Significance
Oxygen delivery by RBC Hb is essential for life. Just three amino acids in Hb are conserved in all mammals and birds, but only two of those are required to carry oxygen. The third, a Cys within the β-chain, βCys93, has been assigned a role in carrying nitric oxide, which mediates vasodilation. However, the physiological importance of RBC-mediated vasoregulation is unknown. We show that blood flow and tissue oxygenation are markedly impaired in mice with a βCys93Ala mutation. The βCys93Ala mutation also results in myocardial ischemia, cardiac decompensation, and enhanced mortality. These findings support a new view of the respiratory cycle wherein, remarkably, RBCs regulate blood flow and (βCys93NO)-Hb is necessary for adequate tissue oxygenation and normal cardiovascular function.
Keywords: S-nitrosylation, S-nitrosohemoglobin, hypoxic vasodilation, nitric oxide
Abstract
Oxygen delivery by Hb is essential for vertebrate life. Three amino acids in Hb are strictly conserved in all mammals and birds, but only two of those, a His and a Phe that stabilize the heme moiety, are needed to carry O2. The third conserved residue is a Cys within the β-chain (βCys93) that has been assigned a role in S-nitrosothiol (SNO)-based hypoxic vasodilation by RBCs. Under this model, the delivery of SNO-based NO bioactivity by Hb redefines the respiratory cycle as a triune system (NO/O2/CO2). However, the physiological ramifications of RBC-mediated vasodilation are unknown, and the apparently essential nature of βCys93 remains unclear. Here we report that mice with a βCys93Ala mutation are deficient in hypoxic vasodilation that governs blood flow autoregulation, the classic physiological mechanism that controls tissue oxygenation but whose molecular basis has been a longstanding mystery. Peripheral blood flow and tissue oxygenation are decreased at baseline in mutant animals and decline excessively during hypoxia. In addition, βCys93Ala mutation results in myocardial ischemia under basal normoxic conditions and in acute cardiac decompensation and enhanced mortality during transient hypoxia. Fetal viability is diminished also. Thus, βCys93-derived SNO bioactivity is essential for tissue oxygenation by RBCs within the respiratory cycle that is required for both normal cardiovascular function and circulatory adaptation to hypoxia.
In vertebrates, life requires the delivery to tissues of O2 conveyed by RBC Hb. O2 binds to iron within the heme prosthetic group of Hb and, within monomeric Hb subunits, the proximal His residues that coordinate the heme moiety and the distal Phe residues that stabilize the heme group are highly conserved (1, 2); without them, Hb cannot carry O2. Remarkably, only one other residue, βCys93, is fully conserved across birds and mammals, that is, across vertebrate species that possess cardiovascular systems evolved to support enhanced aerobic exercise-related metabolic demand (2, 3). βCys93 undergoes oxidative modification by NO to form an S-nitrosothiol (SNO) that retains NO bioactivity in blood (4) (whereas oxygenated hemes in Hb inactivate NO). However, the basis of the apparently essential nature of βCys93 and, more generally, the physiological role of RBC-derived NO vasoactivity remain unknown.
Classically, O2 delivery to tissues has been identified simply with the O2 content of RBCs, transported by Hb from lungs to tissues (2, 5, 6). However, it now is well understood that O2 delivery within the respiratory cycle also is regulated substantially by changes in local blood flow (tissue perfusion) that are coupled to metabolic demand [O2 delivery = (ΔO2 content × blood flow)] (refs. 7 and 8 and reviewed in ref. 6). Thus, delivery of O2 to tissues is a function not only of the O2 content of RBC Hb but also is determined critically by the physiological response known as “blood flow autoregulation” (6, 7), mediated by hypoxic vasodilation that couples blood flow to tissue O2 requirements. Although described more than half a century ago by Guyton and colleagues (7), and accepted as a core principle of cardiovascular and respiratory physiology, the molecular mechanism of blood flow autoregulation remains in question.
Although the essential function of heme-associated Hb residues (His and Phe) may be understood in the context of the O2 content of RBCs, an extensive body of experimental in vitro data supports a model in which βCys93 may subserve blood flow autoregulation by titrating the release of NO bioactivity (4, 6, 8–12). Under this model, βCys93 is oxidatively modified by NO to form an SNO in R-state (oxy) Hb, and the allosteric structural transition of the Hb tetramer to T-state upon deoxygenation promotes the graded release from βCys93 of SNO-based vasodilatory bioactivity (6). Interestingly, an O2-coupled allosteric mechanism also may operate in the generation and release of NO bioactivity from myoglobin in cold-blooded vertebrates (13). Additional findings show that the export of βCys93-derived NO bioactivity is based on an SNO cascade that involves the transfer of NO groups to RBC membrane proteins and to external sites that include small-molecular-weight thiols (10, 14–17). S-nitrosohemoglobin (SNO-Hb) thus may provide a molecular mechanism for hypoxic vasodilation by RBCs and thereby for blood flow autoregulation. However, although SNO-Hb within RBCs actuates hypoxic vasodilation in vitro (9, 14, 18), it has not been possible to manipulate endogenous SNO-Hb selectively in vivo to assess its physiological role.
The creation of mice in which RBCs contain human Hb subunits in which βCys93 either is present or is replaced by Ala (19) has provided an opportunity to explore this role. The results of our analysis indicate that βCys93-derived NO bioactivity mediates the autoregulation of blood flow that is a fundamental component of the respiratory cycle. βCys93 thus is essential for normal cardiovascular function.
Results
Initial Characterization of Mutant Mice.
