP/FRT40A.tkv8. (E) Relative pMad intensity in ectopic spectrosome-containing cells in germaria carrying tkv8 ECs (n = 113) compared with GSCs (n = 10). (F) A tkvi (Bl-40937) germarium exhibits ectopic Dad-lacZ–positive spectrosome-containing cells. (G and H) dpp mRNA is strongly detected in the cap cells of WT (G) or tkvi (H; v3059) germarium. (I and J) knockdown of Dpp in the ECs of a tkvi (v3059) germarium does not suppress the formation of more spectrosome-containing cells. (K–M) Knockdown of Dpp in cap cells causes germ cell loss in a tkvi (BL-40937) germarium. (N) Statistical data for I–M. (O) Knocking down Tkv in germline cells in a tkvi (BL-40937) germarium results in the loss of germ cells. (P) A germarium without cap cell–contacting tkv8 mutant ECs does not exhibit more spectrosome-containing cells. tkv8 mutant clones are indicated by arrowheads. Genotype: c587.UAS-flp; FRT40A.ubiGFP/FRT40A.tkv8. (P′) Cartoon model to illustrate the positions of non cap cell–contacting EC mutant clones (blue). Error bars represent the SEM. ***, P < 0.001. Bars, 10 µm.
Activation of Dpp signaling in GSCs represses the expression of bag of marbles (bam), which is de-repressed in CBs and early cysts to promote differentiation. This dynamics can be monitored with Pbam-GFP (a GFP reporter of bam transcription [Chen and McKearin, 2003]). In controls, Pbam-GFP was expressed at low levels in the CBs located one cell away from the cap cells and was up-regulated in differentiating cysts (Fig. S3 A). Similarly, Bam protein was detected in CBs and differentiating germline cysts (Fig. S3 B). In line with ectopic Dpp signaling in the tkvi germarium, some spectrosome-containing cells outside the niche expressed no/low levels of Pbam-GFP, and the up-regulation of Pbam-GFP was postponed to a more posterior position (Fig. S3 C). Consistently, the majority of those spectrosome-containing cells did not express detectable Bam protein (Fig. S3 D). These results demonstrate that Tkv in the ECs prevents Dpp signal activation in the germline cells outside the niche.
We then investigated whether these pMad- and Dad-lacZ–positive but Pbam-GFP– and Bam-negative cells also proliferate outside the niche. Indeed, the 5-ethynyl-2’-deoxyuridine (EdU; a thymidine analogue) incorporation assay (an indication of S phase) and anti-phosphorylated Histone 3 antibody staining (an indication of mitosis) revealed that these spectrosome-containing cells could undergo cell cycle progression (Fig. S3, E and F). Interestingly, we found that there was a slight increase in GSC proliferation rate in tkvi germaria (33.5% of tkvi germaria [n = 200] harbor EdU-positive GSCs, compared with 26% of control germaria [n = 200]). These data suggest that the observed germline hyperplasia phenotype was caused by both increased GSC self-renewal and proliferation of those ectopic spectrosome-containing cells. Furthermore, the formation of these ectopic spectrosome-containing cells was dependent on Dpp signaling (see the following paragraphs), and forced bam expression in these cells resulted in their differentiation (Fig. S3, G and H), suggesting that these ectopic spectrosome-containing cells possess the potential for differentiation.
Tkv acts independently of EGF receptor (EGFR)/MAPK signaling and Dally activity
In WT germaria, Dpp is mainly produced by the cap cells and acts locally on GSCs within the niche. The activation of ectopic signaling outside the niche may be a consequence of ectopic Dpp production in ECs as reported for germaria with compromised Rho (Kirilly et al., 2011) or Lsd1 (Eliazer et al., 2011) function or as a result of expanded niche-expressed Dpp activity, as shown for germaria with defective EGFR signaling (Liu et al., 2010).
To address whether Tkv functions in the ECs to suppress Dpp expression, we examined dpp expression in WT and tkvi germaria by RNA in situ hybridization. In the WT germarium, dpp transcripts were strongly detected in the cap cells and occasionally in some ECs (Fig. 2 G), consistent with our previous observations (Wang et al., 2008a; Liu et al., 2010). In tkvi germaria, strong dpp expression was also detected in the cap cells, and no elevation of dpp transcripts was observed outside the cap cells (Fig. 2 H). To further address this and to exclude the possibility that the formation of ectopic spectrosome-containing cells in tkvi germaria was a result of the potential up-regulation of Dpp in ECs beyond the detection limit of the method used, we knocked down Dpp in the tkvi germarium and found that further removal of Dpp function from the ECs did not suppress the tkvi phenotype, whereas compromising Dpp function in the cap cells resulted in GSC loss (Fig. 1 C and Fig. 2, I–N). These data indicate that ectopic EC-expressed Dpp, if any exists, is not essential for the formation of ectopic spectrosome-containing cells in tkvi germaria, suggesting that cap cell–expressed Dpp is responsible for the observed germline hyperplasia.
Instead, several lines of evidence indicate that Tkv spatially restricts the activity of cap cell–expressed Dpp. First, although pMad was detected in some ectopic spectrosome-containing cells, higher levels of pMad were detected in the GSCs within the niche (Fig. 2, C–E), indicating that cap cells are likely the major source of Dpp ligands for signaling. Second, the removal of Dpp receptors from germ cells in tkvi germaria resulted in GSC loss, suggesting that the formation of ectopic spectrosome-containing cells was a consequence of Dpp signaling but not of a failure to differentiate (Fig. 2 O). Third, the formation of ectopic spectrosome-containing cells was not observed in germaria bearing tkv mutant EC clones that do not directly contact the cap cells (Fig. 2 P). All germaria with ectopic spectrosome-containing cells contained both cap cell– and non cap cell–contacting mutant EC clones (Figs. 1 E and S1 C). These results suggest that those cap cell–contacting ECs play an important role in the spatial restriction of Dpp signaling activity and support the notion that the cap cells are likely the source of Dpp. This is consistent with the fact that Tkv is expressed in all ECs. Lastly, compromising cap cell–expressed Dpp led to precocious differentiation in tkvi germaria (Fig. 2 M).
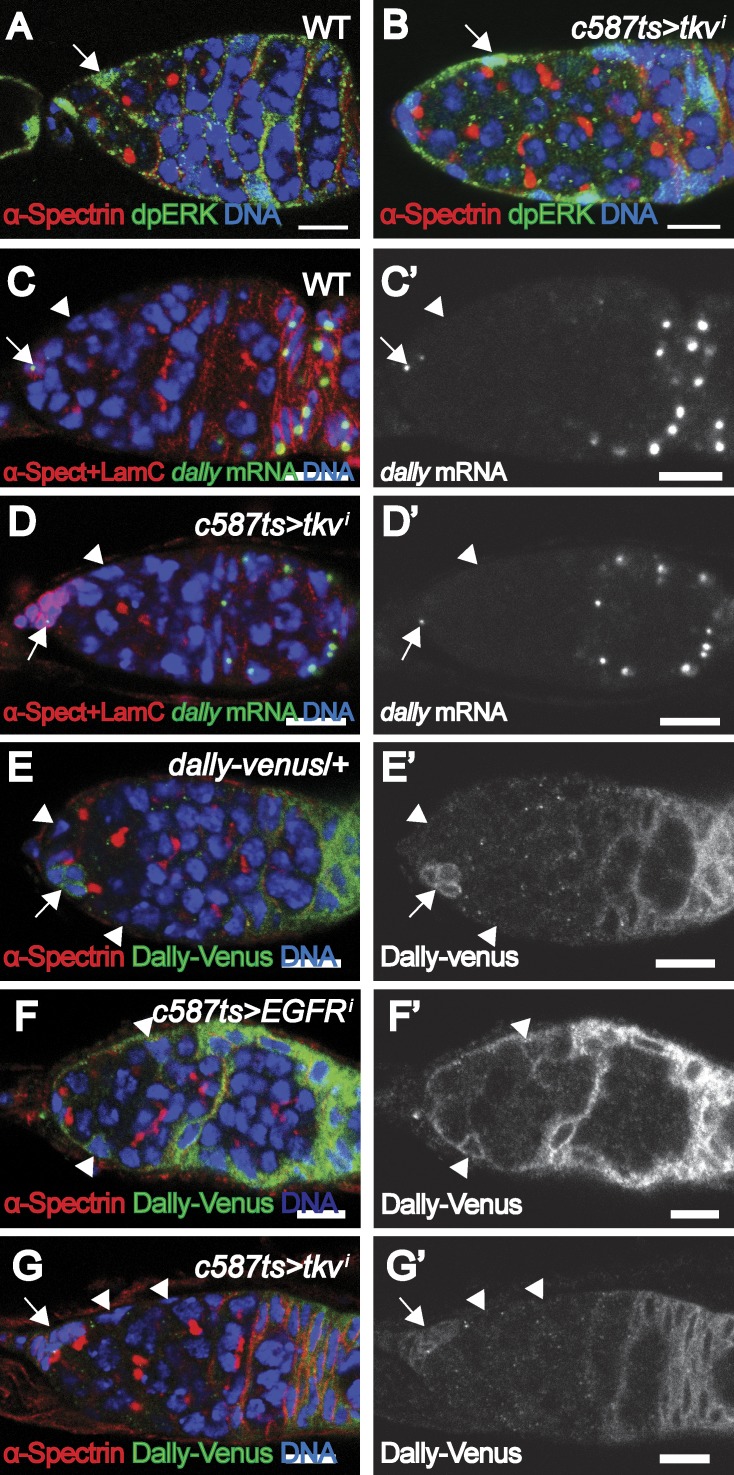
We previously showed that EGFR/MAPK signaling in ECs restricts niche-associated Dpp activity by repressing dally expression (Liu et al., 2010). In vertebrates, it is known that TGFβ receptors can induce MAPK signaling via a noncanonical signaling pathway (Massagué, 2012). Hence we addressed whether Tkv acts through MAPK/Dally to restrict Dpp activity. In WT germaria, the expression of dpERK, an indicator of MAPK signaling, is consistently detected in the ECs (Fig. 3 A). A similar expression pattern of dpERK was observed in the ECs of tkvi germaria (Fig. 3 B), suggesting normal EGFR/MAPK signaling. We further examined dally expression in these backgrounds by RNA in situ hybridization. As previously shown, dally was expressed in the cap cells and prefollicular cells but not in the ECs of control germaria (Guo and Wang, 2009; Hayashi et al., 2009; Liu et al., 2010; Fig. 3 C). In tkvi germaria, no ectopic dally transcripts were detected in ECs (Fig. 3 D). We identified a GFP trap line (Dally-CPTI004473) inserted at the dally locus and found that this reporter can recapitulate the expression pattern of dally transcripts in the germarium (Fig. 3 E). Interestingly, this reporter was up-regulated in the ECs of the EGFRi germarium, consistent with a previous study showing that dally transcripts were ectopically expressed in EGFR signaling-defective ECs (Liu et al., 2010; Fig. 3 F). However, this reporter activity was not detected in the ECs of the tkvi germarium (Fig. 3 G). Thus, the EC-expressed Tkv acts through a novel mechanism, independent of Dally activity, to prevent ectopic Dpp signaling away from the niche.
