Abstract
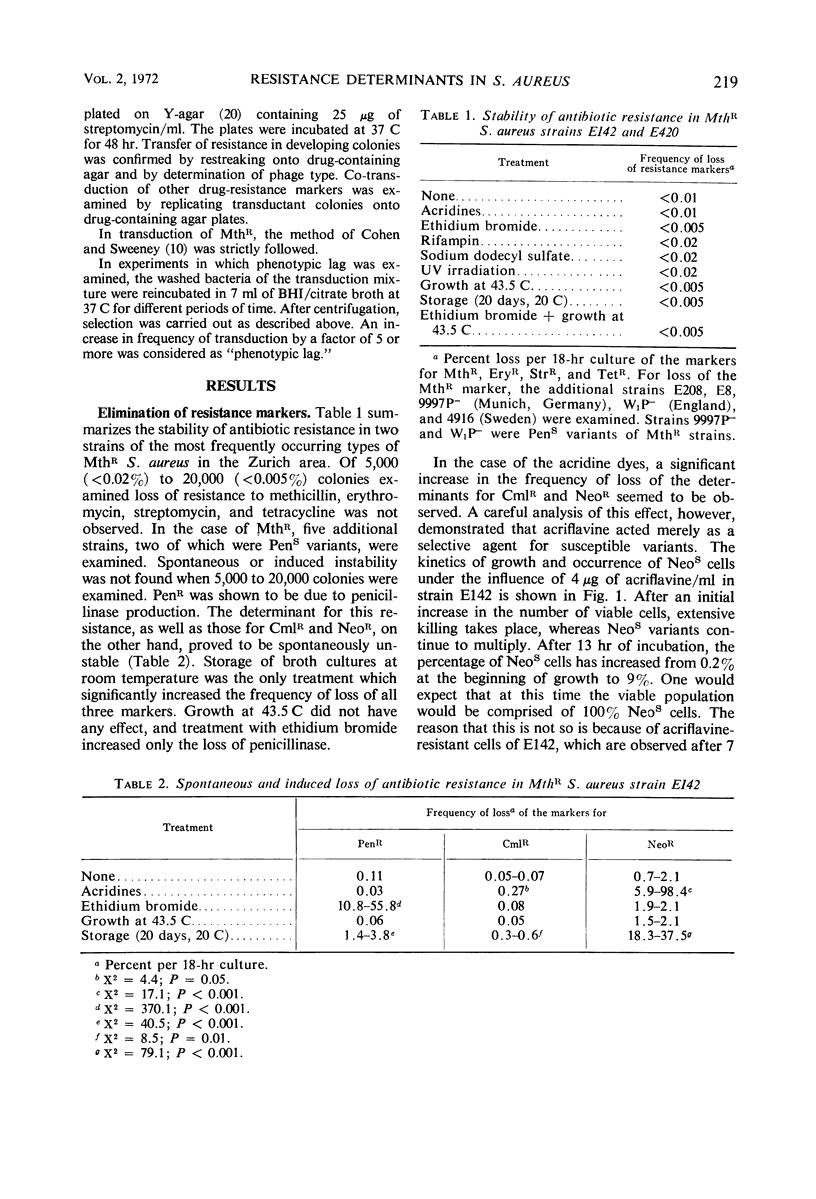
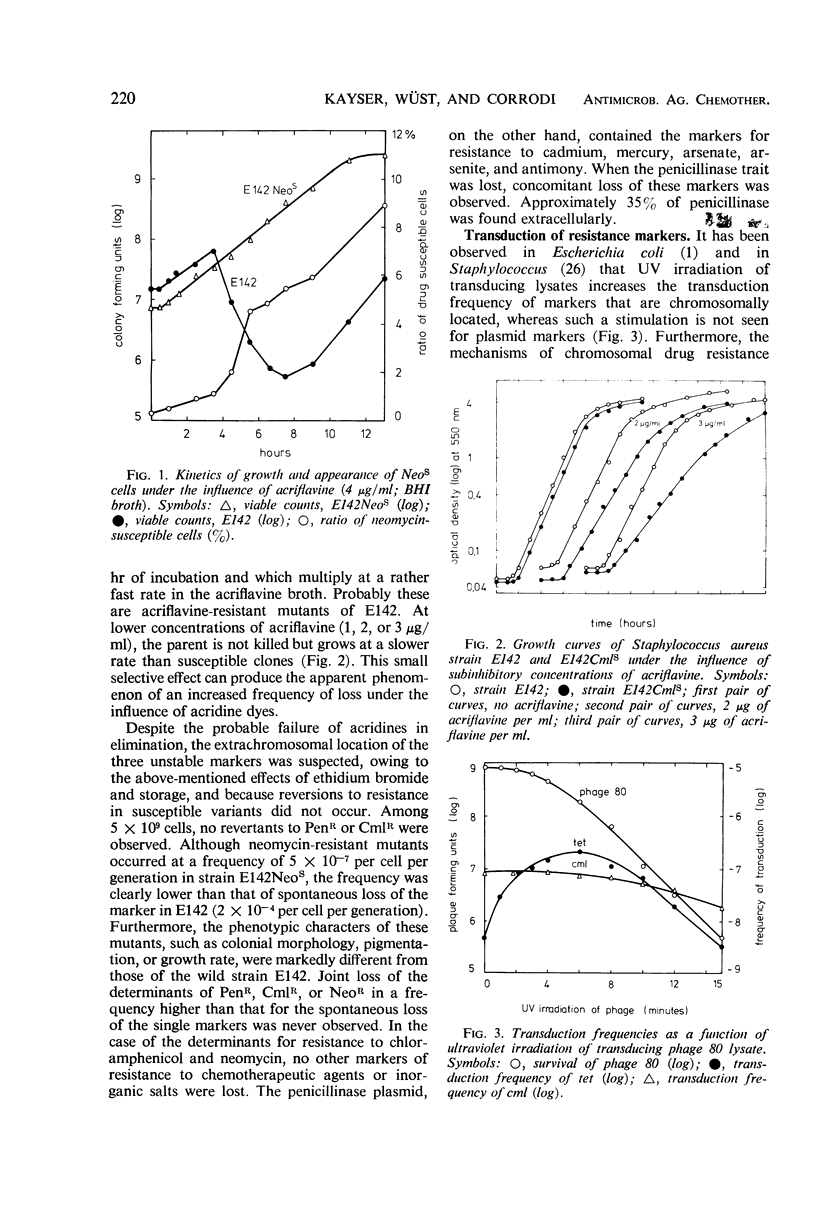
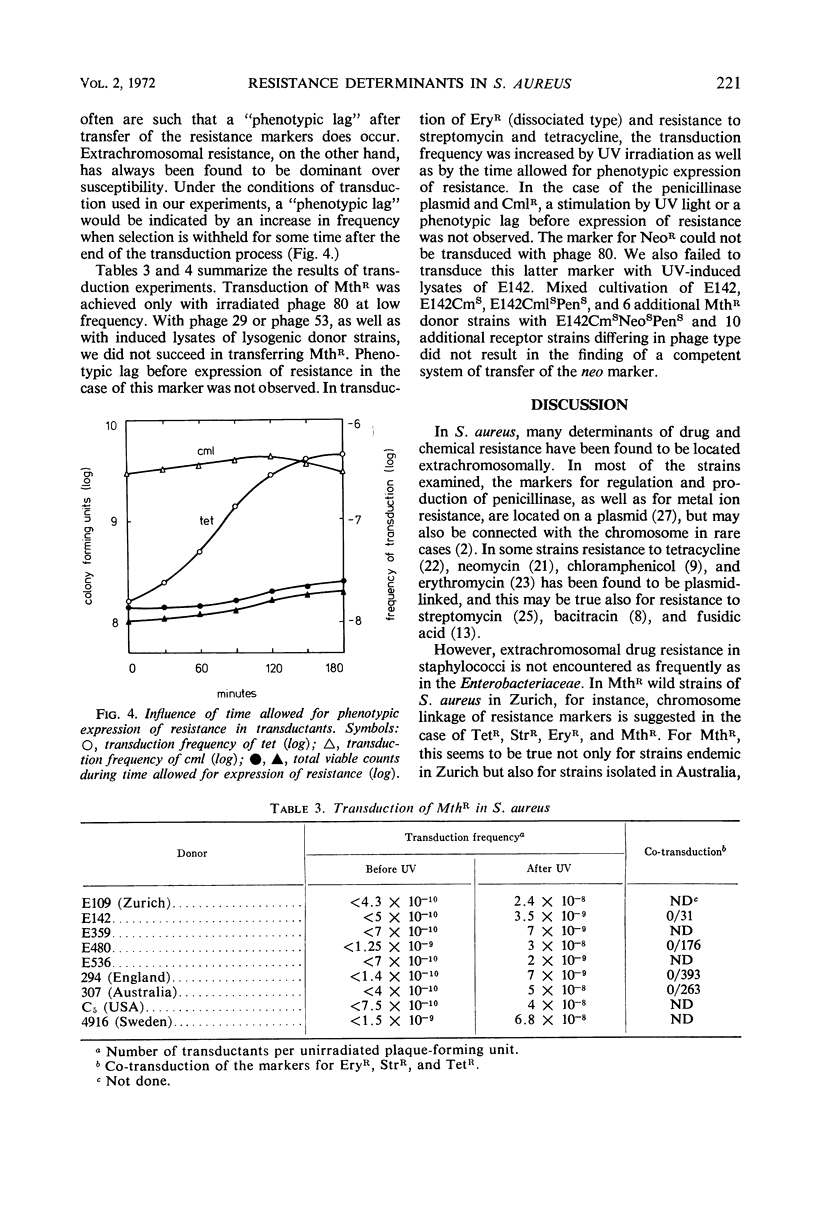
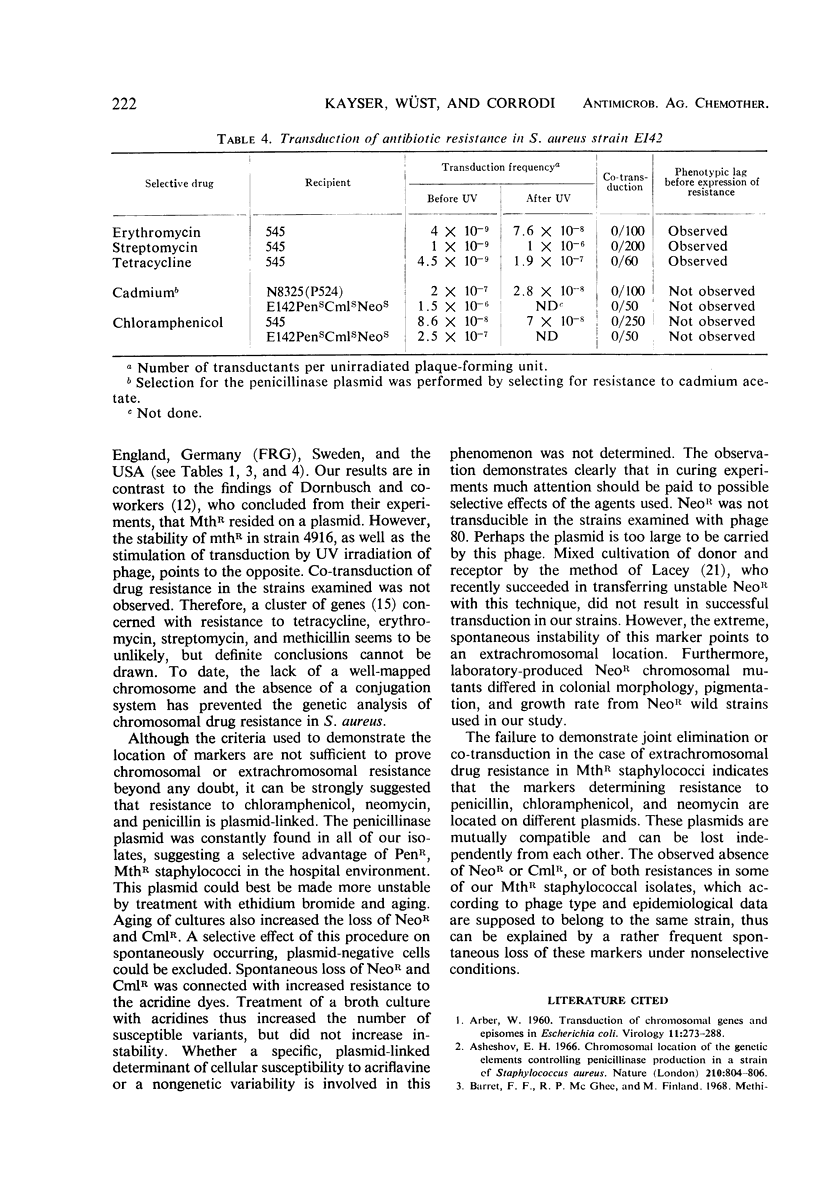
Elimination and transduction of drug resistance was examined in methicillin-resistant strains of Staphylococcus aureus. Irreversible spontaneous loss and “curing” by aging of cultures and by treatment with ethidium bromide indicated that the determinants for penicillinase production and chloramphenicol resistance, and probably also for neomycin resistance, were located extrachromosomally. On the other hand, the determinants of resistance to erythromycin, streptomycin, tetracycline, and methicillin could not be eliminated by acridines, ethidium bromide, rifampin, sodium dodecyl sulfate, ultraviolet (UV) irradiation, growth at 43.5 C, aging of cultures, or combinations of these treatments. The stimulation of transduction frequency by UV irradiation of phage in the case of the stable markers, but not in the case of the unstable ones, supported further the hypothesis of chromosomal location of the markers of methicillin, erythromycin, tetracycline, and streptomycin resistance and extrachromosomal location of the determinants for penicillinase