The mice used in our analyses included three genetically engineered strains expressing “humanized” RBC Hb (19), which we designate “γβC93” (control mice), “γβC93A,” and “βC93A,” as well as C57BL/6J mice representative of the wild type. In all engineered strains, the murine α- and β-subunits of RBC Hb were replaced with the human versions. In γβC93 and γβC93A mice (genetically matched with the exception of βCys93), RBCs also contained the human γ (fetal)-subunit, which was introduced to enhance fetal viability but remains a minor species (Fig. S1) (20), consistent with measurements of O2 affinity that indicate minimal changes in mutant mice (19); in all three strains, RBCs retain the murine γ-subunit. βCys93 was replaced with Ala in βC93A and γβC93A mice. We confirmed that total Hb concentration and blood O2 content of the mutant strains are unchanged from wild type at baseline (room air), as reported (Fig. S2 A and B) (19, 20), and that plasma nitrite levels, a direct measure of circulatory NO production by endothelial nitric oxide synthase (eNOS), was similar in all strains (Fig. S2C). We also determined by telemetry in awake, freely moving mice that blood pressure and heart rate did not differ among the strains used (Fig. S2 D and E).
Reduced Fetal Viability.
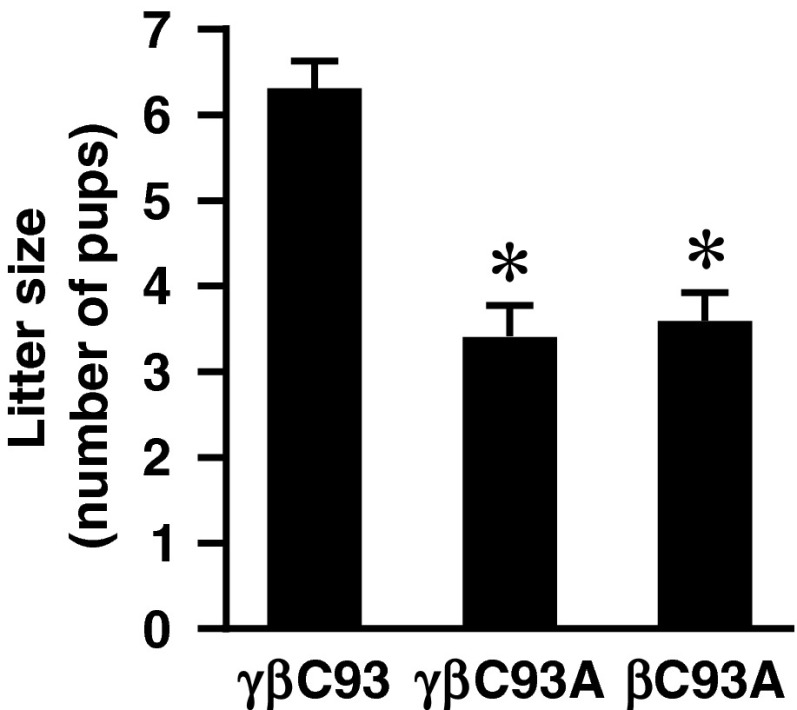
Direct evidence of the evolutionary selection pressure underlying phylogenetic conservation of βCys93 was provided by our finding that embryonic/fetal viability was greatly compromised in the absence of βCys93: Litter size (assessed shortly after birth) was diminished by about 50% in γβC93A and βC93A mice as compared with γβC93 control mice (Fig. 1), and maintaining these lines, particularly the βC93A line, was a significant challenge. This finding is consistent with previous studies that have suggested a role for SNO-Hb in the regulation of fetal blood flow (21) and in the perinatal circulatory transition (22).
Fig. 1.
Diminished viability in the absence of βC93. Embryonic/fetal viability is greatly diminished in βC93A and γβC93A mice versus γβC93 control mice: The average litter size is reduced by ∼50%. Data are presented as mean ± SD; n = 28–56; *P < 0.001 by one-way ANOVA.
Hb S-Nitrosylation in Mutant Mice.
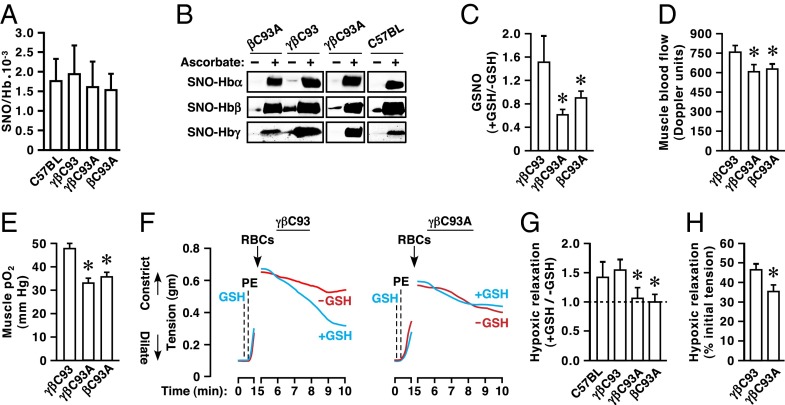
To characterize the status of Hb S-nitrosylation in the strains used, we assessed both overall levels of SNO-Hb and the distribution of SNO across Hb subunits. Analysis of freshly drawn blood by mercury-coupled photolysis-chemiluminescence (23) revealed that absolute levels of SNO-Hb (and of total Hb-bound NO including heme-bound NO) did not differ significantly in samples prepared from βC93A, γβC93, or γβC93A mice (Fig. 2A). Analysis by SNO–resin-assisted capture (SNO-RAC) (24) coupled to Western blotting with subunit-specific antibodies demonstrated S-nitrosylation of the α- and γ-subunits as well as the β-subunit in all engineered strains (Fig. 2B), as also seen in C57BL/6J mice (Fig. 2B).
Fig. 2.