Figure 3.
Tkv does not affect EGFR/MAPK signaling or Dally expression. (A and B) dpERK is detected in the ECs (arrows) of WT (A) and tkvi (BL-40937) germaria. (C and D) dally mRNA is detected in cap cells (arrows) and follicular cells but not ECs (arrowheads) in WT (C) and tkvi (BL-40937) germaria. (E) The Dally-Venus reporter is detected in both cap cells (arrows) and follicular cells but not in ECs (arrowheads) in control (Dally-venus/+) germarium. (F) The Dally-Venus reporter is up-regulated in the ECs (arrowheads) of an EGFRi germarium. (G) The Dally-Venus reporter is not detected in the ECs (arrowheads; cap cells are indicated by arrows) of a tkvi (BL-40937) germaria. Bars, 10 µm.
Tkv acts as a receptor sink to remove excess niche-expressed Dpp
Based on the observations that (a) Tkv was expressed at high levels in the ECs compared with the GSCs, where downstream signaling is transduced (Fig. 1 I); (b) Tkv was present throughout the EC membrane, including the cellular extensions that wrap GSCs, CBs, and differentiating cysts (Fig. 1 I); and (c) ectopic Dpp signaling was observed outside the niche in the tkvi germarium or in germarium carrying tkv mutant ECs (Fig. 2, C–F) although no ectopic Dpp expression was detected in those germaria, our data suggest that the EC-expressed Tkv might function as a barrier to prevent Dpp diffusion beyond the niche. We propose that the EC-expressed Tkv functions as a “receptor sink” to remove excess amount of Dpp produced in the cap cells, thereby restricting the extent of Dpp diffusion. This hypothesis is consistent with previous data showing that ectopic Tkv expression in the wing imaginal discs prevented Dpp diffusion (Lecuit and Cohen, 1998). The disruption of this receptor sink in the germarium would lead to expansion of the Dpp signaling range and, hence, expanded Dpp activity.
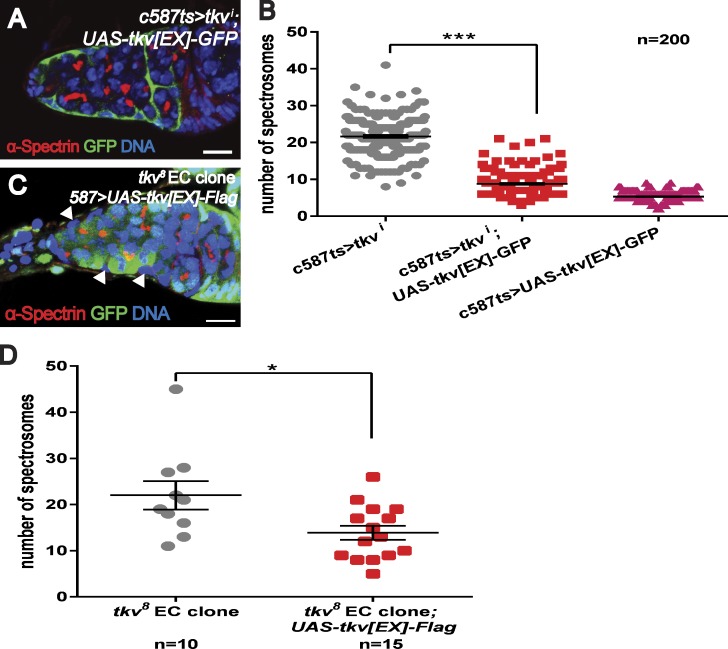
To test this possibility, we conducted rescue experiments by introducing Tkv variants into tkvi or tkv EC mutant germaria. We removed endogenous Tkv using shRNA or dsRNA constructs targeting the intracellular portion of Tkv and then introduced Tkv[EX]-GFP, a variant in which the cytoplasmic portion of Tkv is replaced with a GFP tag, which is unable to transduce Dpp signaling (similarly to the previously reported Tkv[ΔGSK] variant [Haerry et al., 1998]). Our results show that the Tkv[EX]-GFP transgene strongly suppressed the formation of ectopic spectrosome-containing cells in the tkvi germarium (Fig. 4, A and B). Similarly, Tkv[EX]-Flag, a variant in which the cytoplasmic part of Tkv is replaced with a Flag epitope as well as Tkv[ΔGSK] variant, also partially rescued the tkv8 EC mutant phenotype (Fig. 4, C and D, 13.6 ± 1.5; and not depicted). These data support that the extracellular domain of Tkv is functionally important (presumably through its ability to bind Dpp) for suppressing ectopic spectrosome-containing cells.
Figure 4.
Dpp binding is important for the function of Tkv. (A and B) The overexpression of Tkv[EX]-GFP in the ECs of a tkvi (v105834) germarium suppresses the formation of ectopic spectrosome-containing cells. (C and D) The expression of Tkv[EX]-Flag in ECs mutant (C, arrowheads) for the tkv8 allele partially suppresses the formation of ectopic spectrosome-containing cells. Bars, 10 µm. ***, P < 0.001; *, P < 0.05.
Wnt signaling in ECs prevents ectopic Dpp signaling via regulating Tkv expression
To investigate the regulatory mechanism that controls Tkv expression in ECs, we performed another small-scale RNAi-mediated screen for signaling molecules using tkv-lacZ as a reporter and found that knockdown Dsh, a Wnt pathway component, in the ECs resulted in the formation of ectopic spectrosome-containing cells and a strong reduction in tkv-lacZ expression (Fig. S4, A and B).