production and chloramphenicol resistance. Neomycin resistance could not be transduced. Joint elimination and co-transduction of the determinants for penicillinase production and resistance to chloramphenicol and neomycin were not observed, indicating the location of these markers on separate, mutually compatible plasmids. Co-transduction of chromosomal resistance determinants was usually less than 1%, which makes the location of these genes in a circumscribed area of the chromosome improbable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. Chromosomal location of the genetic elements controlling penicillinase production in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):804–806. doi: 10.1038/210804a0. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Benner E. J., Kayser F. H. Growing clinical significance of methcillin-resistant Staphylococcus aureus. Lancet. 1968 Oct 5;2(7571):741–744. doi: 10.1016/s0140-6736(68)90947-1. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Bülow P. Prevalence of extrachromosomal drug resistance. Staphylococci in Danish hospitals during the last decade: factors influencing some properties of predominant epidemic strains. Ann N Y Acad Sci. 1971 Jun 11;182:21–39. doi: 10.1111/j.1749-6632.1971.tb30640.x. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G., GERBAUD G. R. VARIATIONS SOUS L'INFLUENCE DE L'ACRIFLAVINE ET TRANSDUCTION DE LA R'ESISTANCE A LA KANAMYCINE ET AU CHLORAMPH'ENICOL CHEZ LES STAPHYLOCOQUES. Ann Inst Pasteur (Paris) 1964 Nov;107:678–690. [PubMed] [Google Scholar]
- COURTIEU A. L., GUILLERMET F. N., LONGERAY C., MAKA G., CHABBERT Y. A. FR'EQUENCE DES STAPHYLOCOQUES PR'ESENTANT UNE R'ESISTANCE H'ET'EROG'ENE A LA M'ETHICILLINE ET L'OXACILLINE EN MILIEU HOSPITALIER. Ann Inst Pasteur (Paris) 1964 Nov;107:691–697. [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Waterworth P. M. Naturally-occurring fusidic acid resistance in staphylococci and its linkage to other resistances. J Clin Pathol. 1966 Nov;19(6):555–560. doi: 10.1136/jcp.19.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Hashimoto H., Yamagishi S., Mitsuhashi S. Transduction analysis of the genetic determinants for chloramphenicol resistance in Staphylococci. Jpn J Microbiol. 1970 Jul;14(4):261–268. doi: 10.1111/j.1348-0421.1970.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Jessen O., Rosendal K., Bülow P., Faber V., Eriksen K. R. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969 Sep 18;281(12):627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- Johnston J. H., Richmond M. H. The increased rate of loss of penicillinase plasmids from Staphylococcus aureus in the presence of rifampicin. J Gen Microbiol. 1970 Jan;60(1):137–139. doi: 10.1099/00221287-60-1-137. [DOI] [PubMed] [Google Scholar]
- KORMAN R. Z., BERMAN D. T. Genetic transduction with staphylophage. J Bacteriol. 1962 Aug;84:228–236. doi: 10.1128/jb.84.2.228-236.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Wiemer U. Uber die Oxacillinresistenz pathogener Staphylokokken. I. Die Natur der Resistenz gegen Oxyacillin bei Staphylococcus pyogenes aureus. Z Hyg Infektionskr. 1965;150(4):308–316. [PubMed] [Google Scholar]
- Kono M., Hashimoto H., Mitsuhashi S. Drug resistance to staphylococci. 3. Resistance to some macrolide antibiotics and inducible system. Jpn J Microbiol. 1966 Apr;10(1):59–66. [PubMed] [Google Scholar]
- Lacey R. W. High-frequency transfer of neomycin resistance between naturally occurring strains of Staphylococcus aureus. J Med Microbiol. 1971 Feb;4(1):73–84. doi: 10.1099/00222615-4-1-73. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HASHIMOTO H., KONO M., MORIMURA M. DRUG RESISTANCE OF STAPHYLOCOCCI. II. JOINT ELIMINATION AND JOINT TRANSDUCTION OF THE DETERMINANTS OF PENICILLINASE PRODUCTION AND RESISTANCE TO MACROLIDE ANTIBIOTICS. J Bacteriol. 1965 Apr;89:988–992. doi: 10.1128/jb.89.4.988-992.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., OSHIMA H., KAWAHARADA U., HASHIMOTO H. DRUG RESISTANCE OF STAPHYLOCOCCI. I. TRANSDUCTION OF TETRACYCLINE RESISTANCE WITH PHAGE LYSATES OBTAINED FROM MULTIPLY RESISTANT STAPHYLOCOCCI. J Bacteriol. 1965 Apr;89:967–976. doi: 10.1128/jb.89.4.967-976.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura M., Watanabe K., Mori H., Mitsuhashi S. Lability of streptomycin resistance in Staphylococcus aureus. Jpn J Microbiol. 1970 Jul;14(4):253–256. doi: 10.1111/j.1348-0421.1970.tb00521.x. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. T., Hewitt J. H. Methicillin resistance in Staphylococcus aureus. Lancet. 1970 Apr 18;1(7651):800–804. doi: 10.1016/s0140-6736(70)92408-6. [DOI] [PubMed] [Google Scholar]
- Rountree P. M., Beard M. A. Hospital strains of Staphylococcus aureus, with particular reference to methicillin-resistant strains. Med J Aust. 1968 Dec 28;2(26):1163–1168. doi: 10.5694/j.1326-5377.1968.tb83502.x. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