Diminished SNO generation, vasorelaxation, and blood flow in the absence of βC93. (A) The quantity of endogenously S-nitrosylated Hb (SNO-Hb) does not differ significantly in RBCs obtained from C57BL/6J, βC93A, γβC93, or γβC93A mice. Data are presented as mean ± SD; n = 6–12; P < 0.05 by one-way ANOVA. (B) Basal S-nitrosylation of Hbα, Hbβ and Hbγ in βC93A, γβC93, and γβC93A mice as well as in C57BL/6J mice. Ascorbate is omitted as a control for specificity of the SNO-RAC procedure. (C) Deoxygenation of RBCs from γβC93 (control) but not γβC93A or βC93A mice in the presence of GSH formed GSNO. Data are shown as mean ± SEM; n = 3 mice per strain. (D) Basal blood flow and (E) basal pO2 in the gastrocnemius muscle of mice breathing room air demonstrate that tissue perfusion and oxygenation are deficient in βC93A and γβC93A mice versus γβC93 control mice. Data are presented as mean ± SEM; n = 18–23. (F) Representative bioassays of aortic rings (derived from eNOS−/− mice) to assess vasodilation at 1% O2 by RBCs from γβC93 mice and isogenic γβC93A mice. GSH (1 mM) was added before contraction with phenylephrine (PE). (G) GSH-mediated potentiation of vasorelaxation does not differ between wild-type C57BL/6J and γβC93 RBCs but is eliminated in RBCs from βC93A and γβC93A mice. Data are presented as mean ± SEM; n = 4–5; (H) Hypoxic vasodilation by RBCs from γβC93A versus γβC93 mice was significantly diminished in bioassays of endothelium-intact wild-type aortic rings. Data are presented as mean ± SEM; n = 14–17. *P < 0.05 by one-way ANOVA in C–E and G and by Student's t test in H.
Synthesis in vitro of Hb that is S-nitrosylated at the single αCys105 has been reported (25), but endogenous S-nitrosylation of the α-subunit or of the single conserved Cys93 within the γ-subunit has not been observed previously. The human β-subunit also contains a second Cys, βCys112, which can be S-nitrosylated in vitro (25). However, it has been established by previous mutagenic (26), mass spectrometric (27, 28), and X-ray crystallographic analyses (29) that βCys93 is the predominant site of human β-subunit S-nitrosylation, consistent with the oxygen-regulated disposition of NO within Hb in vivo (4, 30, 31). Additionally, the export of βCys93-derived NO bioactivity is based on an SNO cascade that involves the transfer of NO groups to RBC membrane proteins and to external sites that include small-molecular-weight thiols (10, 14–17). We verified directly that the hypoxia-regulated transfer of NO groups from RBCs to extracellular glutathione (GSH), which forms the potent vasodilator S-nitrosoglutathione (GSNO) (11) and which requires S-nitrosylated βCys93 (SNO-βCys93) (6, 9, 11), was compromised in mutant RBCs (Fig. 2C). Therefore, our results (Fig. 2 A and B) suggest that S-nitrosylation of βCys112 is promoted in the absence of βCys93. Enhanced S-nitrosylation of a closely spaced, alternative Cys in the absence of a preferred site also has been observed in other SNO-proteins (32), including invertebrate Hb (33), and a similar shift in the site of other regulatory posttranslational modifications when preferred sites are mutated is well documented. In addition, although γ-subunits reportedly constitute only ∼1% of Hb in γβC93 and γβC93A mice (20) (percentages that we have confirmed qualitatively; Fig. S1), Hbγ concentrations nonetheless far exceed endogenous amounts of SNO-Hb, and all strains will, in fact, retain some SNO-(γ)Cys93. In sum, our analysis of Hb-bound NO indicates that absolute SNO-Hb levels are similar in γβC93, βC93A, and γβC93A mice. However, the overall disposition of Hb SNO is likely different in control and mutant strains in the absence of βCys93, which has been implicated in vasoregulation that is allosterically regulated (6).
Impaired Peripheral Blood Flow and Tissue Oxygenation in Vivo.
To assess vasoregulation by RBCs replete with SNO-Hb but with selective absence of SNO-βCys93, we measured peripheral blood flow and tissue oxygenation in vivo using a combined laser Doppler and reflectance spectroscopy probe inserted into the hindlimb gastrocnemius muscle of anesthetized mice breathing room air. Blood flow (Fig. 2D) and tissue partial pressure of oxygen (pO2) (Fig. 2E) were diminished significantly in βC93A and γβC93A mice as compared with γβC93 mice under these baseline conditions. Thus, although overall levels of SNO-Hb are unaltered, tissue perfusion and pO2 are compromised in the absence of βCys93, indicating that the absence of βCys93 creates a deficit that is not compensated by either S-nitrosylation of an alternative site within the β-subunit or the presence of S-nitrosylated γCys93. Our data point to a major impairment in the autoregulation of blood flow, because tissue hypoxia normally is countered by increases in blood flow (hypoxic vasodilation) (7, 34–36). Indeed, impairments in oxygenation that accompany decreases in blood flow directly reflect diminished perfusion. [Of note, substitution of βCys93 results in only very minor increases in the O2-binding affinity of Hb (3, 19) that would, if anything, conduce to increased blood flow (36).]
Impaired Hypoxic Vasodilation in Vitro.
To confirm that compromised tissue perfusion and pO2 could be ascribed to deficient RBC-derived, SNO-βCys93–based hypoxic vasodilation, we used a well-established method of in vitro RBC bioassay (9). Some of the mechanisms of local vasoregulation differ among mammalian species and among blood vessels in a given species. In mice, unlike humans (9), RBC-mediated vasodilation in vitro involves a significant contribution from eNOS, which reflects, at least in part, the release of ATP by RBCs, resulting in the activation of eNOS-coupled endothelial purinergic receptors (37). To eliminate the contribution of this mechanism in assessing SNO-based hypoxic vasodilation, we used aortic ring segments obtained from eNOS-knockout mice (9). In addition, because βCys93-derived NO bioactivity is exported via transfer from T-state Hb to acceptor thiols (9, 15, 16, 18), we isolated the contribution of βCys93 to SNO-based vasoactivity by evaluating the effects on RBC-mediated vasodilation of extracellular GSH at low pO2, as described previously (6, 11). We found that, although GSH (1 mM) had no intrinsic vasodilatory activity, vasorelaxation by RBCs at low pO2 (1% O2) was significantly potentiated by GSH in γβC93 control mice (as in wild-type C57BL/6J mice) but not in mutant βC93A or γβC93A mice (Fig. 2 F and G), fulfilling the criterion of allosteric potentiation that is uniquely identified with βCys93 (6, 11). This finding is consistent with the failure of βC93A or γβC93A RBCs to support the transfer of NO groups to extracellular thiol (GSH) illustrated above (Fig. 2C). In addition, we found that hypoxic vasodilation by RBCs from γβC93A versus γβC93 mice was significantly diminished in bioassays of endothelium-intact wild-type aortic rings (Fig. 2H), verifying a role for βC93-derived, NO-based bioactivity in the presence of mouse-specific, endothelium-dependent mechanisms. Thus, the SNO-based mechanism of hypoxic vasodilation by RBCs (4, 6, 11, 14) requires βCys93, and the deficits in tissue perfusion and pO2 observed in the absence of βC93 (Fig. 2 D and E) likely may be ascribed to impaired SNO-based vasorelaxation by RBCs.