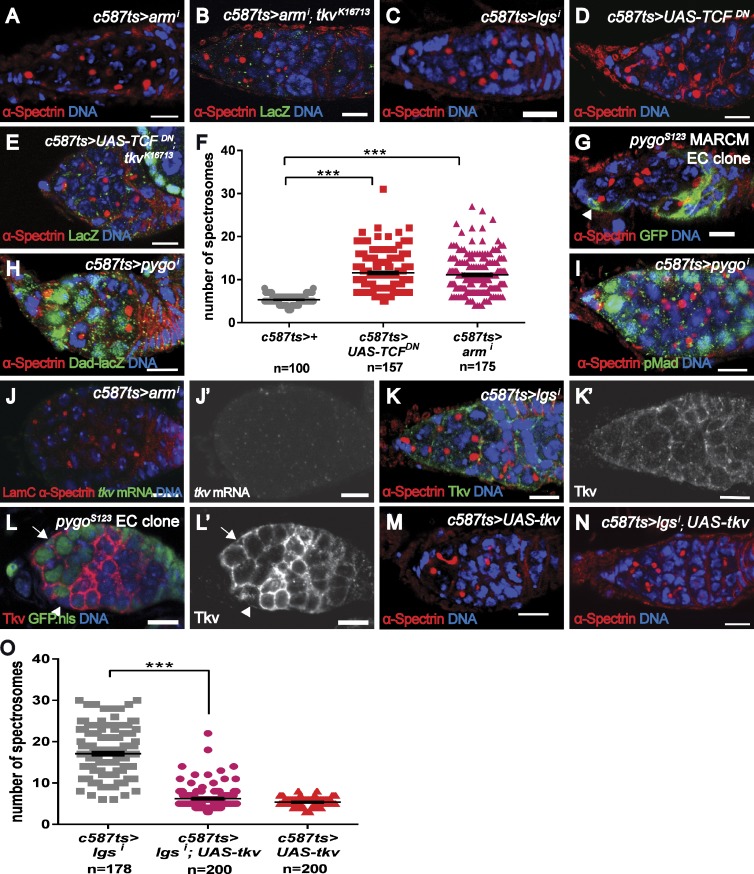
Our results show that the canonical Wnt pathway functions in the ECs because compromising the functions of other canonical signal pathway components, including Arm (Fig. 5, A, B, and F), Pygo (Fig. S4, C and E), and Lgs (Fig. 5, C and O; and Fig. S4, D and E), and the overexpression of a dominant-negative version of dTCF (TCFDN; Fig. 5, D–F) in the ECs led to the formation of ectopic spectrosome-containing cells and to the down-regulation of tkv-lacZ. Similarly, the formation of ectopic spectrosome-containing cells was also observed in the germaria bearing EC mutant clones for pygoS123 (Fig. 5 G). Furthermore, restoring downstream Wnt signaling by expressing ArmS10, a gain-of-function version of Arm that bypasses upstream signaling activation, strongly rescued dshi phenotypes (Fig. S4 F). In contrast, removing Wnt signaling components from the GSCs did not compromise the self-renewal and maintenance of the GSCs (unpublished data), consistent with a previous study (Song et al., 2002) and indicating that Wnt signaling in the GSCs is dispensable for self-renewal. These data demonstrate a role for Wnt signaling in the ECs in controlling germline homeostasis and possibly regulating tkv expression.
Figure 5.
Wnt signaling acts in the ECs to regulate Tkv expression. (A–F) Germaria with compromised Wnt signaling components (Arm [A and B, BL-35004] and Lgs [C, BL-37476]) or with overexpressed TCFDN (D and E) in ECs exhibit more spectrosome-containing cells and reduced tkvk16713 reporter expression. (F) Statistical data for spectrosome-containing cells in armi or TCFDN expressing germarium. (G) A germarium containing ECs mutant for the pygos123 allele (generated using the MARCM system and marked with an arrowhead) exhibits ectopic spectrosome-containing cells. Genotype: hs-flp.UAS-CD8.GFP/+;tub-gal4/+;FRT82B.pygos123/FRT82B.tub-gal80. (H) A pygoi (NIG-11518R) germarium harbors ectopic Dad-lacZ–positive spectrosome-containing cells. (I) A pygoi (NIG-11518R-1) germarium contains more pMad-positive spectrosome-containing cells. (J) A armi (BL-35004) germarium expresses low levels of tkv transcripts (compared with Fig. 1 H). (K) A lgsi (BL-37476) germarium expresses low levels of Tkv in the ECs (compared with Figure 1 I). (L) One EC mutant for pygos123 (arrowhead) exhibits reduced Tkv expression, compared with control EC (arrow) of the same germarium. Genotype: hs-flp;FRT82B.pygos123/FRT82B.ubi-GFP. (M–O) Forced expression of Tkv in lgsi (BL-37476) germaria strongly suppresses the formation of ectopic spectrosome-containing cells. ***, P < 0.001. Bars, 10 µm.
We next investigated whether Wnt signaling also restricts Dpp signaling in the germarium and examined the cell fate of the ectopic spectrosome-containing cells in those Wnt signaling-defective germaria. In WT germaria, Pbam-GFP was detected at low levels in the CBs and was up-regulated in differentiating cysts (Fig. S3 A). In germaria with compromised Wnt signaling in the ECs, some ectopic spectrosome-containing cells exhibited no or low levels of Pbam-GFP expression (6.9 ± 0.3, n = 100, for pygoi vs. 2.2 ± 1.0, n = 101, for controls; P < 0.001), and its up-regulation was postponed to a more posterior position (Fig. S4 G, compared with Fig. S3 A). Additionally, Bam protein was absent in those spectrosome-containing cells although it was still detected in fusome-containing cysts (Fig. S4 H, compared with Fig. S3 B). In contrast, Dad-lacZ was detected in more spectrosome-containing cells (Fig. 5 H, 10.3 ± 0.3, n = 100, for lgsi compared with 6.0 ± 0.1, n = 115, for control; P < 0.001). Similarly, pMad was also detected in some spectrosome-containing cells outside the niche in addition to the GSCs within the niche (Fig. 5 I, 5.9 ± 0.4, n = 101, for lgsi; P < 0.001). Thus, these data show that compromising Wnt signaling in the ECs results in ectopic Dpp signaling outside the niche that is reminiscent of that observed in the tkvi germarium.
Further analyses indicate that the formation of ectopic spectrosome-containing cells in those germaria was not a result of ectopic Dpp expression outside the niche because no ectopic dpp transcripts were detected in the ECs (Fig. S4, I and J), and knockdown of Dpp in the ECs did not rescue the observed phenotypes (Fig. S4, K and L). Our data suggested an expanded function of cap cell–expressed Dpp because those phenotypes were suppressed upon removing Dpp receptors from those spectrosome-containing cells in lgsi germaria (Fig. S4, M–P), similarly to the data for tkvi germaria.
We then addressed whether Wnt signaling acts through EGFR/MAPK signaling or through Dally to restrict Dpp activity. However, two lines of evidence do not support this link. First, MAPK signaling was still activated in the ECs of those germaria as measured by dpERK (Fig. S4 Q and not depicted). Second, no ectopic dally expression (measured by both RNA in situ and reporter expression) was detected in those ECs (Fig. S4, R and S). Lastly, further removal of the function of Dally from the ECs did not suppress the observed phenotypes (Fig. S4 T).
Because our early results showed that Tkv acted independently of Dally to constrain Dpp function to the niche and that tkv-lacZ expression was reduced in the germaria along with compromised Wnt signaling in the ECs (Fig. 5, B and E), we investigated whether Wnt signaling regulates Tkv expression. Indeed, tkv transcripts were strongly down-regulated in the ECs with compromised Wnt signaling (Fig. 5 J and Fig. S4, U and V, compared with Fig. 1 H). Consistently, Tkv protein expression was also reduced in the germaria with defective Wnt signaling in the ECs (Fig. 5 K and Fig. S4 W, compared with Fig. 1 I) and in the ECs mutant for pygoS123 (Fig. 5 L). Supporting this connection, restoring Tkv expression in the ECs of Wnt signaling-defective germaria strongly suppressed the formation of ectopic spectrosome-containing cells (Fig. 5, M–O; and Fig. S4, X–Z). Collectively, these data show that Wnt signaling in the ECs acts through Tkv to constrain the activity of the cap cell–produced Dpp.
