Abstract
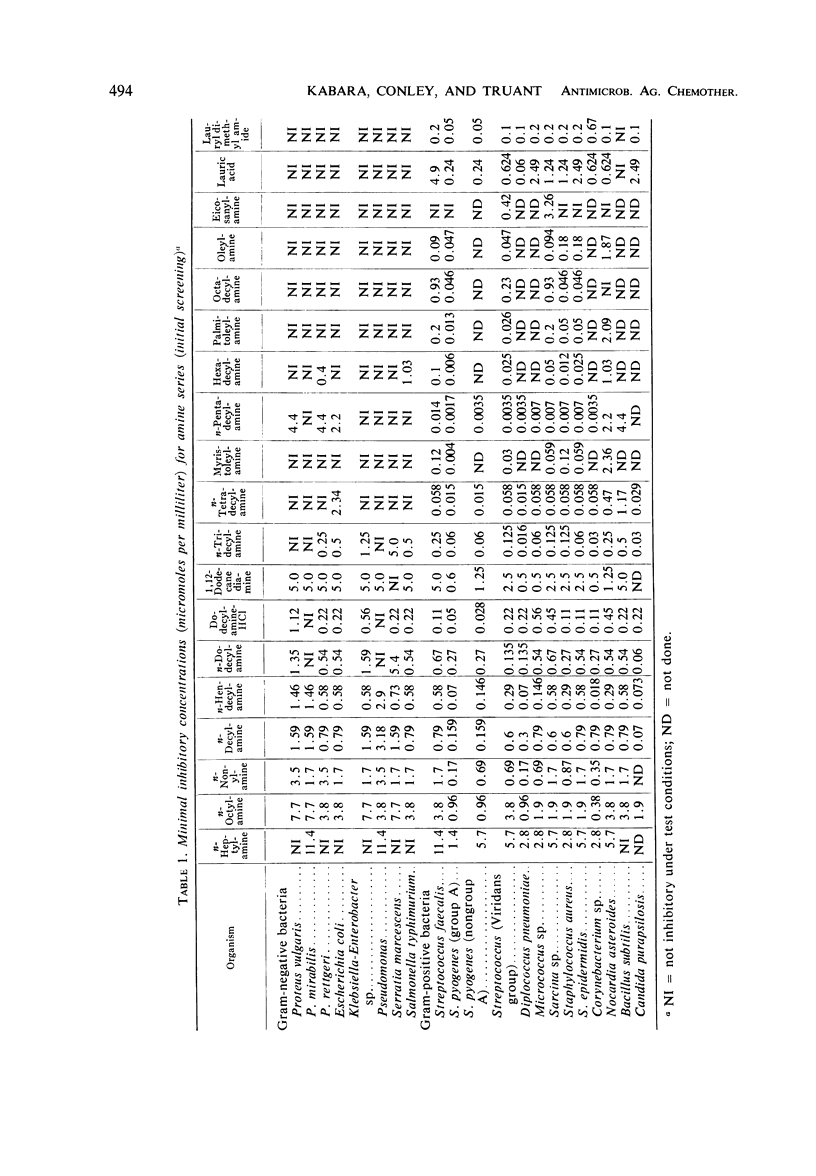
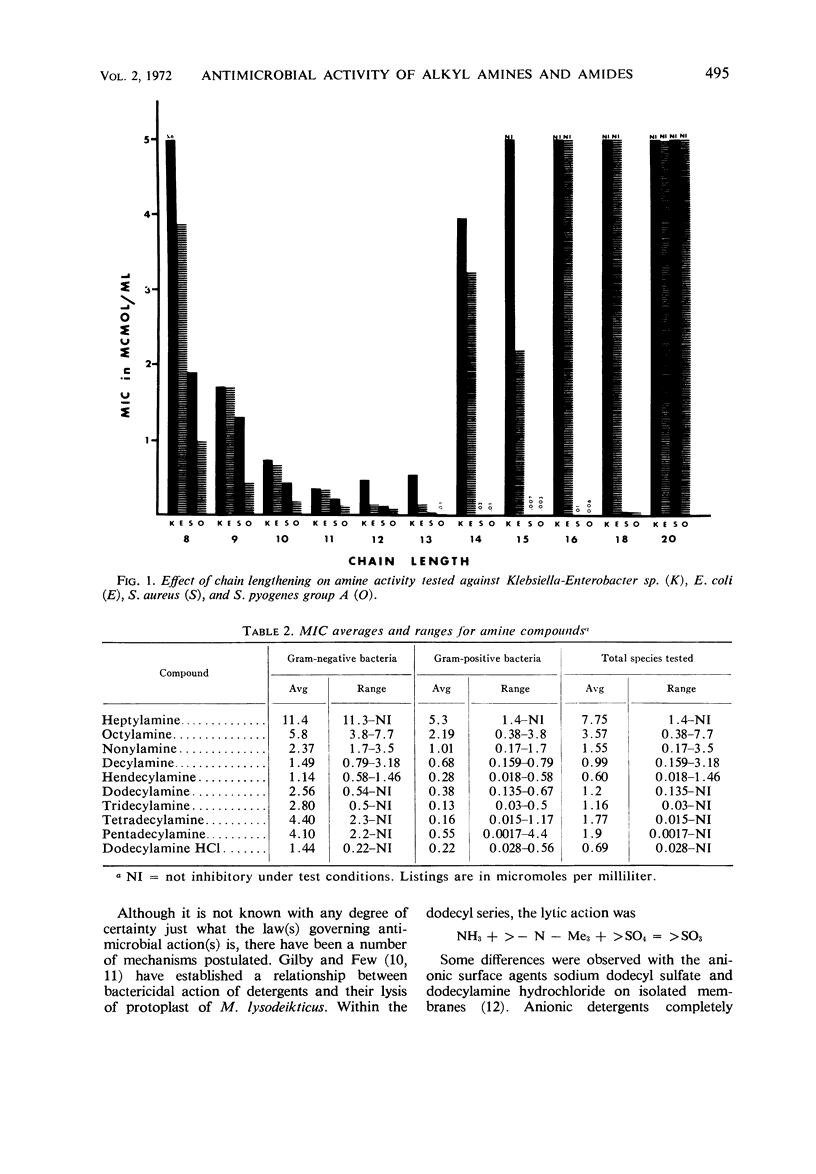
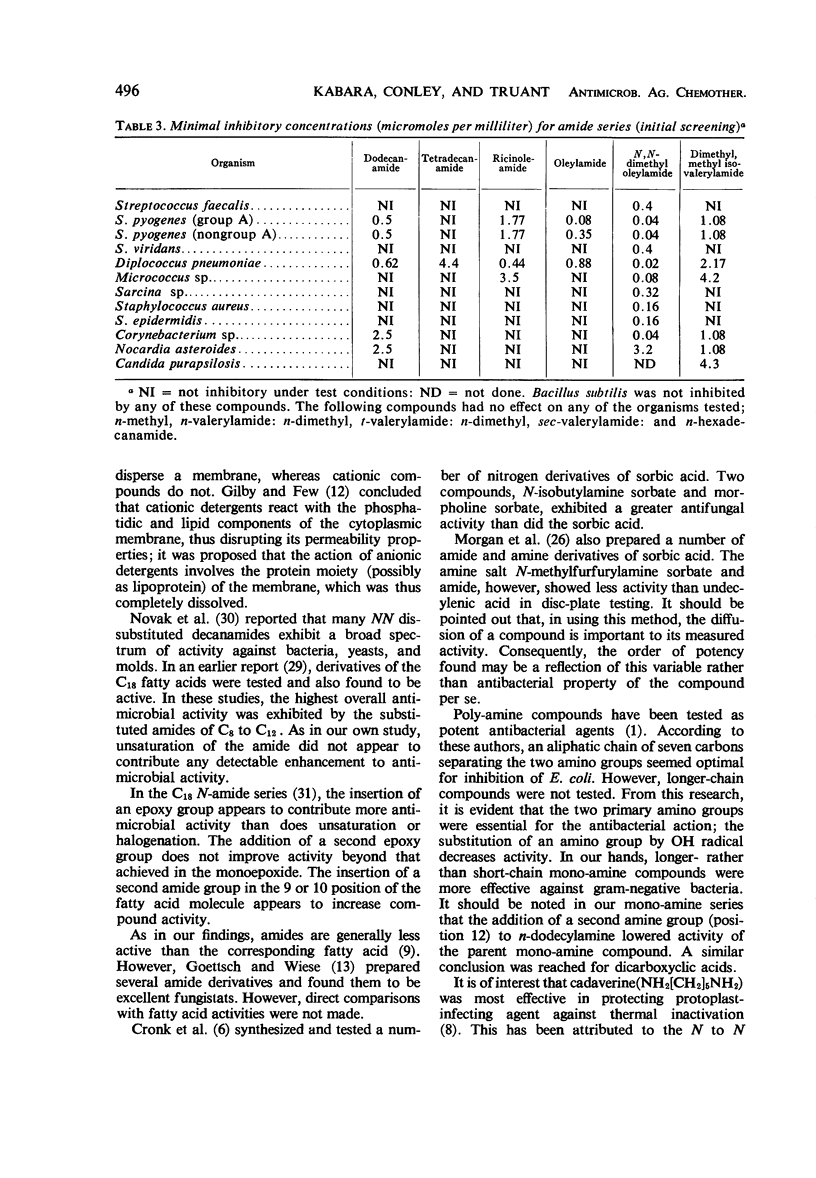
Contrary to the limited effects of alkyl amides and their corresponding N-derivatives, alkyl amines affected both gram-positive and gram-negative organisms. As with other alkyl derivatives the most sensitive gram-negative bacteria were usually more resistant than the most resistant gram-positive bacteria.