Impaired Blood Flow Autoregulation and Tissue Oxygenation in Vivo.
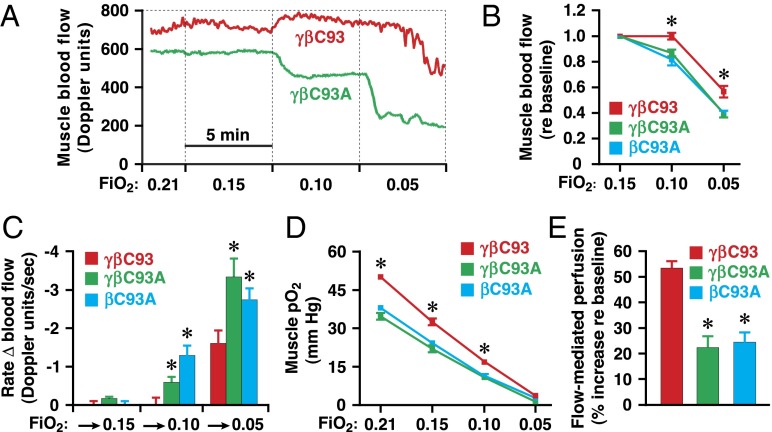
To examine directly the role of βCys93 in the response to changes in blood oxygenation (Hb O2 saturation), we examined peripheral blood flow and tissue oxygenation, measured in the gastrocnemius muscle as in Fig. 2, in individual mice breathing room air followed by a progressively lower inhaled fraction of O2 (FiO2), i.e., progressive global hypoxia. At baseline (room air, FiO2 = 0.21), blood O2 content, as measured in samples drawn from the left ventricle, was comparable across engineered strains (Fig. S2B). In control (γβC93) mice, blood flow was increased and then was maintained at initial levels as blood O2 content was diminished progressively at FiO2 of 0.15, 0.10, and 0.05 (with 5 min at each O2 level, by which time the measured variables had stabilized), whereas blood flow was diminished progressively in βC93A and γβC93A mice (Fig. 3 A and B). The rate of change of blood flow upon transition to lower FiO2 also was attenuated significantly in βC93A and γβC93A mice as compared with γβC93 mice (Fig. 3 A and C). In addition, tissue pO2 was progressively diminished at FiO2 of 0.15, 0.10, and 0.05, and these decreases were significantly greater in βC93A and γβC93A mice than in γβC93 mice (Fig. 3D).
Fig. 3.
Autoregulation of blood flow and reactive hyperemia are dependent on βC93. (A) Representative continuous recordings of muscle blood flow during progressive hypoxia in γβC93 and γβC93A mice. (B) Blood flow is significantly compromised in βC93A and γβC93A mice versus γβC93 control mice. (C) The rate of change of blood flow upon transition to lower FiO2 is significantly greater in βC93A and γβC93A mice than in γβC93 mice. (D) Muscle pO2 was significantly lower in βC93A and γβC93A mice than in γβC93 mice. In B, C, and D, data are presented as mean ± SEM; n = 18–23; *P < 0.05 by one-way ANOVA for differences between βC93A and γβC93A mice versus γβC93 mice. (E) Enhancement of flow-mediated perfusion (blood flow) in gastrocnemius muscle is deficient in βC93A and γβC93A mice versus γβC93 mice. Data are presented as mean ± SEM; n = 14–23; *P < 0.05 by one-way ANOVA.
Impaired Reactive Hyperemia in Vivo.
To assess further the participation of RBC-derived and SNO-based bioactivity in the response to acute, local tissue hypoxia, we measured flow-mediated perfusion (reactive hyperemia), which represents the compensatory increase in blood flow following the release of a temporary, flow-disrupting ligature. Flow-mediated perfusion generally is thought to reflect principally activation of eNOS by shear stress induced by restored flow (38, 39). Surprisingly, after compression of the femoral artery for 5 min, we observed a large (greater than 50%) decrease in the magnitude of flow-mediated perfusion of the gastrocnemius muscle in βC93A and γβC93A mice as compared with γβC93 mice (Fig. 3E), indicating a previously unappreciated influence of RBC-derived and NO-based bioactivity in hypoxic vasodilation under these conditions and suggesting that responses to hypoxia in situ integrate both endothelial and RBC components.
Taken together, our findings that, in the absence of βCys93, tissue perfusion is diminished in the setting of tissue hypoxia in vivo and that hypoxic vasodilation is diminished in vitro unequivocally demonstrate a role for βCys93 in local blood flow regulation. Our findings are not consistent with the conclusion that βCys93 plays no role in hypoxic vasodilation, reached in earlier analyses by other investigators who used these humanized mice (19), but, as explicated elsewhere (40, 41), the experiments adduced as support for that conclusion did not actually assess hypoxic vasodilation or the role of SNO-based mechanisms in particular. Notably, our studies provide, to our knowledge, the first assessments in βCys93-mutant animals of tissue blood flow and tissue oxygenation, which represent the pathognomonic hallmarks of hypoxic vasodilation, as well as the first assessment of SNO-based vasoactivity of RBCs containing mutant Hb.
Impaired Myocardial Contractility Under Hypoxia.