Cap cell–expressed Wg and Wnt6 contribute to regulating tkv expression in the ECs
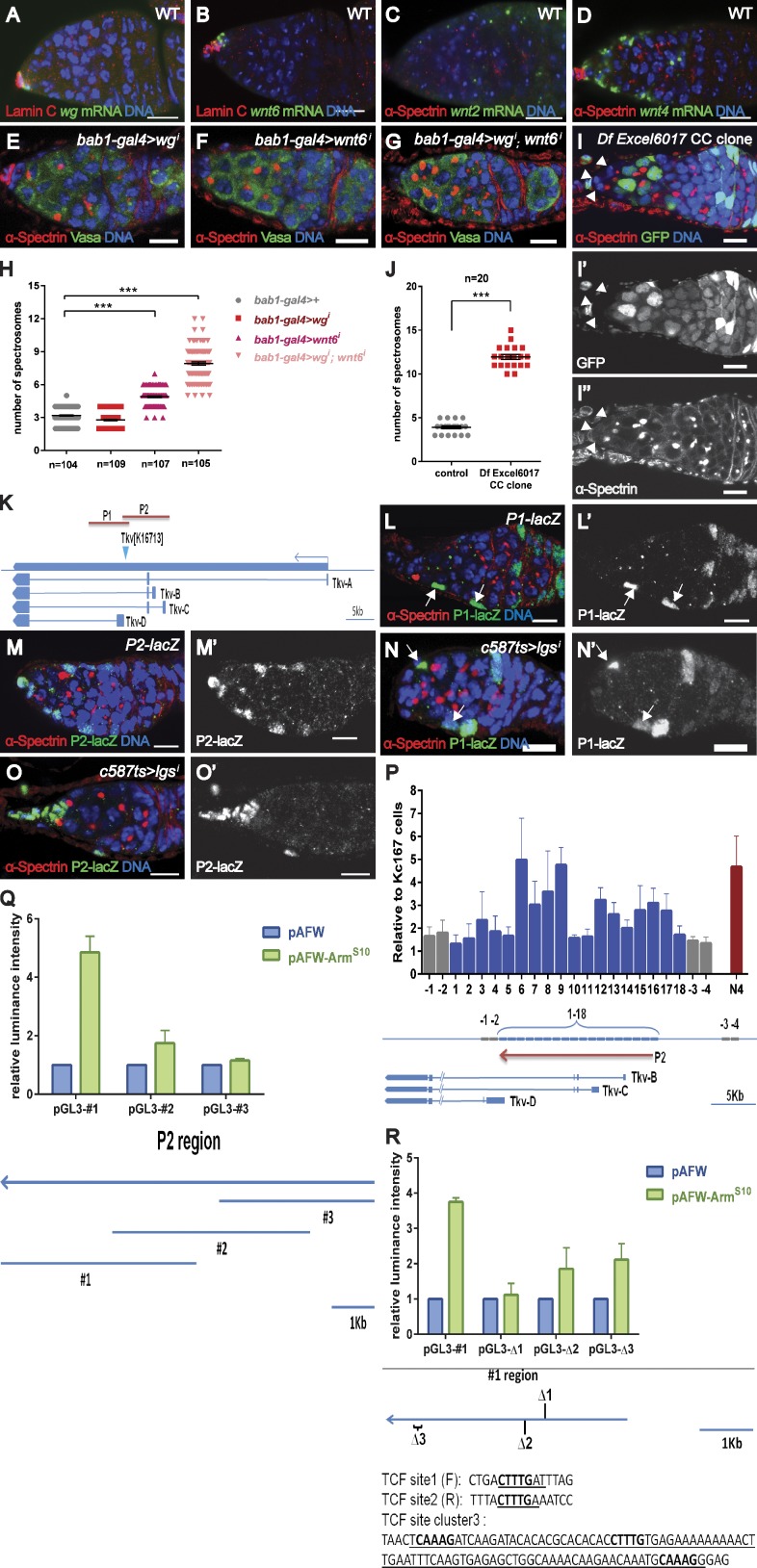
We next investigated the source of Wnt ligands and conducted RNA in situ hybridization to examine the expression pattern of all seven annotated Wnts and found that four of them are expressed in the germarium. As previously reported, wg transcripts were strongly detected in cap cells (Forbes et al., 1996; Song and Xie, 2003; Fig. 6 A). Interestingly, wnt6 mRNA was also highly expressed in cap cells (Fig. 6 B), whereas wnt2 and wnt4 were expressed in both cap cells and ECs, with wnt4 transcripts detected at a higher level and wnt2 transcripts detected at a lower level (Fig. 6, C and D).
Figure 6.
Cap cell–expressed Wnts promote Tkv expression in ECs. wg (A) and wnt6 (B) transcripts are strongly detected in cap cells (indicated by strong LamC expression). Both wnt2 (C) and wnt4 (D) transcripts are detected in cap cells and ECs. (E–H) Knocking down Wnt6 (F) but not Wg (E) from cap cells results in a slight increase in spectrosome-containing cells, whereas removing both Wg and Wnt6 from cap cells leads to the formation of ectopic spectrosome-containing cells (G and H). (I) A germarium with cap cell mutants (lack of GFP signals marked by arrowheads) for a deficiency removing wg, wnt4, wnt6, and wnt10 contains more spectrosomes. Genotype: FRT40A.ubi-GFP/FRT40A.Df(2L)Excel6017;bab1-gal4.UAS-flp. (J) Statistical data from spectrosome-containing cells in I. (K) A schematic of the genomic region used for the generation of two new transgenic reporters (P1-lacZ and P2-lacZ). (L) P1-lacZ is expressed in several ECs (arrows). (M) P2-lacZ is expressed in most ECs. (N and O) P2-lacZ (O) but not P1-lacZ (N) is down-regulated in the ECs of lgsi (BL-37476) germaria (arrows in N indicate ECs). (P) ChIP experiments in KC167 cells showing that ArmS10 is enriched at the P2 region of the tkv enhancer. Regions 1–18 cover the entire P2 region. Regions −1 to −4 outside the P2 region serve as negative controls, and N4 serves as a positive control (see Materials and methods). (Q) A luciferase assay using dissected fragments of the P2 region shows that fragment #1 responds strongly to ArmS10 overexpression. The dissected fragments (#1 to #3) are shown in the bottom panel. (R) Luciferase assay using different variants of fragment #1 with site1, site2, or site cluster3 deleted (deleted sequence is underlined); bold letters indicate the binding consensus sequence (5′-CTTTG-3′). The luminance intensity is the relative ratio of Firefly/Renilla normalized by the value of related luciferase reporter alone. Error bars represent the SEM. ***, P < 0.001. Bars, 10 µm.
We next investigated the functions of these germarium-expressed Wnts. We first used bab1-gal4 and c587-gal4 in combination to knock down these Wnts from both cap cells and ECs. Consistently with a previous study (Song and Xie, 2003), we found that germaria with compromised wg function did not harbor more spectrosome-containing cells (Fig. S5, A and E). Although compromising wnt2 activity did not lead to an increase in spectrosome-containing cells (Fig. S5, B and E), removing wnt4 activity resulted in deformed germaria, some of which contained slightly more spectrosome-containing cells (Fig. S5, C and E). Interestingly, compromising wnt6 function resulted in a weak increase in spectrosome-containing cells (Fig. S5, D and E, 7.8 ± 0.2, n = 200, compared with 5.3 ± 0.1, n = 97, in controls).
We then examined whether these cap cell–expressed Wnts function synergistically and focused our attention on Wg and Wnt6 that are strongly expressed in cap cells, which also produce Dpp. We used bab1-gal4 to knock down these Wnts in the cap cells. Although germarium with Wg knockdown did not exhibit more spectrosome-containing cells (Fig. 6, E and H, 2.8 ± 0.1 for Wg knockdown germaria compared with 3.2 ± 0.1 for control germaria), germaria with Wnt6 knockdown contained slightly more spectrosomes (Fig. 6, F and H, 4.9 ± 0.1). Interestingly, germaria with compromised Wg and Wnt6 functions harbored more spectrosome-containing cells (Fig. 6, G and H, 7.9 ± 0.1), supporting a redundant role of these cap cell–expressed Wg and Wnt6. To further confirm this finding, we generated cap cells mutant for these Wnts by using a small deficiency and found these germaria contained more spectrosome-containing cells (Fig. 6, I and J). These data show that these cap cell–expressed Wnts act non cell autonomously to prevent germline over-proliferation. We then addressed Tkv expression in these germaria. Reinforcing the role of those cap cell–expressed Wnts in regulating Tkv expression, both tkv transcript and protein expression were reduced in germaria with compromised Wg and Wnt6 functions (Fig. S5, F and G; and not depicted). Thus, in contrast to cap cell–expressed Dpp, which maintains GSCs, cap cell–expressed Wnts promote germline differentiation by regulating Tkv expression. However, we cannot exclude the possibility that cap cell–expressed Wnt2 and Wnt4 also have a similar role in restricting germ cell proliferation.
Thus far, our data show that cap cell–expressed Wnts (including Wg and Wnt6) function to modulate Tkv expression in the ECs. To investigate the mechanism underlying this regulation, we used the tkv-lacZ (tkvk16713) reporter line to identify the enhancer/promoter region driving tkv expression in the ECs. We generated new reporter transgenic lines (P1-lacZ and P2-lacZ) by placing the genomic fragments flanking this insertion site in front of a LacZ reporter (Fig. 6 K) and examined the reporter expression in the germarium. Interestingly, both reporter lines were expressed in the ECs; P2-lacZ consistently expressed in the ECs, whereas P1-lacZ was only detected in several ECs (Fig. 6, L and M). Further analyses showed that expression of the P2-lacZ reporter was strongly suppressed in Wnt signaling–defective germaria (Fig. 6, N and O; and Fig. S5, H and I), suggesting that Wnt signaling likely acts on this region to promote Tkv expression. To test whether Wnt signaling may act directly on this enhancer region, we used a cell-based assay and performed chromatin immunoprecipitation (ChIP) experiments to investigate whether ArmS10, upon ectopic expression, could occupy the enhancer region that drives its expression in the ECs. Indeed, ArmS10 occupancy was enriched at this enhancer/promoter region (Fig. 6 P), suggesting a direct link between Wnt signaling and Tkv expression. We further conducted a luciferase-based assay to dissect this region and identified one 4.5-kb fragment that could respond to ArmS10 expression in KC167 cells (Fig. 6 Q). Through sequence analysis, we identified several putative Arm/dTCF-binding consensus sequences (including two binding sites [referred to as site1 and site2] and one binding cluster containing several putative binding sites [referred to as cluster3]; Fig. 6 R) within this region (Waterman et al., 1991). Although deleting the site2 or binding cluster3 somehow reduced the response of this fragment to ArmS10 expression, we found that removing site1 strongly compromised the response of this fragment to ArmS10 expression (Fig. 6 R). Collectively, these data show that cap cell–expressed Wnts restrict cap cell–associated Dpp activity by directly regulating Tkv expression in the ECs.