Compounds with a chain-length of 11 to 15 are most active. Although some of the general properties relating the activity of fatty acids to their antimicrobial action are similar to those of amine compounds, the amines are unique in that monounsaturation does not increase compound activity.
The possible modes of action of these compounds are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U., Weinstein A. Effect of aliphatic polyamines on growth and macromolecular syntheses in bacteria. J Gen Microbiol. 1970 Feb;60(2):159–165. doi: 10.1099/00221287-60-2-159. [DOI] [PubMed] [Google Scholar]
- CRONK D. H., ZOPF L. C., JONES J. W. Synthesis and antifungal studies on sorbic acid derivatives. J Am Pharm Assoc Am Pharm Assoc. 1959 Aug;48(8):455–457. doi: 10.1002/jps.3030480809. [DOI] [PubMed] [Google Scholar]
- Davies J., Gorini L., Davis B. D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol. 1965 Jul;1(1):93–106. [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V. Lysis of protoplasts of Micrococcus lysodeikticus by ionic detergents. J Gen Microbiol. 1960 Aug;23:19–26. doi: 10.1099/00221287-23-1-19. [DOI] [PubMed] [Google Scholar]
- GOETTSCH R. W., WIESE G. A. The synthesis of some substituted thianaphthene-2-carboxamides and their antifungal properties. J Am Pharm Assoc Am Pharm Assoc. 1958 May;47(5):319–322. doi: 10.1002/jps.3030470506. [DOI] [PubMed] [Google Scholar]
- GOOD N. E. Activation of the Hill reaction by amines. Biochim Biophys Acta. 1960 Jun 3;40:502–517. doi: 10.1016/0006-3002(60)91391-3. [DOI] [PubMed] [Google Scholar]
- Gershon H., Rodin R. L., Parmegiani R., Godfrey P. K. Organic fluorine compounds. IV. Synthesis and antifungal properties of 2-fluoro fatty acid amides. J Med Chem. 1970 Nov;13(6):1237–1239. doi: 10.1021/jm00300a057. [DOI] [PubMed] [Google Scholar]
- HELLER C. G., FLAGEOLLE B. Y., MATSON L. J. HISTOPATHOLOGY OF THE HUMAN TESTES AS AFFECTED BY BIS(DICHLOROACETYL)DIAMINES. Exp Mol Pathol Suppl. 1963 Dec;2:107–114. [PubMed] [Google Scholar]
- Hueck H. J., Adema D. M., Wiegmann J. R. Bacteriostatic, fungistatic, and algistatic activity of fatty nitrogen compounds. Appl Microbiol. 1966 May;14(3):308–319. doi: 10.1128/am.14.3.308-319.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara J. J., Swieczkowski D. M., Conley A. J., Truant J. P. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972 Jul;2(1):23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J., BENEDICT M., ARTMAN M. A common site of action of polyamines and streptomycin. Biochim Biophys Acta. 1962 Jul 30;62:202–204. doi: 10.1016/0006-3002(62)90519-x. [DOI] [PubMed] [Google Scholar]
- Merola A. J., Brierley G. P. Inhibition of mitochondrial oxidation and uncoupling of phosphorylation by antispermatogenic bis-dichloroacetamides. Biochem Pharmacol. 1970 Apr;19(4):1429–1442. doi: 10.1016/0006-2952(70)90058-4. [DOI] [PubMed] [Google Scholar]
- Mills J., Dubin D. T. Some effects of spermine on Escherichia coli. Mol Pharmacol. 1966 Jul;2(4):311–318. [PubMed] [Google Scholar]
- Morgan L. W., Cronk D. H., Knott R. P. Synthesis and in vitro fungistatic evaluation of some N-substituted amides and amine salts of sorbic acid. J Pharm Sci. 1969 Aug;58(8):942–945. doi: 10.1002/jps.2600580806. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JAGENDORF A. T. LIGHT-INDUCED PH CHANGES RELATED PHOSPHORYLATION BY CHLOROPLASTS. Arch Biochem Biophys. 1964 Jul;107:109–119. doi: 10.1016/0003-9861(64)90276-0. [DOI] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A. F., Solar J. M., Mod R. R., Magne F. C., Skau E. L. Antimicrobial activity of some N-substituted amides of long-chain fatty acids. Appl Microbiol. 1969 Dec;18(6):1050–1056. doi: 10.1128/am.18.6.1050-1056.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. D. The mechanism of action of some antibacterial agents. Prog Med Chem. 1969;6:135–199. doi: 10.1016/s0079-6468(08)70198-x. [DOI] [PubMed] [Google Scholar]
- Silver S., Kralovic M. L. Fatty acid-conjugated polyamines that alter cell permeability and active transport properties of Escherichia coli. Mol Pharmacol. 1969 May;5(3):300–302. [PubMed] [Google Scholar]
- Silver S., Levine E. Action of steroidal diamines on active transport and permeability properties of Escherichia coli. J Bacteriol. 1968 Aug;96(2):338–345. doi: 10.1128/jb.96.2.338-345.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