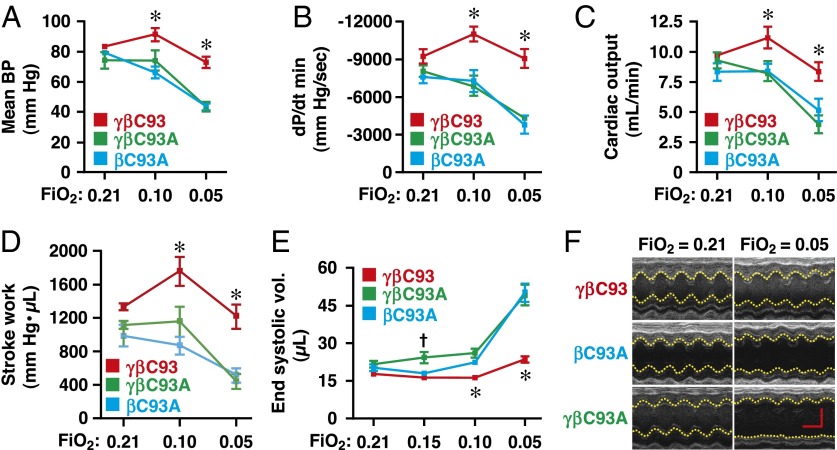
We then extended this analysis to examine the effects of hypoxia on principal hemodynamic parameters, including direct measurements of cardiac function using both a pressure–volume catheter in the left ventricle and direct assessment by echocardiography. Mean blood pressure (Fig. 4A), rate of change of left ventricular pressure (dP/dt) (Fig. 4B and Fig. S3A), cardiac output (Fig. 4C), and stroke work (Fig. 4D) were diminished substantially with progressive hypoxia in βC93A and γβC93A mice but not in γβC93 control mice. Similarly, progressive decreases in inspired O2 resulted in progressive diminishment of echocardiographic measures of cardiac function that were significantly greater in βC93A and γβC93A mice than in γβC93 mice (Fig. 4 E and F and Fig. S3), including left ventricular ejection fraction (Fig. S3B), fractional shortening (Fig. S3C), end systolic volume (Fig. 4E), and left ventricular inner dimension (Fig. 4F and Fig. S3D). It is important to emphasize that, when analyzed by individual strain, decrements in both hemodynamic and cardiac parameters were observed in βC93A and γβC93A mice at all levels of transient hypoxia, whereas no significant decrement in any measured parameter was seen in γβC93 control mice except for relatively minor changes at 5% O2; that is, βC93-bearing mice were uniquely able to compensate for all but extreme hypoxia (Fig. 4 and Fig. S3).
Fig. 4.
Impaired hemodynamic and cardiac responses to hypoxia in βCys93-deficient mice. (A–D) Invasive hemodynamic monitoring. Changes in (A) mean blood pressure, (B) rate of change of left ventricular pressure (dP/dt) (diastolic is shown), (C) cardiac output, and (D) stroke work during progressive hypoxia [FiO2 of 0.21 (room air), 0.10, and 0.05]. Declines in all measures (A–D) are significantly greater in βC93A and γβC93A mice versus γβC93 control mice. (E and F) Echocardiography demonstrates that ventricular function is significantly compromised during progressive global hypoxia in βC93A and γβC93A mice versus γβC93 mice (see Fig. S3 for additional measures). (E) Left ventricular end systolic volume. (F) Representative recordings from mice at FiO2 = 0.21 and FiO2 = 0.05 that illustrate left ventricular dilation in βC93A and γβC93A versus γβC93 control mice. Data are quantified in Fig. S3. (In F, the vertical scale bar represents 2 mm, and the horizontal scale bar represents 100 ms.) Data in A–E are presented as mean ± SEM; n = 9–12; *P < 0.05 by one-way ANOVA for differences between βC93A and γβC93A mice versus γβC93 mice; †P < 0.05 by one-way ANOVA for differences between γβC93A mice versus γβC93 mice.
Myocardial Ischemia Under Normoxia and Hemodynamic Collapse and Enhanced Mortality Under Transient Hypoxia.
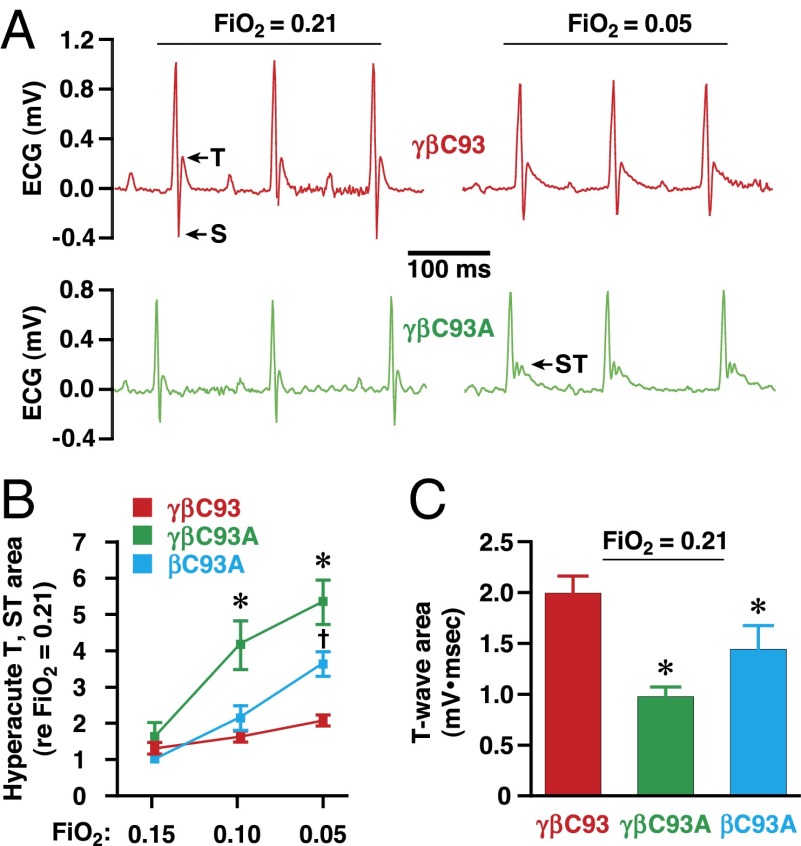
Myocardial function is exquisitely sensitive to ischemia. We monitored the heart by electrocardiography, which provides a clinically relevant measure of tissue oxygenation, and analysis of those measurements demonstrated that, during progressive hypoxia (5 min at each O2 level, as above), the frequency and magnitude of hyperacute T-wave and ST-wave elevation, electrocardiographic signatures of acute myocardial ischemic injury (42), were significantly greater in βC93A and γβC93A mice than in γβC93 mice (Fig. 5 A and B and Fig. S4). Strikingly, analysis of electrocardiographic recordings at baseline (room air) demonstrated that T-wave amplitude was decreased significantly in βC93A and γβC93A mice as compared with γβC93 mice (Fig. 5C). Decreased T-wave amplitude is a signature of myocardial ischemia, which, under normoxia, reflects diminished coronary blood flow. Therefore, these findings in room air indicate a chronic, uncompensated inadequacy of myocardial perfusion in the absence of βC93.