Discussion
In this study, we show that Tkv functions as a receptor sink in the ECs to remove excess diffusible cap cell–expressed Dpp, thereby locally restricting its activity. We further show that the expression levels of Tkv are transcriptionally regulated by canonical Wnt signaling via multiple Wnt ligands produced by the cap cells, including Wg and Wnt6 (Fig. 7). This mechanism by which a niche, through the use of multiple signaling pathways, defines its own boundary (a “self-restraining” niche), may be a general feature of stem cell systems (Watt and Hogan, 2000; Moore and Lemischka, 2006; Morrison and Spradling, 2008; Li and Clevers, 2010; Hsu and Fuchs, 2012).
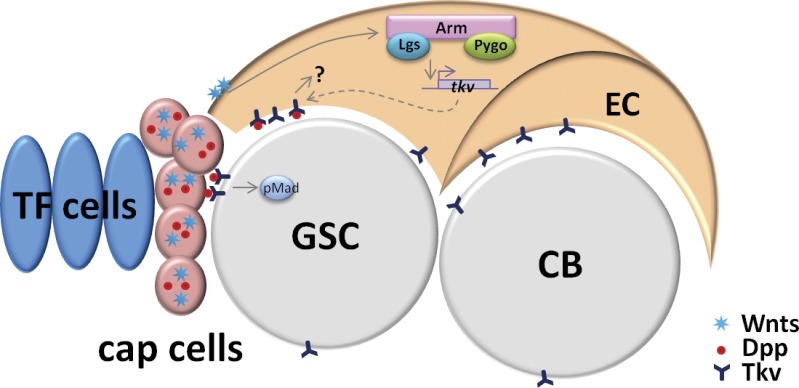
Figure 7.
The working model. In Drosophila ovarian stem cell niche, cap cells produce several signaling molecules including Dpp and Wnts. Dpp promotes GSC self-renewal by activating downstream (indicated by pMad). Wnts function in ECs via the canonical signaling pathway to promote Tkv expression, which in turn removes excess of cap cell–produced Dpp independent of the canonical Dpp signaling pathway and prevents Dpp activity outside the niche. Thus, the Drosophila GSC niche helps to define its own niche activity.
Function of Tkv in ECs
In addition to its expression in germ cells, Tkv is also expressed in the ECs (Fig. 1, H and I). Removing Tkv in ECs (either by RNAi or by the generation of mutant clones) leads to ectopic Dpp signaling outside the niche (Fig. 2, C and F), indicating that Dpp forms a long-range gradient in these germaria and suggesting that Tkv normally prevents Dpp signaling outside the niche. Our data here are consistent with a previous observation suggesting that in wing imaginal discs Tkv expression levels may play a role in Dpp diffusion (Lecuit and Cohen, 1998). This mechanism of receptor-mediated sequestration of ligands is not unique to Dpp. During early Drosophila embryogenesis, the receptor Torso functions to sequester its ligand, Trunk (Casanova and Struhl, 1993). In wing imaginal discs, the transmembrane receptor Patched binds to and limits the diffusion of its ligand, Hedgehog (Chen and Struhl, 1996). In Caenorhabditis elegans, Let-23 (the nematode EGFR homologue) has been proposed to restrict the diffusion of its ligand, Lin3 (the EGF homologue) during vulva induction (Hajnal et al., 1997).
Although these receptors prevent further diffusion of their ligands, the binding of ligands to their cognate receptors inadvertently activates downstream signaling cascades that initiate specific developmental programs (Casanova and Struhl, 1993; Chen and Struhl, 1996; Hajnal et al., 1997). Although Tkv is a bona fide Dpp receptor (Penton et al., 1994; Haerry, 2010), its function of restricting the diffusion of Dpp outside the GSC niche appears to be independent of the canonical Dpp signaling pathway. Supporting this, removing downstream signaling components or expressing a dominant-negative form of Tkv in the ECs does not result in a similar phenotype. Therefore, restricting the diffusion of Dpp away from the niche is an important task of EC-expressed Tkv; however, EC-expressed Tkv may have additional functions that have yet to be identified.
Mechanisms that confine Dpp activity within the GSC niche
Dpp, a well-studied morphogen, has the ability to function over a long range (Tabata and Takei, 2004). However, in the germarium, cap cell–expressed Dpp acts over a one-cell-diameter distance within the niche; the range of Dpp function is not likely to be limited by the amounts of Dpp produced because facilitating its transport (by ectopically expressing Dally in the ECs) leads to its long-range action (Guo and Wang, 2009; Hayashi et al., 2009; Liu et al., 2010). This raises the question of how Dpp activity is spatially restricted to ensure proper GSC lineage development.
Overall, two strategies are used to maintain this tight spatial control. The first strategy, which involves the GSC lineage itself, is to control signal receiving in the germline in such a way that promotes signaling activation in the GSCs but dampens it in the CBs. A variety of mechanisms involving the posttranscriptional regulation of Dpp signaling components in CBs have been identified, including the degradation of activated Tkv (via the Fused–dSmurf complex [Xia et al., 2010]) and the translational repression of Mad (via the Brat–Pum complex [Harris et al., 2011]) or Sax (by miR-184 [Iovino et al., 2009]). Additionally, genetic evidence shows that Bam (which is repressed by Dpp signaling) works redundantly with dSmurf to down-regulate Dpp signaling via an unknown mechanism (Casanueva and Ferguson, 2004).
However, much less is known about the second strategy, which limits Dpp diffusion to confine high concentrations of Dpp within the niche. The glypican Dally is specifically expressed in cap cells, and ectopic Dally expression in the ECs leads to expanded Dpp activity (Guo and Wang, 2009; Hayashi et al., 2009; Liu et al., 2010). Interestingly, the suppression of Dally expression is mediated by the GSC lineage-initiated EGFR signaling in the ECs. Thus, the GSC lineage helps to define the range of the niche activity. The type IV collagen Viking, which binds and promotes Dpp activity in embryos, acts instead to limit the functional range of Dpp in the germarium by sequestering Dpp around the GSCs (Wang et al., 2008b). Here, we reveal a novel mechanism by showing that niche-derived Wnts constrain the diffusion of Dpp by promoting Tkv expression in the ECs to remove excess cap cell–produced Dpp. Our data also demonstrate that, in addition to its well-established role in promoting GSC self-renewal by producing niche-associated (Dpp) signaling, the Drosophila ovarian niche also restrains its own activity by using a different (Wnt) signal. Thus, multiple mechanisms function in concert to ensure the integrity of GSC lineage development.
Function of Wnt signaling in the germarium
In vertebrates, Wnt signaling is implicated in various types of stem cells, including embryonic, hair follicle, and intestinal epithelial stem cells (Reya and Clevers, 2005; Lien and Fuchs, 2014). In the fly, the Wnt pathway also plays a role in stem cell systems such as hematopoietic precursors, intestinal stem cells, and follicular stem cells (Forbes et al., 1996; Song and Xie, 2003; Lin et al., 2008; Lee et al., 2009; Sinenko et al., 2009; Sahai-Hernandez and Nystul, 2013).
Here, we show that the Wnt ligands Wg, Wnt2, Wnt4, and Wnt6 are expressed in the germarium. Their overlapping expression pattern suggests functional redundancy. Consistent with this notion, knocking down Wnt6 produces a weak increase in the spectrosome-containing cell phenotype; this effect is enhanced by further removing Wg function. Because of the technical limitations, we were unable to remove all four Wnts from the cap cells to address whether they are all involved in this process. However, we observed that compromising downstream signaling in the ECs results in a stronger phenotype than the single or double knockdown of these Wnts in the cap cells.
We then asked how Wnt signaling in the ECs promotes germline homeostasis. In this study, we show that compromising Wnt signaling in the ECs leads to ectopic cap cell–associated Dpp activity outside the niche, without affecting Dpp expression. We provide evidence that the Wnt pathway does not act through EGFR signaling or through Dally. Instead, Tkv expression (measured by both in situ hybridization and antibody staining) is reduced in ECs with compromised Wnt signaling, suggesting that the Wnt pathway regulates its expression at the transcriptional level. Supporting this, restoring Tkv expression in these Wnt signaling-defective germaria partially suppresses those defects. Thus, our data define a novel mechanism functioning in the ECs to prevent ectopic niche-associated Dpp activity outside the niche. The partial rescue of Wnt signaling defects by Tkv expression suggests the existence of other targets of Wnt signaling. Indeed, a recent study showed that EC-expressed Wnt4 acts through Arm to regulate germline development by promoting piwi expression in the ECs that indirectly controls germline differentiation (Hamada-Kawaguchi et al., 2014). Thus, it appears Wnt signaling may regulate a plethora of targets in the ECs to maintain germline homeostasis.
Materials and methods
Fly stocks
Information about the strains used in this study is provided in the text or in FlyBase: y1w1118, c587-gal4 (c587, a driver that strongly expresses in ECs; a gift from T. Kai, Temasek Life Sciences Laboratory, Singapore, Singapore); Pbam-gfp (a GFP reporter under the control of bam promoter that recapitulates bam transcription pattern; a gift from D. Chen [Institute of Zoology, Beijing, China] and D. McKearin [University of Texas Southwestern Medical Center, Dallas, TX]); Babl-gal4.UAS-flp (flippase expression under the control of Bab1-gal4, which is expressed in terminal filament cells and cap cells; a gift from A. Gonzalez-Reyes, Universidad Pablo de Olavide, Sevilla, Spain); UAS-tkv (a gift from S. Cohen, Institute of Molecular and Cell Biology, Singapore, Singapore); and UAS-tkvDN (a Tkv variant lacking of GS motif and kinase domain; a gift from J. Zhou and M. Boutros, German Cancer Research Center, Heidelberg, Germany). Other stocks were obtained from Bloomington Stock Center, Kyoto Stock Center, NIG-Fly, or Vienna Drosophila RNAi Center: armdsRNA (BL-35004, v107344), bamΔ86, bab1-Gal4 (BL-6802), dally (CPTI0044773; a YPF fusion line inserted at dally locus; 115624; Drosophila Genomics Resource Center), dppdsRNA (BL-25782), dshdsRNA (BL-31306 and BL-31307), lgsdsRNA (BL-37476), maddsRNA (BL-35648, BL-43183, and BL-31315), mad12 (an amorphic EMS-induce allele with premature stop at amino acid Q417), puntdsRNA (BL-35195, BL-39025, and BL-27514), medeadsRNA (v19688), saxdsRNA (BL-36131), pygodsRNA (11518R2 and 11518R3), pygoS123 (BL-7209), pygodsRNA (BL-38208, v100724), tkvdsRNA (BL-40937, Bl-35653, Bl-31040, v105834, and v3059), tkv mutant (tkv8, an EMS-induced amorphic allele with premature stop as amino acid C144, and tkv4, an EMS-induced amorphic allele with premature stop at amino acid W476), wgdsRNA (BL-31249, BL-31310, v13351, and v104579), tkv-lacZk16713 (a lacZ enhancer trap line inserted in tkv locus; BL-11191), ubi-GFP.nls.FRT40A, ubi-GFP.nls.FRT82B, ubi-GFP.nls.FRT19A, ubi-Gal80TS, FRT82B.tubP-Gal80 (BL-5135), wnt2dsRNA (BL-28892, BL-29441, and v104338), wnt4dsRNA (BL-29442), wnt5dsRNA (BL-28534 and BL-29443), wnt6dsRNA (BL-30493 and v104020), and wntDdsRNA (BL-28947 and BL-29560).
Experimental design to knock down gene functions
To address gene function in adult ECs, c587 was used in combination with Ubi-Gal80ts (c587ts for short), which suppresses Gal4 activity at low temperature (18°C), and crosses (unless stated otherwise) were maintained at 18°C to bypass potential requirements during early developmental stages. Progeny with the desired genotypes were collected upon eclosion and fattened at 31°C to inactivate Gal80ts before dissection and immunostaining. Both UAS-dsRNA and UAS-shRNA transgene stocks were used in this study. If available, several dsRNA or shRNA lines were tested for each gene; and the lines listed in the Fly stocks section showed similar phenotypes.
To knock down pygo function in the ECs, c587ts was used, and crosses were maintained at room temperature. Progeny were fattened at 31°C after eclosion.
To knock down wnts from cap cells and ECs, a combination of c587 and bab1-gal4 was used, and crosses were maintained at room temperature. Progeny were fattened at 31°C after eclosion.
To knock down wnts from the cap cells, bab1-gal4 was used, and crosses were maintained at room temperature. Progeny were fattened at 31°C after eclosion.
To ectopically express Bam in tkvi germaria, crosses of c587; hs-bam; tkvi were raised at room temperature. Progeny with the proper genotype were collected, fattened at 31°C for 3 d, and split into two groups. One group was subjected to two heat-shock treatments at 31°C for 1 h per treatment at 10-h intervals; the other group was kept at room temperature as a control. Flies were dissected 24 h after the second heat-shock treatment.
Generation of GSC clones
To generate GSC mutant clones, crosses were set up and maintained at 25°C. Progeny with the proper genotypes were collected and incubated at 37°C for 1 h at 12-h intervals for three consecutive days and fattened at 25°C. Flies were dissected at the indicated time points and GSC clones were examined.
Generation of cap cell and EC clones
To generate cap cell clones for small deficiency line uncovering wg and wnt6 or EC clones for mad12, tkv4, and tkv8, crosses were maintained at 25°C and progeny with the proper genotypes (see figure legends) were collected and fattened for 4 d before examination.
To perform the rescue of tkv4 and tkv8 EC clones using different tkv variants, crosses were maintained at 25°C and progeny with the proper genotypes (see figure legends) were collected and fattened at 31°C for 4 d before dissection and examination.
To generate the MARCM EC clone for pygos123, crosses were maintained at 25°C and third instar larvae were heat shocked six times at 37°C at 8–12-h intervals. Progeny with the proper genotype (see figure legends) were collected and examined.
Generation of transgenic stocks
To generate the UASp-tkv[EX]-GFP or UASp-tkv[EX]-Flag transgenes, cDNA corresponding to isoform B of tkv was amplified and cloned in a pPWG or pPWF vector, respectively, using the Gateway system (Invitrogen). The off-target variant construct was generated by replacing the coding sequence (5′-CAAGCAGTTTGAAGAGTTCAA-3′) of tkv, which is targeted by shRNA (BL-40937), with 5′-TAAACAATTCGAGGAATTTAA-3′. The enhancer/promoter fragments of tkv were amplified using the primers (listed in Table S1) and digested with NotI before being inserted into the pattBLacZ vector (a gift from K. Basler, University of Zurich, Zurich, Switzerland). The injection of these constructs and transgene generation were performed by BestGene Inc.
Cell culture, biochemistry, and ChIP
Drosophila S2 and KC167 cell lines were obtained from the Drosophila Genomics Resource Center and cultured in Shields and Sang M3 Drosophila insect medium (Sigma-Aldrich) at 25°C.
To test the efficiency of the shRNA knockdown construct for BL40937, shRNA was designed according to the TRiP construct (Drosophila RNAi Screening Center) and inserted into a pVALIUM20 (a UASt-based vector; a gift of J. Ni, Tsinghua University, Beijing, China) to construct pVALIUM20-miTkv. The “off-target” variant of Tkv was created by modifying the nucleotide sequence without changing the coding amino acid (see Generation of transgenic stocks) and cloned into a pUASt vector (designated pUASt-Tkv[off-target]). Then, 5 µg of each plasmid (pAc5.1C-Tkv-flag or pUASt-Tkv[off-target], pAc5.1C-act-gal4, pVALIUM20-miTkv, or the pVALIUM20 vector only) was transfected into 2.5 × 105 S2 cells. Cells were harvested in 2 d, and Western blotting was performed following a standard protocol. Primary antibodies used were mouse anti-Flag (1:3,000; Sigma-Aldrich) and rabbit anti-tubulin (1:1,000; Sigma-Aldrich).
For ChIP experiments, 2 × 106 cells were transfected using the Effectene transfection reagent (QIAGEN) to express ArmS10, a constitutively active form of Arm. The cells were harvested 52 h after transfection. ChIP samples were prepared using EZ-Magna ChIP G (EMD Millipore) according to the manufacturer’s protocol and quantitative PCR was performed using the KAPA SYBR FAST quantitative PCR reagent (KAPA Biosystems) on a 7900HT Fast Real-Time PCR system (Applied Biosystems), following standard protocol. The primers used for detection are listed in Table S2. Region −1 to −4, located outside the P2 region, was used as a negative control, whereas region N4, a published region in nkd that responds to dTCF in KC167, was used as a positive control (Fang et al., 2006).
Synthesis of complementary DNA and quantitative real-time PCR analysis
RNA was extracted from 100 ovaries using TRIzol (Invitrogen). cDNAs were generated using the RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific). Quantitative PCR was performed using the primers listed in Table S3.