Fig. 5.
Cardiac ischemia and myocardial injury in βCys93-deficient mice. (A) Representative electrocardiographic recordings in a γβC93 (control) mouse (Upper) and a γβC93A mouse (Lower) at FiO2 = 0.21 and FiO2 = 0.05. T- and ST-waves are indicated. (B) During transient progressive hypoxia, ST-wave elevation (and hyperacute T-waves), indicative of acute ischemic injury, are significantly greater and far more frequent in βC93A and γβC93A mice than in γβC93 control mice (see also Fig. S4). (C) At FiO2 = 0.21 (room air), T-wave amplitude is significantly reduced in βC93A and γβC93A mice versus γβC93 mice, indicative of myocardial ischemia. In B and C data are presented as mean ± SEM; n = 13–21 mice; *P < 0.05 by one-way ANOVA for differences between βC93A and γβC93A mice versus γβC93 mice; †P < 0.05 by one-way ANOVA for differences between βC93A mice versus γβC93 mice.
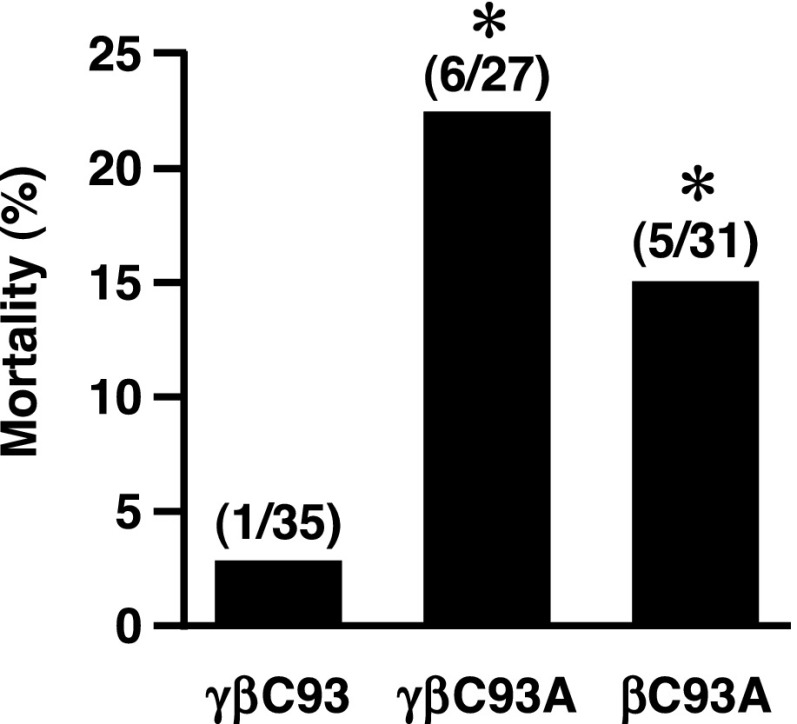
The ischemic injury, decrement in blood pressure, and decline in direct measures of cardiac function during transient hypoxia constitute a signature of cardiogenic shock—a clinical state with very high mortality. We found that a proportion of mice could not be recovered after transient exposure to 5% O2 and that mortality was much greater in βC93A and γβC93A mice than in γβC93 control mice (Fig. 6). Thus, the exacerbated decrement in cardiac function under transient hypoxia in the absence of βC93 frequently is fatal.
Fig. 6.
Increased hypoxia-induced mortality in βCys93-deficient mice. βC93A and γβC93A mice are significantly more likely than γβC93 (isogenic control) mice to die during or following brief hypoxic challenge, i.e., respiration at FiO2 = 0.05. n = 27–35 per strain as indicated; *P < 0.05 by Fisher’s exact test.
Discussion
The ability of hemes of Hb to bind and inactivate NO in vitro, thereby constricting blood vessels, played a substantial role in the identification of NO as the endothelium-derived relaxing factor (EDRF) and catalyzed recognition of the central role of NO in vasoregulation. However, the demonstration that, in RBCs under physiological conditions, the heme iron within the β-subunit could transfer the NO group allosterically to βCys93 to generate an SNO that retains NO bioactivity (4, 26) supported a reconceptualization of the interactions of NO and Hb and, more broadly, of the chemical biology of NO. This reconceptualization foreshadowed S-nitrosylation as a ubiquitous redox-based posttranslational protein modification (43, 44) with diverse roles throughout the cardiovascular system (and, more broadly, across phylogeny and cell type). Notably this analysis included a role for sequential transnitrosylation (NO group transfer) among SNOs in the delivery of NO bioactivity (4). However, the physiological or signaling role played by SNO-Hb–derived NO bioactivity has remained unclear. Our results establish a central role for SNO-Hb in the classic physiology of blood flow autoregulation, which subserves tissue oxygenation within the integrated cardiovascular system. Our findings also provide a new perspective of the vascular unit according to which RBC-derived SNO contributes to vasoregulation previously ascribed to endothelial mechanisms.
The findings presented here demonstrate the essential role played by βCys93 in mammalian respiratory cycle physiology, where O2 delivery by Hb depends not only on blood O2 content but also, importantly, on tissue perfusion (7). The results support the model in which hypoxia shifts the equilibrium between SNOs in RBCs and vasculature by off-loading NO groups from βCys93 in T-structured Hb to autoregulate blood flow (4, 8). Mechanistically, hemoglobin allostery mediates both the sensing of O2 concentration and the transduction of O2-coupled SNO-based signaling. Our observation that Cysβ93-derived NO bioactivity participates in both hypoxia- and flow-coupled responses, ascribed previously to endothelium-derived NO, provides a unifying explanation for the recent findings that GSNO and other SNOs in equilibrium with SNO-Hb are exported from RBCs under hypoxia (4, 10, 15, 17) and that, at least in part, EDRF is identified in vivo with GSNO (by strict genetic criteria) (45, 46). Thus, RBC–endothelium interactions that regulate microvascular blood flow and tissue oxygenation, and thereby maintain organ function, are best understood in terms of coupled equilibria that govern SNO-based bioactivity in blood and vasculature viewed as an integrated system.
Cells rarely rely on single mechanisms to effect important functions. Thus, endothelium-dependent vasodilation may involve prostacyclin, H2S, CO, and H2O2 in addition to NO/SNO (47). Similarly, in some contexts, RBC-mediated vasodilation may involve release of ATP (48) or NO bioactivity derived from erythrocytic eNOS (including SNO or nitrite) (49). Our work does not exclude roles for SNO-Hb–independent mechanisms of RBC bioactivity. However, it is important to recognize that autoregulation of blood flow is largely unaffected by eNOS inhibition (which would exclude a direct role for ATP, erythrocytic NOS, and nitrite) (6) and that the physiological roles of alternative mediators remain to be established.
Altered levels of SNO in RBCs and of SNO-Hb in particular have been found in a spectrum of cardiovascular and blood disorders, including sickle cell disease (18), diabetes (50), sepsis (51), and congestive heart failure (52), as well as in pulmonary hypertension (31), and have been implicated in the deleterious consequences of blood transfusion (12). Notably, these apparently diverse conditions are characterized by impairments of tissue blood flow. However, the conventional view holds that blood flow regulation is the purview of eNOS and that the deficiency of NO-based vasoactivity that characterizes these conditions primarily reflects endothelial dysfunction (53). Because the NO processed by RBCs derives in part from eNOS (4), assignment of a specific role for RBCs in deficient blood flow regulation has not been possible until now. Thus, our results support a causal role for altered SNO-Hb levels in impaired tissue blood flow that impacts multiple pathophysiologies. More directly, our finding that mice lacking βCys93 are subject to myocardial infarction and cardiogenic shock under hypoxia raises the possibility that RBCs may play a general role in ischemic coronary syndromes and heart failure, the most common causes of death in Western societies. A role for RBC-derived NO bioactivity in protection from myocardial damage also has been described in the context of an ex vivo model of ischemia–reperfusion injury (49). That NO deficiency may be a common contributing factor in myocardial injury and death is, in fact, well accepted (54); however, current tenets hold that endothelial cells (rather than RBCs) are the root cause.
The selective pressure underlying conservation of βCys93 (2, 3) is demonstrated by our findings that mice lacking βCys93 are ischemic in room air and are much less likely to survive in utero or in the face of transient hypoxia. It should be noted that, despite a long list of human Hb mutants (55), which include mutations that markedly affect Hb O2 affinity, function, and stability, homozygous mutation of human βCys93 (which minimally affects traditional Hb functions) has never been reported, and no other mutation of Hb (or any other protein of which we are aware) is known to cause myocardial ischemia in normoxia. Recently, it has been shown that the ventilatory response to hypoxia is abnormal in βC93A-mutant mice, implicating SNO-Hb in the central control of breathing (15, 20). Further, SNO-Hb levels in humans increase with ascent to altitude and are correlated strongly with exercise performance under hypoxia (34). Our findings, in combination with these results, suggest that βCys93 integrates both central and peripheral responses to hypoxia and thus support a key role for RBC-derived, SNO-based bioactivity in the respiratory cycle. This role for SNO-Hb may have broad implications for the understanding of heart, lung, and blood function and may offer new approaches for the treatment of the many pathophysiological conditions characterized by impaired blood flow and tissue oxygenation.
Materials and Methods
Detailed materials and methods, including in vivo assay of cardiovascular function, in vitro assay of vasoactivity, and biochemical methods, can be found in SI Materials and Methods.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University School of Medicine and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (56).
Supplementary Material
Acknowledgments
We thank Dr. Tim Townes, University of Alabama at Birmingham, for providing the transgenic mice used in this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 6254.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502285112/-/DCSupplemental.
References
- 1.Perutz MF. Blood. Taking the pressure off. Nature. 1996;380(6571):205–206. doi: 10.1038/380205b0. [DOI] [PubMed] [Google Scholar]
- 2.Perutz MF. 2001. Molecular anatomy and physiology of hemoglobin. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management, eds Steinberg MH, Forget BG, Higgs DR, Nagel RL (Cambridge Univ Press, Cambridge, UK), pp 174–196.