Immunostaining
Collection, fixation, antibody staining, and fluorescent RNA in situ hybridization of ovaries were performed as previously described (Liu et al., 2010). In brief, ovaries were fixed with 4% paraformaldehyde/PBS for 20–40 min at room temperature, rinsed with PBST (0.1% Triton X-100 in PBS) three times, and blocked in 5% NGS buffer (5% normal goat serum in PBST; Jackson ImmunoResearch Laboratories, Inc.) for 30 min before incubation with primary antibodies (diluted in blocking buffer) overnight at 4°C. On the second day, ovary samples were washed with PBST three times, incubated with secondary antibody (diluted in PBST) for 2–4 h, and then washed with PBST two times before staining with TOPRO-3 DNA dye. The primary antibodies used in this study are as follows: mouse monoclonal anti–α-Spectrin (3A9, 1:100; Developmental Studies Hybridoma Bank), rabbit anti–α-Spectrin (1:3,000; generated in our laboratory), rabbit anti-pMad (1:500; Cell Signaling Technology), guinea pig anti-Vasa (a gift from T. Kai), mouse anti-Bam (1:5; Developmental Studies Hybridoma Bank), chicken anti-GFP (1:5,000; Abcam), rabbit anti-Tkv (against the extracellular domain; 1:2,000; generated in this study), rabbit anti-phosphohistone H3(Ser10) (1:2,000; Cell Signaling Technology). Fluorescein (FITC), Cy3- and Alexa Fluor 647–conjugated goat against rabbit, mouse, chicken, and guinea pig secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. The DNA dyes used were TOPRO-3 (1:5,000; Invitrogen) or Hoechst 33258 (1:5,000; Invitrogen). Samples were analyzed with an upright confocal microscopy (LSM510 Meta; Carl Zeiss), and single-section images were collected.
To perform in situ hybridization, ovaries were fixed in 4% paraformaldehyde in PBS at 4°C overnight, washed with PBST (0.1% Tween 20 in PBS) three times, treated with proteinase K (50 µg/ml; Sigma-Aldrich) for 5 min, and subsequently washed again with PBST three times followed by prehybridization in hybridization solution (50% formamide, 5× SSC, 0.1% Tween 20, 50 µg/ml heparin, and 100 µg/ml of salmon sperm DNA) for at least 1 h at 65°C. Ovaries were then hybridized with Dig-labeled probe in hybridization solution overnight at 65°C. After washing out the probe by PBST, ovaries were incubated with anti-Dig-POD (1:200; Roche) and, subsequently, fluorescent color was developed using fluo dye according to the manufacturer’s instructions (fluorescent tyramide; PerkinElmer). The primers used to generate the templates for in situ probes are listed in Table S4.
Microscopy
Ovary samples were stored in Vectashield mounting medium (Vector Laboratories) before mounting. The immunostaining signals were detected using either an Eclipse 80I microscope (Nikon) or an upright confocal system (LSM510 Meta) with Axioplan 2 microscope (Carl Zeiss) at room temperature. Confocal images were captured at room temperature using the upright confocal system with a NEOFLUAR 40×/1.3 NA oil objective lens and the acquisition software LSM Image Brower (Carl Zeiss). The images were exported and then processed in Photoshop 7.0.1 and Illustrator CS6 (Adobe Systems).
Luciferase assay
Putative enhancer/promoter (#1, #2, or #3) fragments of the P2 region (Fig. 6 Q) were amplified from genomic DNA using the primers listed in Table S5 and were subcloned into pGL3-Basic (Promega). Two binding sites (TCF site1 and site2) and one binding cluster (TCF site cluster3) were identified in fragment #1 (Fig. 6 R). Three #1 variants (each carrying a deletion of the binding site or binding cluster [see underlined nucleotides in Fig. 6 R]) were generated and inserted into a pGL3-Basic vector for the luciferase assay. The coding sequence of ArmS10 was cloned into the pAFW vector using the Gataway vector collection system (Invitrogen). Then, 2.5 × 105 KC167 cells were transfected with 200 ng of luciferase reporter construct, 50 ng pAC-Renilla, and 100 ng pAFW-ArmS10 or pAFW vector using the Effectene system (QIAGEN). Transfected cells were collected after 72 h for the duo-luciferase assay (Promega).
EdU detection
Ovaries were dissected in Shields and Sang M3 insect medium, incubated in medium containing 20 µM EdU for 1 h, and fixed before performing Edu staining (Liu et al., 2010). The EdU staining was performed using the Click-iT EdU Alexa Fluor 555 imaging kit (Invitrogen) according to the manufacturer’s instructions. After three 15-min washes, the ovaries were further stained with other antibodies.
Generation of the anti-Tkv antibody
The rabbit anti-Tkv antibody was raised against a GST fusion protein containing the extracellular domain of isoform B (1–120 aa) with a GST tag at the N terminus. The rabbit anti α-Spectrin antibody was raised against a His tag protein containing N terminus 9–367 aa. Corresponding cDNA fragments of Tkv and α-Spectrin were amplified by PCR using primers listed in Table S6 and cloned into pDEST15 and pDEST17 (Invitrogen), respectively. Fusion proteins were purified according to the manufacturer’s protocol and immunizations were performed by GeneScript.
Statistical and quantification analyses
Spectrosome numbers were counted by fluorescence microscopy. Dividing GSCs in late mitosis (telophase) with two separated nuclei (identified by Hoechst staining) were counted as two spectrosome-containing cells, one as a new-born GSC and one as a CB. All statistical data were recorded in Excel (Microsoft) and graphed in Prism 6.0 (GraphPad Software). P-values were calculated using unpaired t tests in GraphPad Prism. P < 0.05 was considered statistically significant. Error bars represent the SEM. To analyze pMad intensity, all images were taken under the same confocal setting and Z stack slices were reasonably summed. Each pMad-positive cell was manually outlined and the intensity was measured using ImageJ. For each germarium, the intensity of pMad in two GSCs was measured and averaged. The intensity of pMad in those ectopic spectrosome-containing cells was normalized to the mean of two GSCs in the same germarium.
Online supplemental material
Fig. S1 shows that Tkv functions in ECs to restrict germline proliferation non cell autonomously. Fig. S2 shows that Tkv acts independently of the canonical Dpp signaling pathway. Fig. S3 shows that EC-expressed Tkv prevents ectopic Dpp signaling outside the niche. Fig. S4 shows that Wnt signaling in ECs maintains germline homeostasis via modulating Tkv expression in ECs. Fig. S5 shows that Wnts function in the germarium. Table S1 lists primers used to generate tkv reporter lines. Table S2 lists primers used for ChIP experiment. Table S3 lists primers used for quantitative real-time PCR. Table S4 lists primers used in in situ hybridization experiments. Table S5 lists primers used for generating constructs of the luciferase assay. Table S6 lists primers used for antibody generation. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201409142/DC1. Additional data are available in the JCB DataViewer at http://dx.doi.org/10.1083/jcb.201409142.dv.
Supplementary Material
Acknowledgments
We thank Drs. K. Basler, E. Martin Blanco, M. Boutros, D. Chen, S. Cohen, A. Gonzalez-Reyes, T. Kai, D. McKearin, J.Q. Ni, A. Spradling, T. Tabata, J. Zhou, and T. Xie; the Developmental Studies Hybridoma Bank, the Bloomington Drosophila Stock Center, the Vienna Drosophila Resource Center, the NIG-Fly (Japan) Stock Center, and TRiP at Harvard Medical School (National Institutes of Health/NIG R01-GN084947) for reagents; Temasek Life Sciences Laboratory confocal facility and sequencing facility for support; the members of the Cai laboratory for suggestions; and P. Chai, W. Chia, T. Kai, and C.T. Ong for their critical reading of the manuscript.