- 3.Nagai K, Perutz MF, Poyart C. Oxygen binding properties of human mutant hemoglobins synthesized in Escherichia coli. Proc Natl Acad Sci USA. 1985;82(21):7252–7255. doi: 10.1073/pnas.82.21.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380(6571):221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 5.Payne JP. Oxygen measurements in blood and tissue. Ann R Coll Surg Engl. 1965;37(5):297–309. [PMC free article] [PubMed] [Google Scholar]
- 6.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 7.Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. Am J Physiol. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Stamler JS, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276(5321):2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 9.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: Evidence for an s-nitrosothiol-based signal. Circ Res. 2008;103(5):545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doctor A, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci USA. 2005;102(16):5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon TJ, Exton Stone A, Bonaventura J, Singel DJ, Solomon Stamler J. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275(22):16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds JD, et al. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA. 2007;104(43):17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helbo S, Fago A. Allosteric modulation by S-nitrosation in the low-O2 affinity myoglobin from rainbow trout. Am J Physiol Regul Integr Comp Physiol. 2011;300(1):R101–R108. doi: 10.1152/ajpregu.00374.2010. [DOI] [PubMed] [Google Scholar]
- 14.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409(6820):622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 15.Lipton AJ, et al. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413(6852):171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 16.Palmer LA, et al. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117(9):2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallakunta VM, Slama-Schwok A, Mutus B. Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells. Redox Biol. 2013;1(1):373–380. doi: 10.1016/j.redox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA. 2005;102(7):2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isbell TS, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14(7):773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaston B, et al. Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice. J Appl Physiol (1985) 2014;116(10):1290–1299. doi: 10.1152/japplphysiol.01050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funai EF, Davidson A, Seligman SP, Finlay TH. S-nitrosohemoglobin in the fetal circulation may represent a cycle for blood pressure regulation. Biochem Biophys Res Commun. 1997;239(3):875–877. doi: 10.1006/bbrc.1997.7565. [DOI] [PubMed] [Google Scholar]
- 22.Gaston B, et al. Umbilical arterial S-nitrosothiols in stressed newborns: Role in perinatal circulatory transition. Biochem Biophys Res Commun. 1998;253(3):899–901. doi: 10.1006/bbrc.1998.9865. [DOI] [PubMed] [Google Scholar]
- 23.Stamler JS, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester MT, et al. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol. 2009;27(6):557–559. doi: 10.1038/nbt.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolzt M, et al. Biochemical characterization of S-nitrosohemoglobin. Mechanisms underlying synthesis, no release, and biological activity. J Biol Chem. 1999;274(41):28983–28990. doi: 10.1074/jbc.274.41.28983. [DOI] [PubMed] [Google Scholar]
- 26.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391(6663):169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 27.Ferranti P, Malorni A, Mamone G, Sannolo N, Marino G. Characterisation of S-nitrosohaemoglobin by mass spectrometry. FEBS Lett. 1997;400(1):19–24. doi: 10.1016/s0014-5793(96)01258-6. [DOI] [PubMed] [Google Scholar]
- 28.Hao G, Gross SS. Electrospray tandem mass spectrometry analysis of S- and N-nitrosopeptides: Facile loss of NO and radical-induced fragmentation. J Am Soc Mass Spectrom. 2006;17(12):1725–1730. doi: 10.1016/j.jasms.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Chan NL, Rogers PH, Arnone A. Crystal structure of the S-nitroso form of liganded human hemoglobin. Biochemistry. 1998;37(47):16459–16464. doi: 10.1021/bi9816711. [DOI] [PubMed] [Google Scholar]
- 30.McMahon TJ, et al. A nitric oxide processing defect of red blood cells created by hypoxia: Deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci USA. 2005;102(41):14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 32.Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129(3):511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 33.Minning DM, et al. Ascaris haemoglobin is a nitric oxide-activated ‘deoxygenase’. Nature. 1999;401(6752):497–502. doi: 10.1038/46822. [DOI] [PubMed] [Google Scholar]
- 34.Beall CM, Laskowski D, Erzurum SC. Nitric oxide in adaptation to altitude. Free Radic Biol Med. 2012;52(7):1123–1134. doi: 10.1016/j.freeradbiomed.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janocha AJ, et al. Nitric oxide during altitude acclimatization. N Engl J Med. 2011;365(20):1942–1944. doi: 10.1056/NEJMc1107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu B, Bloch KD, Zapol WM. Hemoglobin-based red blood cell substitutes and nitric oxide. Trends Cardiovasc Med. 2009;19(3):103–107. doi: 10.1016/j.tcm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington LS, et al. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol. 2007;72(5):1132–1136. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- 38.Joannides R, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 39.Schuler D, et al. Measurement of endothelium-dependent vasodilation in mice—brief report. Arterioscler Thromb Vasc Biol. 2014;34(12):2651–2657. doi: 10.1161/ATVBAHA.114.304699. [DOI] [PubMed] [Google Scholar]
- 40.Stamler JS, Singel DJ, Piantadosi CA. SNO-hemoglobin and hypoxic vasodilation. Nat Med. 2008;14(10):1008–1009, author reply 1009–1010. doi: 10.1038/nm1008-1008. [DOI] [PubMed] [Google Scholar]
- 41.Palmer LA, Doctor A, Gaston B. SNO-hemoglobin and hypoxic vasodilation. Nat Med. 2008;14(10) doi: 10.1038/nm1008-1009a. 1009. [DOI] [PubMed] [Google Scholar]
- 42.Caligiuri G, Levy B, Pernow J, Thorén P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci USA. 1999;96(12):6920–6924. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 44.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 46.Beigi F, et al. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci USA. 2012;109(11):4314–4319. doi: 10.1073/pnas.1113319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013;20(3):239–247. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprague RS, Ellsworth ML. Erythrocyte-derived ATP and perfusion distribution: Role of intracellular and intercellular communication. Microcirculation. 2012;19(5):430–439. doi: 10.1111/j.1549-8719.2011.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Gonon AT, Sjöquist PO, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci USA. 2013;110(37):15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: Reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94(7):976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 51.Crawford JH, et al. Transduction of NO-bioactivity by the red blood cell in sepsis: Novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104(5):1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 52.Datta B, et al. Red blood cell nitric oxide as an endocrine vasoregulator: A potential role in congestive heart failure. Circulation. 2004;109(11):1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 53.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 54.Erdmann J, et al. CARDIoGRAM Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504(7480):432–436. doi: 10.1038/nature12722. [DOI] [PubMed] [Google Scholar]
- 55.Giardine B, et al. HbVar database of human hemoglobin variants and thalassemia mutations: 2007 update. Hum Mutat. 2007;28(2):206. doi: 10.1002/humu.9479. [DOI] [PubMed] [Google Scholar]
- 56. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.