This work is supported by Temasek Life Sciences Laboratory and the Singapore Millennium Foundation.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- Arm
- Armadillo
- Bam
- bag of marbles
- CB
- cystoblast
- ChIP
- chromatin immunoprecipitation
- Dally
- division abnormally delayed
- Dpp
- Decapentaplegic
- Dsh
- Dishevelled
- EC
- escort cell
- EdU
- 5-ethynyl-2’-deoxyuridine
- EGFR
- EGF receptor
- GSC
- germline stem cell
- Lgs
- legless
- pMad
- phosphorylated Mad
- Pygo
- pygopus
- TCF
- T cell factor
- Tkv
- Thickveins
- Wg
- Wingless
- WT
- wild type
References
- Angers S., and Moon R.T.. 2009. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10:468–477. [DOI] [PubMed] [Google Scholar]
- Casanova J., and Struhl G.. 1993. The torso receptor localizes as well as transduces the spatial signal specifying terminal body pattern in Drosophila. Nature. 362:152–155. 10.1038/362152a0 [DOI] [PubMed] [Google Scholar]
- Casanueva M.O., and Ferguson E.L.. 2004. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 131:1881–1890. 10.1242/dev.01076 [DOI] [PubMed] [Google Scholar]
- Chen D., and McKearin D.M.. 2003. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 130:1159–1170. 10.1242/dev.00325 [DOI] [PubMed] [Google Scholar]
- Chen S., Wang S., and Xie T.. 2011. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr. Opin. Genet. Dev. 21:684–689. 10.1016/j.gde.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Chen Y., and Struhl G.. 1996. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 87:553–563. 10.1016/S0092-8674(00)81374-4 [DOI] [PubMed] [Google Scholar]
- Clevers H., and Nusse R.. 2012. Wnt/β-catenin signaling and disease. Cell. 149:1192–1205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- de Cuevas M., Lilly M.A., and Spradling A.C.. 1997. Germline cyst formation in Drosophila. Annu. Rev. Genet. 31:405–428. 10.1146/annurev.genet.31.1.405 [DOI] [PubMed] [Google Scholar]
- del Álamo Rodríguez D., Terriente Felix J., and Díaz-Benjumea F.J.. 2004. The role of the T-box gene optomotor-blind in patterning the Drosophila wing. Dev. Biol. 268:481–492. 10.1016/j.ydbio.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Eliazer S., Shalaby N.A., and Buszczak M.. 2011. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc. Natl. Acad. Sci. USA. 108:7064–7069. 10.1073/pnas.1015874108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Li J., Blauwkamp T., Bhambhani C., Campbell N., and Cadigan K.M.. 2006. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J. 25:2735–2745. 10.1038/sj.emboj.7601153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A.J., Spradling A.C., Ingham P.W., and Lin H.. 1996. The role of segment polarity genes during early oogenesis in Drosophila. Development. 122:3283–3294. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Tumbar T., and Guasch G.. 2004. Socializing with the neighbors: stem cells and their niche. Cell. 116:769–778. 10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- Fuller M.T., and Spradling A.C.. 2007. Male and female Drosophila germline stem cells: two versions of immortality. Science. 316:402–404. 10.1126/science.1140861 [DOI] [PubMed] [Google Scholar]
- Guo Z., and Wang Z.. 2009. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 136:3627–3635. 10.1242/dev.036939 [DOI] [PubMed] [Google Scholar]
- Haerry T.E. 2010. The interaction between two TGF-β type I receptors plays important roles in ligand binding, SMAD activation, and gradient formation. Mech. Dev. 127:358–370. 10.1016/j.mod.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Haerry T.E., Khalsa O., O’Connor M.B., and Wharton K.A.. 1998. Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development. 125:3977–3987. [DOI] [PubMed] [Google Scholar]
- Hajnal A., Whitfield C.W., and Kim S.K.. 1997. Inhibition of Caenorhabditis elegans vulval induction by gap-1 and by let-23 receptor tyrosine kinase. Genes Dev. 11:2715–2728. 10.1101/gad.11.20.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada-Kawaguchi N., Nore B.F., Kuwada Y., Smith C.I., and Yamamoto D.. 2014. Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila. Science. 343:294–297. 10.1126/science.1244512 [DOI] [PubMed] [Google Scholar]
- Harris R.E., and Ashe H.L.. 2011. Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 12:519–526. 10.1038/embor.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Pargett M., Sutcliffe C., Umulis D., and Ashe H.L.. 2011. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev. Cell. 20:72–83. 10.1016/j.devcel.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kobayashi S., and Nakato H.. 2009. Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 187:473–480. 10.1083/jcb.200904118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.C., and Fuchs E.. 2012. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 13:103–114. 10.1038/nrm3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino N., Pane A., and Gaul U.. 2009. miR-184 has multiple roles in Drosophila female germline development. Dev. Cell. 17:123–133. 10.1016/j.devcel.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Kirilly D., Wang S., and Xie T.. 2011. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 138:5087–5097. 10.1242/dev.067850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T., and Cohen S.M.. 1998. Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development. 125:4901–4907. [DOI] [PubMed] [Google Scholar]
- Lee W.C., Beebe K., Sudmeier L., and Micchelli C.A.. 2009. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 136:2255–2264. 10.1242/dev.035196 [DOI] [PubMed] [Google Scholar]
- Li L., and Clevers H.. 2010. Coexistence of quiescent and active adult stem cells in mammals. Science. 327:542–545. 10.1126/science.1180794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., and Xie T.. 2005. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21:605–631. 10.1146/annurev.cellbio.21.012704.131525 [DOI] [PubMed] [Google Scholar]
- Lien W.H., and Fuchs E.. 2014. Wnt some lose some: transcriptional governance of stem cells by Wnt/β-catenin signaling. Genes Dev. 28:1517–1532. 10.1101/gad.244772.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G., Xu N., and Xi R.. 2008. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 455:1119–1123. 10.1038/nature07329 [DOI] [PubMed] [Google Scholar]
- Lin H., Yue L., and Spradling A.C.. 1994. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 120:947–956. [DOI] [PubMed] [Google Scholar]
- Liu M., Lim T.M., and Cai Y.. 2010. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci. Signal. 3:ra57 10.1126/scisignal.2000740 [DOI] [PubMed] [Google Scholar]
- Logan C.Y., and Nusse R.. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781–810. 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Losick V.P., Morris L.X., Fox D.T., and Spradling A.. 2011. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell. 21:159–171. 10.1016/j.devcel.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 2012. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13:616–630. 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.A., and Lemischka I.R.. 2006. Stem cells and their niches. Science. 311:1880–1885. 10.1126/science.1110542 [DOI] [PubMed] [Google Scholar]
- Morrison S.J., and Spradling A.C.. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 132:598–611. 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C., Hausmann G., and Basler K.. 2009. β-Catenin hits chromatin: regulation of Wnt target gene activation. Nat. Rev. Mol. Cell Biol. 10:276–286. 10.1038/nrm2654 [DOI] [PubMed] [Google Scholar]
- Nellen D., Affolter M., and Basler K.. 1994. Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell. 78:225–237. 10.1016/0092-8674(94)90293-3 [DOI] [PubMed] [Google Scholar]
- Penton A., Chen Y., Staehling-Hampton K., Wrana J.L., Attisano L., Szidonya J., Cassill J.A., Massagué J., and Hoffmann F.M.. 1994. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell. 78:239–250. 10.1016/0092-8674(94)90294-1 [DOI] [PubMed] [Google Scholar]
- Reya T., and Clevers H.. 2005. Wnt signalling in stem cells and cancer. Nature. 434:843–850. 10.1038/nature03319 [DOI] [PubMed] [Google Scholar]
- Sahai-Hernandez P., and Nystul T.G.. 2013. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development. 140:4490–4498. 10.1242/dev.098558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden D.T. 2006. The stem-cell niche as an entity of action. Nature. 441:1075–1079. 10.1038/nature04957 [DOI] [PubMed] [Google Scholar]
- Schofield R. 1978. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 4:7–25. [PubMed] [Google Scholar]
- Sinenko S.A., Mandal L., Martinez-Agosto J.A., and Banerjee U.. 2009. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev. Cell. 16:756–763. 10.1016/j.devcel.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., and Xie T.. 2003. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 130:3259–3268. 10.1242/dev.00524 [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C.H., Doan C., and Xie T.. 2002. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 296:1855–1857. 10.1126/science.1069871 [DOI] [PubMed] [Google Scholar]
- Tabata T., and Takei Y.. 2004. Morphogens, their identification and regulation. Development. 131:703–712. 10.1242/dev.01043 [DOI] [PubMed] [Google Scholar]
- Wang L., Li Z., and Cai Y.. 2008a. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J. Cell Biol. 180:721–728. 10.1083/jcb.200711022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Harris R.E., Bayston L.J., and Ashe H.L.. 2008b. Type IV collagens regulate BMP signalling in Drosophila. Nature. 455:72–77. 10.1038/nature07214 [DOI] [PubMed] [Google Scholar]
- Wang X., Pan L., Wang S., Zhou J., McDowell W., Park J., Haug J., Staehling K., Tang H., and Xie T.. 2011. Histone H3K9 trimethylase Eggless controls germline stem cell maintenance and differentiation. PLoS Genet. 7:e1002426 10.1371/journal.pgen.1002426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M.L., Fischer W.H., and Jones K.A.. 1991. A thymus-specific member of the HMG protein family regulates the human T cell receptor Cα enhancer. Genes Dev. 5:656–669. 10.1101/gad.5.4.656 [DOI] [PubMed] [Google Scholar]
- Watt F.M., and Hogan B.L.. 2000. Out of Eden: stem cells and their niches. Science. 287:1427–1430. 10.1126/science.287.5457.1427 [DOI] [PubMed] [Google Scholar]
- Weissman I.L., Anderson D.J., and Gage F.. 2001. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 17:387–403. 10.1146/annurev.cellbio.17.1.387 [DOI] [PubMed] [Google Scholar]
- Xia L., Jia S., Huang S., Wang H., Zhu Y., Mu Y., Kan L., Zheng W., Wu D., Li X., et al. . 2010. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 143:978–990. 10.1016/j.cell.2010.11.022 [DOI] [PubMed] [Google Scholar]
- Xie T. 2013. Control of germline stem cell self-renewal and differentiation in the Drosophila ovary: concerted actions of niche signals and intrinsic factors. Wiley Interdiscip. Rev. Dev. Biol. 2:261–273. [DOI] [PubMed] [Google Scholar]
- Xie T., and Spradling A.C.. 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 94:251–260. 10.1016/S0092-8674(00)81424-5 [DOI] [PubMed] [Google Scholar]
- Xie T., and Spradling A.C.. 2000. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 290:328–330. 10.1126/science.290.5490.328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.